Back to Journals » Drug Design, Development and Therapy » Volume 12
Islet protection and amelioration of type 2 diabetes mellitus by treatment with quercetin from the flowers of Edgeworthia gardneri
Authors Zhuang MJ, Qiu HH, Li P, Hu LH, Wang YY, Rao L
Received 12 October 2017
Accepted for publication 8 February 2018
Published 23 April 2018 Volume 2018:12 Pages 955—966
DOI https://doi.org/10.2147/DDDT.S153898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Manjiao Zhuang,1 Honghong Qiu,2 Ping Li,2 Lihua Hu,2 Yayu Wang,3 Lei Rao2
1Department of Pharmacy, Sichuan University, Chengdu, People’s Republic of China; 2Collaborative Innovation Center of Sichuan for Elderly Care and Health, Chengdu Medical College, Chengdu, China; 3Department of Cell Biology, Institute of Biological Medicine, Jinan University, Guangzhou, People’s Republic of China
Background and purpose: The traditional Chinese medicine – the flower of Edgeworthia gardneri – is reported as an effective therapeutic for type 2 diabetes mellitus (T2DM). Nevertheless, most constituents of the flowers of E. gardneri have not yet been studied. This study was conducted to investigate the effect of quercetin extracted from the flowers of E. gardneri on islet protection and amelioration in T2DM and explore its mechanism.
Method: Quercetin was extracted from the flowers of E. gardneri and verified by high-performance liquid chromatography. Quercetin or crude extract’s effect on insulin secretion was investigated. ERK1/2 and phospho-ERK1/2 were detected by Western blot analysis, and fluo-3 AM was used to detect intracellular Ca2+. The anti-apoptosis effect of quercetin or crude extract on MIN-6 cells was investigated by thiazolyl blue tetrazolium bromide (MTT) assay and flow cytometry analysis. Activation of caspases and expression of Bcl-2 and BAX were tested by Western blot analysis. In addition, the mitochondrial membrane potential was determined by JC-1 probe. Moreover, in vivo activity was also tested in db/db mice.
Results: A quercetin level of >10 μmol/L could induce insulin secretion. Intracellular Ca2+ and ERK1/2 were involved in the signaling pathway of quercetin-induced insulin secretion. We also observed that quercetin could inhibit palmitic acid-induced cell apoptosis via suppressing the activation of caspase-3, -9, -12; increasing the ratio of Bcl-2/BAX and reversing the impaired mitochondrial membrane potential. Crude extract’s effect on insulin secretion was similar to that of pure extracted quercetin, while it possessed higher anti-apoptosis activity. Additionally, intraperitoneal glucose tolerance, plasma insulin level, hepatic triglyceride, hepatic glycogen and the pathological histology of both pancreatic islet and liver in db/db mice were significantly improved by the administration of the extracted quercetin.
Conclusion: Our study indicated that quercetin extracted from the flowers of E. gardneri exerted excellent properties in islet protection and amelioration.
Keywords: quercetin, Edgeworthia gardneri, insulin secretion, anti-apoptosis, type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM), a common chronic disease, has spread rapidly as a grave threat to people’s health in the past few decades.1 It is characterized by β-cell dysfunction and chronic insulin resistance.2,3 The decline in β-cell function is characterized as impaired insulin secretion and increased β-cell decrease.4 Therefore, improving insulin secretion as well as inhibiting β-cell apoptosis are considered as two main strategies to treat T2DM.
The traditional Chinese medicine – the flower of Edgeworthia gardneri – is a class of herbal teas found in Tibet that is believed to provide a range of benefits. It contains various active components including coumarins, flavones, edgeworin and daphnoretin.5 Previous studies have demonstrated that the extracts of the flowers of E. gardneri display broad pharmacological activities for the treatment of diabetes, inflammation and cardiovascular disease.6,7 For example, Zhao et al demonstrated that coumarin from the flowers of E. gardneri exhibits α-glucosidase and α-amylase inhibitory activities.8 Ma et al showed that phenolics from the flowers of E. gardneri possess α-glucosidase inhibition and antihyperglycemic activity.9 Nevertheless, most constituents of the flowers of E. gardneri have not yet been studied.
Quercetin is a compound of flavones in many Chinese traditional herbal medicines with good biologic value. It is known to exhibit a broad variety of biological effects that have potential applications in medical treatment, including anticancer, antioxidant, anti-inflammatory and, in particular, protective effect in diabetes.10–12 Quercetin was also found to be abundant in the flowers of E. gardneri, while its antidiabetic activity has yet to be investigated systematically.9
Therefore, in the present study, the isolation and biological activity of quercetin from the flowers of E. gardneri were reported. This is the original report on islet protection and amelioration of T2DM by treatment with quercetin from the flowers of E. gardneri both in vitro and in vivo.
Materials and methods
Materials
The flowers of E. gardneri were purchased from ZangXiTang Co., Ltd (Tibet, People’s Republic of China). Quercetin standard, rutin standard and isoquercetin standard were purchased from Solarbio (Beijing, People’s Republic of China). MIN-6 cell line was purchased from Cell Bank of Chinese Academy Sciences (Beijing, People’s Republic of China). Both RPMI-1640 medium and fetal bovine serum were purchased from Gibco (Gaithersburg, MD, USA). Glimepiride, AZD8330 (inhibitor of ERK1/2), nifedipine (inhibitor of Ca2+ channel) and fluo-3 AM were obtained from Sigma-Aldrich (St Louis, MO, USA). Thiazolyl blue tetrazolium bromide (MTT) was purchased Merck (Darmstadt, German). Insulin concentrations in cell supernatant were determined using the Mouse Insulin Elisa Kit obtained from Crystal Chem (Downers Grove, IL, USA). Rabbit anti-ERK1/2 antibody, rabbit anti-phospho-ERK1/2 antibody (Thr202/Tyr204) and apoptosis-related antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). Annexin V-FITC/PI Apoptosis Detection Kit was purchased from Merck. MitoProbe JC-1 Assay Kit was a product from Thermo Fisher Scientific (Waltham, MA, USA). Triglyceride Quantification Assay Kit (Colorimetric/Fluorometric) and Glycogen Colorimetric/fluorometric Assay Kit were obtained from Abcam (Cambridge, UK).
Extraction and isolation
Dried powder of the flowers of E. gardneri (500 g) was extracted by using methanol at room temperature for 3 days. The resultant extracts were combined and concentrated under reduced pressure, and the residue was partitioned into water and extracted with petroleum ether, ethyl acetate and n-butano in succession. Each fraction was evaporated in vacuo to yield residue extracts. The ethyl acetate fraction was used to isolate quercetin (3 mg) by using column chromatography over Sephadex LH-20 according to Ma et al’s study.9
High-performance liquid chromatography (HPLC) analysis
HPLC analysis of quercetin extracted from the flowers of E. gardneri was implemented by a Waters Alliance 2695-2487 HPLC system with an Agilent C18 column (Waters, Milford, MA, USA) in gradient elution. The effluent was detected at 280 nm.13
MIN-6 cell culture
The insulin-secreting cell line MIN-6 was cultured in RPMI-1640 in accordance with previous studies. MIN-6 cells were cultured and treated with palmitic acid for 24 h to establish oxidative damage model.14
Measurement of insulin secretion
For insulin secretion studies, 1×104 MIN-6 cells were plated in a 96-well microplate and cultured for 48 h. After that, the medium was removed from each well and 1 mL of fresh medium was added. Increasing concentrations of quercetin from E. gardneri were added to the medium, and after 1 h of incubation, the medium was collected. The insulin concentration in the medium was measured by a commercial Mouse Insulin Elisa Kit in accordance with the manufacturer’s instructions. Glimepiride was taken as a positive control. Additionally, to test the role of Ca2+ channel and ERK1/2 in quercetin-induced insulin secretion, relevant inhibitors (AZD8330 or Nifedipine) were added in the medium together with quercetin.15
Measurement of cell viability
Cell viability was determined by MTT assay according to the manufacturer’s instructions.16 Briefly, MIN-6 cells were plated in 96-well plates in DMEM (high glucose) for 24 h. Cells were incubated with increasing concentrations of quercetin or crude extract (0, 0.001, 0.01, 0.1, 1, 10, and 100 μmol/L) in the presence of palmitic acid for 24 h. After incubation, cells were washed twice with Krebs-Ringer bicarbonate HEPES (KRBH, NaCl 118.5 mmol/L, CaCl2-2H2O 2.54 mmol/L, KHP2O4 mmol/L, KCl 4.74 mmol/L, NaHCO3 25 mmol/L, MgSO4 1.19 mmol/L, HEPES 10 mmol/L, 0.1% BSA, pH 7.4) and incubated with 0.5 mg/mL MTT for further 3 h in the dark in a humidified atmosphere (5% CO2, 37°C). Then cells were washed with KRBH and precipitates were dissolved in 50 μL dimethyl sulfoxide (DMSO). The absorbance of the reduced intracellular formazan product was measured at 490 nm on a microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA).
Flow cytometric measurement
Cell apoptosis was detected by using Annexin V-FITC staining. Briefly, cells were harvested after treated with 0.8 mmol/L palmitic acid in combination with quercetin for 24 h and washed twice with PBS followed by staining with Annexin V-FITC at 37°C for 15 min. Data acquisition and analysis were carried out by flow cytometer detection.17
Measurement of intracellular Ca2+
The intracellular Ca2+ concentration was evaluated by fluo-3 AM. The cells were incubated with 2.5 mmol/L fluo-3 AM in dark at 37°C to load fluo-3 AM into the cell. After loading, cells were washed twice with KRBH. Thereafter, 10 μmol/L quercetin was applied onto the cells using a perfusion system. Fluorescence imaging was performed on Olympus-IX711. The image at excitation wavelengths of 506 nm was recorded every 30 min.18
Western blot
The MIN-6 cells were plated in a six-well microplate. After 24 h of incubation with both 0.8 mmol/L palmitic acid and different concentrations of extracted quercetin, cells were collected and total protein was extracted. The expression of ERK1/2 and ERK1/2 phosphorylation level was identified by Western blot analysis, which was performed as previously described by using anti-ERK1/2 antibody or anti-phospho-ERK1/2 antibody (Thr202/Tyr204).19 The activation of caspase-3, -9, -12 and the expression of BAX and Bcl-2 were also measured by Western blot analysis.
Assessment of mitochondrial membrane potential
The mitochondrial membrane potential was determined by JC-1 probe. MIN-6 cells were treated with 0.8 mmol/L palmitic acid in combination with quercetin for 24 h, and then the cells were loaded with 1 mg/L of JC-1 at 37°C for 10 min. Then a confocal microscope (Axiovert 200; Carl Zeiss Meditec AG, Jena, Germany) was used to analyze fluorescence intensity.20
Glucose tolerance test and insulin secretion in db/db mice
Db/db mice with T2DM (BKS.Cg-m+/+Leprdb/J) and their lean wild-type control (C57BLKS/J db/+) were purchased from Model Animal Research Center of Nanjing University. Mice, 8 weeks old, were used in this study. The animals had access to diet and water ad libitum. The animal experiments were conducted in compliance with the guidelines of Chengdu Medical College Animal Studies Committee. The institutional review board of Chengdu Medical College Animal Studies Committee also approved this study.
The mice were randomly divided into four groups of six mice each:
Group 1: normal mice were intragastrically administrated with 0.4 mL distilled water per day for 4 weeks.
Group 2: db/db mice were intragastrically administrated with 0.4 mL equivalent distilled per day water for 4 weeks.
Group 3: db/db mice were intragastrically administrated with 0.5 g/kg glibenclamide per day for 4 weeks.
Group 4: db/db mice were intragastrically administrated with 0.5 g/kg quercetin per day for 4 weeks.
At the end of quercetin treatment, IPGTT was performed. Each animal was fasted overnight, and IPGTTs were carried out. For the initial 15 min after the last intragastric administration, db/db mice received an intraperitoneal injection of 15% glucose solution (2 g/kg body weight). Tail blood was collected before (time 0) the administration of the glucose and 15, 30, 60, 90 and 120 min later for IPGTT and detecting blood insulin level. The concentration of blood glucose was determined with One Touch Ultra Meter (Johnson & Johnson, New Buren Zwick, NJ, USA). Meanwhile, plasma insulin level at different time points was also evaluated by using mouse insulin Elisa Kit. In addition, after the experimental period, the animals were sacrificed. Liver segments and other organs from each animal were quickly removed for the quantification of hepatic fat, hepatic glycogen content and histopathological studies.21
Quantification of hepatic triglyceride and hepatic glycogen content
Triglyceride (TG) level in hepatic tissue was measured with Triglyceride Quantification Assay Kit (Abcam), according to the manufacturer’s instructions. Absorbance was measured with a microplate spectrophotometric system at OD 570 nm (colorimetric). The result of TG was expressed as mg TG per g tissue (mg/g). A Glycogen Colorimetric/Fluorometric Assay Kit (Abcam) was used in accordance with the manufacturer’s protocol to measure the hepatic glycogen content. Results were expressed as mg glycogen per g liver weight (mg/g).22
Histopathological studies
The pancreatic and liver tissues were fixed in 10% formaldehyde, subsequently dehydrated in a graded series of ethanol and embedded in paraffin. The obtained pancreatic or liver sections (5 μm thick) were then dewaxed, rehydrated and stained with hematoxylin–eosin. The staining sections were observed by the microscope (400×).23
Statistical analysis
Experimental data are presented as mean values ± SEM. Statistical difference was assessed by one-way analysis of variance. Unpaired two-tailed t-tests and paired two-tailed t-tests were used to determine the statistical significance between test groups as appropriate. P < 0.05 was considered statistically significant.
Results
Isolation and qualification of quercetin
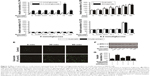
The quercetin extracted from E. gardneri was identified with standard quercetin combined with HPLC. As shown in Figure 1, the retention time of extracted quercetin was similar with standard substance, showing that the composition of the extracted product was the same as the composition of standard quercetin. Quercetin identified in the extract of E. gardneri naturally exists in two forms: aglycone quercetin and glycoside quercetin. Rutin and isoquercetin are glycoside forms of quercetin extracted from E. gardneri.9 The content of quercetin glycosides accounted for about 72.2% of the quercetin extracted from E. gardneri according to Ma et al’s study.9 The extracted quercetin identified in our study was aglycone quercetin, which was characterized by HPLC as shown in Figure 1C and D.
Effect of quercetin from the flowers of E. gardneri on insulin secretion
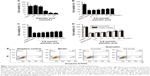
The decline in β-cell function is identified as a major cause of T2DM; thus, insulin secretagogue effect of extracted quercetin was evaluated. The extracted quercetin induced a slight increase in insulin secretion in the absence of glucose. While in the presence of glucose (8.3 mmol/L), quercetin (10 μmol/L) provoked threefold increase in insulin secretion compared with the control group showing that quercetin induced insulin secretion in a glucose-dependent manner. However, with the quercetin concentration reaching 100 μmol/L, the insulin secretagogue effect was significantly decreased (Figure 2A). In addition, as illustrated in Figure 2B, glimepiride as well as the extracted quercetin (10 μmol/L) could induce a time-dependent increase in insulin secretion in MIN-6 cells. Moreover, the effect of crude extract of E. gardneri on insulin secretion was similar to that of pure quercetin (Figure 2C and D).
The high content of Ca2+ in insulin secretory granules is crucial for the secretory function of pancreatic β cells.19 To investigate whether the insulin secretagogue effect of the quercetin involves in the change of Ca2+, 10 μmol/L quercetin was applied on MIN-6 cells. The intracellular Ca2+ levels in MIN-6 were monitored by using Ca2+ indicator fluo-3 AM. As illustrated in Figure 2E, Ca2+ influx in response to quercetin stimulation (10 μmol/L) was significantly greater than control group.
Youl et al reported that quercetin can potentiate ERK1/2 phosphorylation in INS-1 cells;15 thus, the ERK1/2 phosphorylation level trigged by the extracted quercetin was investigated in MIN-6 cells. As depicted in Figure 2F, the extracted quercetin increased ERK1/2 phosphorylation. Notably, the extracted quercetin (10 μmol/L) promoted a remarkable phosphorylation of the ERK1/2, which was consistent with the insulin secretagogue effect of quercetin.
The inhibitors of Ca2+ channel and ERK1/2 were also used to evaluate the quercetin-induced insulin secretion. As depicted in Figure 2G, in the presence of 10 μmol/L AZD8330 or nifedipine, the elevation of insulin secretion induced by extracted quercetin was significantly inhibited.
Anti-apoptosis effect of quercetin from the flowers of E. gardneri
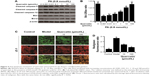
To establish the apoptosis model, palmitic acid was used to incubate MIN-6 cells. The administration of palmitic acid (0.05, 0.2, 0.8, 3.2 and 12.8 mmol/L) in the presence of 8.3 mmol/L glucose resulted in a concentration-dependent cell viability inhibition (Figure 3A). According to the results, 0.8 mmol/L palmitic acid induced ~50% cell viability decrease. Therefore, quercetin was tested on MIN-6 cells’ viability in the presence of 0.8 mmol/L palmitic acid as well as 8.3 mmol/L glucose. As shown in Figure 3B, 10 μmol/L extracted quercetin obviously improved MIN-6 cell viability in the presence of 0.8 mmol/L palmitic acid. Additionally, viability of MIN-6 cells was significantly increased 16 h after treatment with quercetin under the induction of palmitic acid (Figure 3C). According to Figure 3D, the crude extract was found more active in anti-apoptosis activity than the pure extract quercetin. As shown in Figure 3E, the percentage of apoptotic cells increased approximately by 40%, following treatment with 0.8 mmol/L palmitic acid, as compared with the control group. Quercetin had an obvious protective effect on palmitic acid-induced cell apoptosis in a dose-dependent manner (Figure 3E). Caspase-3, -9, -12 and Bcl-2/BAX are the important regulators of cell apoptosis, and therefore, we investigated their involvement during quercetin treatment in MIN-6 cells. It was observed that quercetin inhibited the activation of caspase-3, -9, -12 induced by palmitic acid. Additionally, quercetin increased the expression of Bcl-2 and decreased the expression of BAX (Figure 4A and B).
The decline of mitochondrial membrane potential is an early landmark event of apoptosis. Therefore, JC-1 fluorescent probe was used to monitor the effect of quercetin on the mitochondrial membrane potential of treated MIN-6 cells. As depicted in Figure 4C and D, the palmitic acid-treated cells stained green indicating partial dissipation of the mitochondrial membrane potential. Quercetin reversed the repression of mitochondrial membrane potential and suppressed apoptosis induced by palmitic acid in the MIN-6 cells.
Effect of quercetin from the flowers of E. gardneri in the treatment of db/db mice
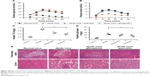
To evaluate the effect of quercetin from the flowers of E. gardneri on glucose tolerance and insulin secretion, IPGTT and the plasma insulin were both evaluated. The result of the IPGTTs is shown in Figure 5A; as expected, db/db mice exhibited impaired glucose tolerance accompanied by a significantly diminished glucose-stimulated insulin secretion (Figure 5B). Treatment of db/db mice with quercetin resulted in a remarkable improvement in glucose tolerance, as proved by a much more rapid decrease in blood glucose level compared with db/db mice. Following intraperitoneal injection of glucose, there was an immediate increase in plasma insulin levels of normal mice. Compared with db/db mice, an improved insulin secretion was observed in both glimepiride and quercetin groups (Figure 5B). The peak insulin level was significantly higher in the quercetin group as compared with the db/db mice.
To assess whether fat accumulation in the liver was improved by quercetin, TG content in liver tissues was determined. TG was 2.3-fold higher in the db/db mice in comparison with normal mice. Quercetin treatment was sufficient to suppress TG accumulation in the liver substantially (Figure 5C). As impaired hepatic fat is commonly associated with impaired glycogen metabolism in the liver; therefore, glycogen content in the liver was also evaluated. The hepatic glycogen stores were reduced in both quercetin and glimepiride treatment groups as compared with db/db mice (Figure 5D).
According to microscopic examination, β cells with granulated cytoplasm and uniform nuclei were observed in the normal mice. However, the β cells were found degranulated, with marked vacuolation and dark scanty cytoplasm in db/db mice. Db/db mice given quercetin revealed a variable degree of improvement in the number of pancreatic islets and degree of vacuolations (Figure 5E). Moreover, as compared with the liver of normal mice, the liver of db/db mice showed predominantly large lipid-filled vacuoles and macrovesicular steatosis. Treatment of db/db mice with quercetin exhibited less lipid-filled vacuoles and was distinguishable from db/db mice (Figure 5E). The aforementioned changes in quercetin group were similar to those in the positive control group.
Discussion
T2DM is a serious chronic metabolic disorder with its patient number increasing dramatically in recent decades.1 Insulin, metformin, sulfonylureas, peroxisome proliferator-activated receptor-γ agonists and α-glucosidase inhibitors are considered as main therapies for T2DM.24 Notably, many traditional Chinese medicines including herbal medicines have been widely used for thousands of years in the treatment of T2DM.25 The flower of E. gardneri is a commonly used herbal tea in Tibet, possessing beneficial effects in the treatment of diabetes and obesity. Hoping to realize the modernization of traditional Chinese medicine, modern science and technology has been used to study the pharmacodynamic components and mechanisms of them. Therefore, in the present study, we isolated quercetin from E. gardneri and evaluated its effects on islet protection and amelioration of T2DM both in vitro and in vivo.
As shown in the HPLC, the retention time of the major peak of the extracted product corresponded to the standard quercetin with less impure peaks (Figure 1). It can be concluded that high-purity quercetin was successfully obtained from the flowers of E. gardneri. The extracted quercetin identified in our study was aglycone quercetin, which was characterized by HPLC as shown in Figure 1C and D. However, the glycosides forms of quercetin, rutin and isoquercetin, are the major forms of quercetin from E. gardneri. Generally, aglycone form of quercetin exerts better activity in insulin secretagogue or anti-apoptosis; therefore, a further study to improve the extraction process for obtaining more aglycone quercetin is needed.
Glucose, a crucial factor in regulating insulin secretion, induces insulin secretion by generating and triggering signals in β cells.26 Our study demonstrated that the extracted quercetin (10 μmol/L) stimulated a remarkable insulin secretion in MIN-6 cells in the presence of 8.3 mmol/L glucose. While in the absence of glucose, insulin secretagogue effects induced by extracted quercetin were significantly decreased. According to the results, the effect of crude extract of E. gardneri on insulin secretion was similar to that of pure extracted quercetin, indicating that quercetin was the only effective ingredient with insulin secretagogue activity in E. gardneri. Previous studies have reported that impaired acute stimulation of insulin secretion induced by glucose can be compensated for by medicines directly opening Ca2+ channels.26,27 In our study, the extracted quercetin (10 μmol/L) was shown to significantly enhance the intracellular Ca2+ in MIN-6 cells, indicating that Ca2+ participated in quercetin-induced insulin secretion (Figure 2E). Quoyer et al reported that ERK1/2 participates in the regulation of insulin secretion.19 Additionally, Youl et al have reported that quercetin potentiates ERK1/2 phosphorylation in INS-1 cells.15 Accordingly, the extracted quercetin (10 μmol/L) was observed to induce a remarkable increase in ERK1/2 phosphorylation, which was consistent with the concentration of quercetin in stimulating insulin secretion (Figure 2F). Therefore, ERK1/2 was also suggested to take part in quercetin-induced insulin secretion.
To further clarify the role of intracellular Ca2+ levels and ERK1/2 in quercetin-induced insulin secretion, the inhibitors of Ca2+ channel and ERK1/2 were used. Both were observed to inhibit quercetin-induced insulin secretion (Figure 2G). The inhibited action of AZD8330 and nifedipine proved that intracellular Ca2+ and ERK1/2 were involved in quercetin-induced insulin secretion.
Increased β-cell apoptosis and β-cell deficit were commonly found in T2DM patients.28 Next, our research was focused on studying the effects of extracted quercetin on palmitic acid-induced MIN-6 cell apoptosis, since palmitic acid was widely used in vitro to establish the apoptosis model.29 The optimal concentration of palmitic acid was found to be 0.8 mmol/L (Figure 3A). Our study revealed that 10 μmol/L quercetin obviously improved MIN-6 cell viability in the presence of palmitic acid (Figure 3B and C). This result could be explained by recently published research showing that quercetin exerts the antioxidant activity.10 However, the crude extract was found to be more active in the anti-apoptosis activity than the pure extracted quercetin (Figure 3D). It has been reported that E. gardneri consists of a variety of antioxidant ingredients with excellent anti-apoptosis activity, including quercetin, ferulic acid, kaempferol and isoquercetin, which also exist in the crude extract of E. gardneri.9 Therefore, the anti-apoptosis activity of E. gardneri was partly from quercetin. Detection of apoptosis by flow cytometry shows that quercetin exerted potential anti-apoptosis benefits. Our current results provided new insights into the mechanisms of quercetin-mediated anti-apoptosis effect. The results revealed that quercetin could not only inhibit the activation of caspase-3, -9, -12 induced by palmitic acid but also increase the ratio of Bcl-2/BAX in the presence of palmitic acid. (Figure 4A and B). The level of mitochondrial function is highly correlated with cell apoptosis. Our results found that quercetin could inhibit the apoptosis of MIN-6 by reversing the decreased mitochondria membrane potential. The results proved that quercetin exerted outstanding anti-apoptosis capacities for MIN-6 cells by suppressing the activation of caspase-3, -9, -12, decreasing the ratio of Bcl-2/BAX and reversing the impaired mitochondrial membrane potential.
The effects of quercetin on islet protection and amelioration of T2DM were also verified in vivo. Both glimepiride- and quercetin-treated db/db mice showed a remarkable improvement in glucose tolerance and had significantly enhanced plasma insulin levels throughout the IPGTT (Figure 5A and B).
The liver plays an important role in controlling carbohydrate metabolism and lipid homeostasis, and dysfunction in hepatic glucose metabolism can be observed in T2DM.30 Our results showed that decreased liver TG and glycogen stores were detected after the treatment of quercetin (Figure 5C and D), showing the liver function of db/db mice was improved by quercetin. According to Jo et al’s study, quercetin possesses a strong inhibitive activity in α-amylase and α-glucosidases, which are involved in the digestion and absorption of carbohydrates.31 Thereafter, quercetin is correlated greatly with carbohydrate metabolism, which probably influences the liver TG and glycogen stores, although a further understanding of the molecular mechanisms underlying this function is still in need of evaluation. Furthermore, treatment with extracted quercetin could obviously improve the pathological injury in liver tissues and pancreatic islets of db/db mice (Figure 5E). The in vivo results revealed that quercetin isolated from the flowers of E. gardneri could effectively improve the fat metabolism disorder and decrease the levels of blood sugar in diabetic mice.
Conclusion
In summary, quercetin isolated from the flowers of E. gardneri could significantly enhance insulin secretion via Ca2+ and ERK1/2 signaling pathway in MIN-6 cell. Additionally, it could inhibit palmitic acid-induced cell apoptosis by suppressing the activation of caspase-3, -9, -12, increasing the ratio of Bcl-2/BAX and reversing impaired mitochondrial membrane potential. Furthermore, the treatment effect of quercetin in vivo was validated. Quercetin was demonstrated to be beneficial in the treatment of T2DM; however, further understanding of its molecular mechanisms in the treatment of T2DM is still in need of evaluation. Additionally, the role of quercetin from E. gardneri was well clarified. The insulin secretagogue activity of E. gardneri was entirely from quercetin, while its anti-apoptosis activity was partly due to quercetin. This study provided a base for the further development of quercetin from the flowers of E. gardneri in the treatment of T2DM.
Acknowledgments
This work was supported by the Collaborative Innovation Center of Sichuan for Elderly Care and Health (number YLZBZ1517), the Education Department of Sichuan province (number 16ZA0292) and the Chengdu Medical College Foundation (number CYZ15-07).
Disclosure
The authors report no conflicts of interest in this work.
References
Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(10):2568–2569. | ||
DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37(6):667–687. | ||
Malchoff CD. Diagnosis and classification of diabetes mellitus. Conn Med. 1991;55:625–629. | ||
Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–S107. | ||
Xu P, Xia Z, Lin Y. Chemical constituents from Edgeworthia gardneri (Thymelaeaceae). Biochem Syst Ecol. 2012;45:148–150. | ||
Wang QY, Xu HY, Xu ZH, Lu ZM, Liu M, Shi JS. Hypoglycemic effect of water extracts from Edgeworthia gardneri (Wall.) Meissn on type 2 diabetic mice. Nat Prod Res Dev. 2014;26(9):1385–1388. | ||
Gao D, Zhang YL, Xu P, et al. In vitro evaluation of dual agonists for PPARγ/β from the flower of Edgeworthia gardneri (wall.) Meisn. J Ethnopharmacol. 2015;162:14–19. | ||
Zhao DG, Zhou AY, Du Z, Zhang Y, Zhang K, Ma YY. Coumarins with α-glucosidase and α-amylase inhibitory activities from the flower of Edgeworthia gardneri. Fitoterapia. 2015;107:122–127. | ||
Ma YY, Zhao DG, Zhou AY, Zhang Y, Du Z, Zhang K. α-glucosidase inhibition and antihyperglycemic activity of phenolics from the flowers of Edgeworthia gardneri. J Agric Food Chem. 2015;63(37):8162–8169. | ||
Lamson DW, Brignall MS. Antioxidants and cancer, part 3: quercetin. Altern Med Rev. 2000;5(3):196–208. | ||
Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2–3):325–337. | ||
Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C(3):357–364. | ||
Rao L, Ma Y, Zhuang M, Luo T, Wang Y, Hong A. Chitosan-decorated selenium nanoparticles as protein carriers to improve the in vivo half-life of the peptide therapeutic BAY 55-9837 for type 2 diabetes mellitus. Int J Nanomedicine. 2014;9:4819–4828. | ||
Bell E, Cao X, Moibi JA, et al. Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes. 2003;52(11):2731–2739. | ||
Youl E, Bardy G, Magous R, et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol. 2010;161(4):799–814. | ||
Präbst K, Engelhardt H, Ringgeler S, Hübner H. Basic colorimetric proliferation assays: MTT, WST, and Resazurin. Methods Mol Biol. 2017;1601:1–17. | ||
Matias AC, Manieri TM, Cerchiaro G. Zinc chelation mediates the lysosomal disruption without intracellular ROS generation. Oxid Med Cell Longev. 2016;2016:6724585. | ||
Zhang WH, Rengel Z, Kuo J. Determination of intracellular Ca in cells of intact wheat roots: loading of acetoxymethyl ester of Fluo-3 under low temperature. Plant J. 2010;15:147–151. | ||
Quoyer J, Longuet C, Broca C, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem. 2010;285(3):1989–2002. | ||
Ganta KK, Mandal A, Chaubey B. Depolarization of mitochondrial membrane potential is the initial event in non-nucleoside reverse transcriptase inhibitor efavirenz induced cytotoxicity. Cell Biol Toxicol. 2017;33(1):69–82. | ||
Mori N, Kurata M, Yamazaki H, et al. Intragastric administration of allyl isothiocyanate reduces hyperglycemia in intraperitoneal glucose tolerance test (IPGTT) by enhancing blood glucose consumption in mice. J Nutr Sci Vitaminol (Tokyo). 2013;59(1):56–63. | ||
Lin KT, Hsu SW, Lai FY, Chang TC, Shi LS, Lee SY. Rhodiola crenulata extract regulates hepatic glycogen and lipid metabolism via activation of the AMPK pathway. BMC Complement Altern Med. 2016;16:127. | ||
Gawish AM, Issa AM, Ali MA, Ismail GA. Histopathological, histochemical and biochemical studies on the effects of lorsban on the liver of Nile tilapia and the possible declaring effect of antioxidants. Aust J Basic Appl Sci. 2011;5(12):75–95. | ||
Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414(6865):821–827. | ||
Liu JP, Zhang M, Wang WY, Grimsgaard S. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2004;(3):CD003642. | ||
Hedeskov CJ. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980;60(2):442–509. | ||
Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes. 1999;48(7):1402–1408. | ||
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. | ||
Xiong FL, Sun XH, Gan L, Yang XL, Xu HB. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur J Pharmacol. 2006;529(1–3):1–7. | ||
Leung PS. The potential protective action of vitamin D in hepatic insulin resistance and pancreatic islet dysfunction in type 2 diabetes mellitus. Nutrients. 2016;8(3):147. | ||
Jo SH, Ka EH, Lee HS, Apostolidis E, Jang HD, Kwon YI. Comparison of antioxidant potential and rat intestinal a-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. Int J Appl Res Nat Prod. 2009;2:52–60. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.