Back to Journals » Infection and Drug Resistance » Volume 13
Is There Emergence of β-Lactam Antibiotic-Resistant Streptococcus pyogenes in China?
Authors Yu D , Zheng Y , Yang Y
Received 9 May 2020
Accepted for publication 25 June 2020
Published 14 July 2020 Volume 2020:13 Pages 2323—2327
DOI https://doi.org/10.2147/IDR.S261975
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Dingle Yu,1,2,* Yuejie Zheng,2,* Yonghong Yang1,2
1Laboratory of Microbiology and Immunology, Beijing Children’s Hospital Affiliated to Capital Medical University, Beijing, People’s Republic of China; 2Department of Respiratory, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yonghong Yang Email [email protected]
Abstract: Streptococcus pyogenes is regarded as susceptible to β-lactam antibiotics. The guidelines of the Clinical and Laboratory Standards Institute (CLSI) are widely recognized and have long-recommended penicillin for treatment of S. pyogenes infections. There is no CLSI guideline for the treatment of S. pyogenes infections that have intermediate susceptibility or resistance to penicillin. However, there have been several reports of S. pyogenes isolates that are nonsusceptible or even resistant to β-lactam antibiotics, mostly from Chinese journals. The purpose of this commentary is to show data from the literature which suggests the presence of S. pyogenes isolates that are not susceptible to β-lactam antibiotics and whether these strains are really nonsusceptible to β-lactam antibiotics and the presence of mutation in the pbp2x gene requires further research and confirmation.
Keywords: Streptococcus pyogenes, GAS, β-lactam, antibiotic resistance, China
Introduction
Streptococcus pyogenes, also called group A Streptococcus (GAS), is a major human pathogen that can cause a broad spectrum of acute infections. Traditionally, S. pyogenes was regarded as susceptible to β-lactam antibiotics, including penicillins and cephalosporins. Thus, penicillin is administered as a first-line antibiotic, and macrolides are an alternative option.1 However, there have been several reports of the emergence of S. pyogenes isolates with resistance to β-lactam antibiotics or reduced susceptibility to penicillin. These findings require confirmation. What is the actual situation? We will address this issue by reviewing the literature.
Search Strategy and Selection Criteria
Data for this review were identified by searches of MEDLINE, Current Contents, PubMed, Wanfang, and references from relevant articles using the search terms “antibiotic”, “resistance”, “surveillance”, “Streptococcus pyogenes” and “group A streptococci”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in the English language between 1995 and 2019 were included. Moreover, the references of all identified articles were searched for further articles. Finally, the search was restricted to manuscripts that were published in China up to May 2020.
Reports of S. pyogenes That is Nonsusceptible to β-Lactam Antibiotics
There have been several reports of the emergence of S. pyogenes isolates that are nonsusceptible or even resistant to β-lactam antibiotics, most of which were published in Chinese journals between 2002 and 2018. Most of these reports were from the large Antimicrobial Surveillance Network (CHINET) in China and were published in Chinese Journals (Table 1). Our examination of the literature indicated only a few isolates of S. pyogenes outside of China were not susceptible to β-lactam antibiotics. A study in Mexico2 reported diminished susceptibility (increased MIC) to penicillin (0.25 to 0.75 μg/mL) in 10 (5%) isolates, a study in India3 identified 7 of 34 strains (20.6%) that were nonsusceptible to penicillin (MICs of 0.19 to 0.25 μg/mL), and a study in Japan4 found 2 of 93 strains that were “resistant” to penicillin (MIC > 2.0 U/mL).
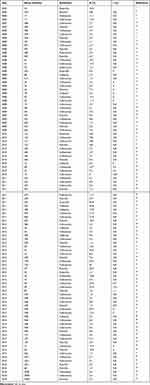 |
Table 1 Publications Reporting the Percentages of Resistance (R) and Intermediate Susceptibility (I) to β-Lactam Antibiotics in Isolates of Streptococcus pyogenes in China |
The standards of the Clinical and Laboratory Standards Institute (CLSI) are widely recognized, and its standard for treatment of Streptococcus infections with penicillin has not changed for many years. These standards consider an inhibition zone diameter of 24 mm or more or a MIC of 0.12 μg/mL or less as indicating susceptibility to penicillin, and by extension to other β-lactam antibiotics (ampicillin, amoxicillin, and cefaclor). The breakpoints for nonsusceptibility are slightly different for penicillin (MIC > 0.12 µg/mL), ampicillin (MIC > 0.25 µg/mL), and cefotaxime/ceftriaxone (MIC > 0.5 µg/mL). However, there is no specific CLSI standard for the use of penicillin for the treatment of patients who have isolates with intermediate susceptibility or resistance.
We read with great interest of a study that investigated 7025 genome sequences of S. pyogenes strains and identified 137 strains that had 37 nonsynonymous mutations in 36 codons in the pbp2x gene.5 The authors proposed that decreased β-lactam susceptibility was geographically widespread in strains with common emm gene subtypes. Coincidentally, Vannice et al6 also recently reported two nearly identical GAS isolates, each with the same rare mutation that led to elevated β-lactam MICs and an invasive infection. The two nearly identical clinical S. pyogenes isolates had the subtype emm43.4 and a pbp2x missense mutation (T553K).
Conclusion
Traditionally, S. pyogenes was regarded as susceptible to β-lactam antibiotics. However, many publications, mostly from China (Table 1), have reported intermediate susceptibility or even resistance to β-lactam antibiotics, but without confirmation. Whether these strains are really nonsusceptible to β-lactam antibiotics, and whether they really have pbp2x mutations will require further research and confirmation.
Author Contributions
YY conceived the idea. DY and YZ were responsible for the concept and contributed to the manuscript. All authors reviewed and agreed with the final manuscript.
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Yonghong Yang reports grants from Shenzhen city government, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Camara M, Dieng A, Boye CSB. Antibiotic susceptibility of Streptococcus pyogenes isolated from respiratory tract infections in Dakar, Senegal. Microbiol Insights. 2013;6:71–75. doi:10.4137/MBI.S12996
2. Amábile-Cuevas CF, Hermida-Escobedo C, Vivar R. Comparative in vitro activity of moxifloxacin by E-test against Streptococcus pyogenes. Clin Infect Dis. 2001;null(Supplement_1):S30–32. doi:10.1086/319373
3. Capoor MR, Nair D, Deb M, et al. Resistance to erythromycin and rising penicillin MIC in Streptococcus pyogenes in India. Jpn J Infect Dis. 2006;59(5):334–336.
4. Ogawa T, Terao Y, Sakata H, et al. Epidemiological characterization of Streptococcus pyogenes isolated from patients with multiple onsets of pharyngitis. FEMS Microbiol Lett. 2011;318(2):143–151. doi:10.1111/j.1574-6968.2011.02252.x
5. Musser JM, Beres SB, Zhu L, et al. Reduced in vitro susceptibility of streptococcus pyogenes to β-lactam antibiotics associated with mutations in the pbp2x gene is geographically widespread. J Clin Microbiol. 2020;58(4):e01993–01919. doi:10.1128/JCM.01993-19
6. Vannice K, Ricaldi J, Nanduri S, et al. Streptococcus pyogenes pbp2x mutation confers reduced susceptibility to β-lactam antibiotics. Clin Infect Dis. 2019.
7. Shanghai surveillance of bacterial resistance working group. Surveillance of bacterial resistance in Shanghai. Chinese J Infect Chemother. 2002;2:1–9.
8. Zhu D, Wang F, Zhang Y. Surveillance of bacterial resistance in hospitals of Shanghai during 2005. Chinese J Infect Chemother. 2006;6:371–376.
9. Wang F. CHINET 2005 surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2006;6:289–295.
10. Zhu D, Zhang Y, Wang F. Surveillance of bacterial resistance in Shanghai hospitals during 2006. Chinese J Infect Chemother. 2007;7:393–399.
11. Wang F. CHINET 2006 surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2008;8:1–9.
12. Wang F, Zhu D, Hu F, et al. CHINET 2007 surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2008;8:325–333.
13. Zhu D, Zhang Y, Wang F. Surveillance of bacterial resistance from hospitals in Shanghai in 2007. Chinese J Infect Chemother. 2008;8:401–410.
14. Wang J, Xiao Y. Mohnarin report 2006–2007: bacterial distribution and resistance in bloodstream infections. Chin J Nosocomiology. 2008;18:1238–1242.
15. Xiao Y, Wang J, Zhao C, et al. Mohnarin bacterial resistance surveillance 2006–2007. Chin J Nosocomiology. 2008;18:1051–1056.
16. Zhao C, Xiao Y. Mohnarin report 2006–2007: bacterial resistant surveillance among inpatients of non-ICU departments in China. Chin J Nosocomiology. 2008;18:1228–1232.
17. Wang J, Xiao Y. Mohnarin report 2006–2007: bacterial distribution and resistance in outpatients and emergency patients. Chin J Nosocomiology. 2008;18:1233–1237.
18. Wang C, Xue J, Zhang H, et al. Distribution and antibiotic resistance of Streptococcus spp.: results of national CHINET program 2007. Chinese J Infect Chemother. 2009;9:180–184.
19. Wang F, Zhu D, Hu F, et al. CHINET 2008 surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2009;9:321–329.
20. Zhu D, Zhang Y, Wang F, et al. Surveillance of bacterial resistance from hospitals in Shanghai during 2008. Chinese J Infect Chemother. 2009;9:401–411.
21. Wang J, Xiao Y. 2008 Mohnarin report: results of Streptococcus, Haemophilus and Moraxella catarrhalis resistance. Chin J Antibiot. 2010;35:543–547.
22. Zhu D, Zhang Y, Wang F, et al. Surveillance report of bacterial resistance from hospitals in Shanghai in 2009. Chinese J Infect Chemother. 2010;10:403–413.
23. Wang C, Wang A, Zhang H, et al. CHINET 2009 surveillance of antibiotic resistance in Streptococcus spp. in China. Chinese J Infect Chemother. 2010;10:426–429.
24. Wang J, Xiao Y. 2008 Mohnarin report: bacterial drug resistance surveillance of emergency outpatients. Chin J Nosocomiology. 2010;20:2393–2398.
25. Zhu D, Wang F, Hu F, et al. CHlNET 2009 surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2011;11:321–329.
26. Zhu D, Zhang Y, Wang F, et al. Surveillance of bacterial resistance in Shanghai hospitals during 2010. Chinese J Infect Chemother. 2011;11:436–445.
27. Zheng B, Lv Y, Wang S. Mohnarin report 2010: surveillance for antimicrobial resistance of gram-positive cocci. Chin J Nosocomiology. 2011;21:5128–5132.
28. Hu F, Zhu D, Wang F, et al. 2011 CHINET surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2012;12:321–329.
29. Zhang X, Yang Q, Sun H, et al. Surveillance of bacterial resistance in Peking Union Medical College Hospital during the period from 2005 to 2010. Chinese J Infect Chemother. 2012;12:330–339.
30. Luo J, Yang Q, Yu Y, et al. Distribution and antimicrobial resistance of common pathogens isolated from respiratory secretions in CHINET 2010 surveillance in China. Chinese J Infect Chemother. 2012;12:340–347.
31. Chen Y, Shen P, Wei Z, et al. Mohnarin report of 2010: surveillance of bacterial resistance of outpatient and emergency patients. Chin J Nosocomiology. 2012;22:491–496.
32. Xiao Y, Shen P, Wei Z, et al. Mohnarin report of 2011: monitoring of bacterial resistance in China. Chin J Nosocomiology. 2012;22:4946–4952.
33. Yang Q, Chen X, Kong H, et al. Mohnarin report of 2010: surveillance of bacterial resistance in patients under 14 years old. Chin J Nosocomiology. 2012;22:497–502.
34. Chen Y, Shen P, Wei Z, et al. Mohnarin report of 2011: bacterial resistance surveillance of emergency and outpatient isolates. Chin J Nosocomiology. 2012;22:5482–5487.
35. Yang Q, Chen X, Kong H, et al. Mohnarin report of 2011: surveillance of bacterial resistance in patients aged between 0 and 14. Chin J Nosocomiology. 2012;22:5488–5492.
36. Wang F, Zhu D, Hu F, et al. 2012 CHINET surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2013;13:321–330.
37. Zhou W, Kuang L, Su M, et al. 2012 annual report of Sichuan provincial antimicrobial resistant investigation net: surveillance of bacterial resistance in patients aged between 0 and 14. Pract J Clin Med. 2013;10:66–71.
38. Yang Y, Zhang L, Wang L, et al. Ministry of health national antimicrobial resistant investigation net report of 2011: bacterial resistance surveillance in patients under 14 years old. Chin J Clin Pharmacol. 2014;30:83–88.
39. Hu F, Zhu D, Wang F, et al. CHINET 2013 surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2014;14:365–374.
40. Fan J, Dong L, Chen Z, et al. Clinical characteristics and antimicrobial resistance of invasive group A phemolytic streptococcus infection in children. Chin J Pediatr. 2014;52(1):46–50.
41. Hu F, Zhu D, Wang F, et al. CHINET 2014 surveillance of bacterial resistance in China. Chinese J Infect Chemother. 2015;15:401–410.
42. Hu F, Zhu D, Wang F, et al. Report of CHINET antimicrobial resistance surveillance program in 2015. Chinese J Infect Chemother. 2016;16:685–694.
43. Li Y, Lv Y, Xue F, et al. Antimicrobial susceptibility of gram-positive organisms: results from china antimicrobial resistance surveillance trial program, 2013–2014. Chin J Lab Med. 2016;39:120–129.
44. Hu F, Guo Y, Zhu D, et al. Antimicrobial resistance profile of clinical isolates in hospitals across China: report from the CHINET surveillance program, 2017. Chinese J Infect Chemother. 2018;18:241–251.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
