Back to Journals » Research and Reports in Urology » Volume 15
Is Size All That Matters? New Predictors of Complications and Bleeding in Renal Angiomyolipoma
Authors Combes A , McQueen S, Palma CA, Benz D, Leslie S, Sved P, Boulas J, Vasilaras A, Rogan C , Drivas I, Eisinger DR, Waugh R
Received 5 January 2023
Accepted for publication 4 March 2023
Published 20 March 2023 Volume 2023:15 Pages 113—121
DOI https://doi.org/10.2147/RRU.S400730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Alexander Combes,1 Simon McQueen,2 Catalina Alejandra Palma,1 David Benz,2 Scott Leslie,1,3– 5 Paul Sved,1,5 John Boulas,1,5 Arthur Vasilaras,1,5 Chris Rogan,2,5 Ilias Drivas,2 David Robert Eisinger,1 Richard Waugh2
1Department of Urology, Royal Prince Alfred Hospital, Camperdown, NSW, 2050, Australia; 2Department of Radiology, Royal Prince Alfred Hospital, Camperdown, NSW, 2050, Australia; 3RPA Institute of Academic Surgery, Sydney, NSW, Australia; 4Faculty of Medicine, University of Sydney, Sydney, NSW, Australia; 5Department of Urology, Chris O’Brien Lifehouse, Sydney, NSW, Australia
Correspondence: Alexander Combes, Department of Urology, Royal Prince Alfred Hospital, 50 Missenden Road, Camperdown, NSW, 2050, Australia, Tel +612 9515 7773, Fax +612 9515 7774, Email [email protected]
Purpose: Renal angiomyolipoma (AML) is the most common benign renal tumor. Whilst generally asymptomatic, they can cause life-threatening bleeding. Selective angioembolization (SAE) may be used to treat large symptomatic and asymptomatic AMLs. We aimed to evaluate the efficacy of SAE for symptomatic and asymptomatic renal AMLs and determine characteristics that predict spontaneous bleeding.
Patients and Methods: Data were retrospectively collected from a prospectively maintained database from July 2011 to April 2022. Patients were included if AML was > 4cm and they underwent subsequent SAE. Follow-up imaging was analyzed to calculate mean reduction in AML size. Clinical notes were reviewed to analyze lesion characteristics including vascularity, fat content and presence of aneurysm as well as post-procedural complications.
Results: 26 patients with 30 AMLs were identified. Interval of follow-up imaging ranged from 1 to 60 months. 25 AMLs were embolized electively with 5 emergency embolizations performed for bleeding. Mean reduction in AML volume was 41% at 3 months (p=0.013) and 63% at 12 months (p=0.007). All 5 bleeding AMLs had a rich vascularity with 60% also having either aneurysms or a low fat content. Complications included post-embolic syndrome (n=9), segmental renal parenchyma devascularization (n=3), acute bleeding requiring re-embolization (n=2), nephrectomy for ongoing bleeding (n=1) and delayed bleeding managed conservatively (n=1). No deterioration in renal function was observed.
Conclusion: SAE is an effective procedure for managing symptomatic and asymptomatic renal AML, with minimal significant complications. AML vascularity, fat content and aneurysms may be useful characteristics to assess future risk of bleeding in patients with renal AML.
Keywords: selective angioembolization, aneurysm, fat content, tuberosclerosis complex
Introduction
Renal angiomyolipoma (AML) is a benign renal neoplasm comprised of fat, blood vessels, and smooth muscle which can occur sporadically or as part of the tuberous sclerosis complex (TSC).1 It has a prevalence of approximately 0.5% and a strong female predilection (2:1). Most renal AMLs are found incidentally, however may be associated with pain or retroperitoneal bleeding requiring urgent intervention.2 Current management guidelines comprise active surveillance, selective angioembolization (SAE) or surgical management (through either nephron sparing surgery (NSS) or nephrectomy) depending on the clinical situation and presence of symptoms.3
SAE has been demonstrated to decrease tumor volume, decrease risk of bleeding, and successfully manage acute bleeding in several retrospective articles and systematic reviews.3–6 Yet SAE is not effective for all patients with some achieving very minimal reduction in overall tumor volume and others presenting with pain or bleeding post-embolization.7,8 Current theories state the fat, muscle and vascular composition of the AML are linked to the success of SAE, however this is yet to be demonstrated in the literature.9 Furthermore, determining which patients are at higher risk of becoming symptomatic or at risk of bleeding has yet to be well established. Current EAU guidelines state risk factors for bleeding are tumor size, grade of angiogenic component and the presence of TSC. Yet other literature also states that the presence and size of aneurysms within the AML have also been proposed as a determinant of poorer outcomes and higher risk of sporadic bleeding.5,6,10 There is a scarcity of knowledge pertaining to other AML characteristics that may better identify high risk patients who would benefit from early intervention.
This article aimed to provide a review of renal AMLs which underwent selective angioembolization and their short- and long-term outcomes, as well as to identify key characteristics of renal AMLs that predict spontaneous bleeding and complications.
Patients and Methods
Our cohort of patients was collected from a prospectively maintained database of renal angiomyolipomas which have undergone selective angioembolization at this institution from 2011 until April 2022. Ethics approval was obtained from the Royal Prince Alfred Hospital Research Governance Office (X21-0472 and 2021/ETH12306) with a waiver for informed consent of the individuals for the use of their medical records granted by the ethics review committee in accordance with the Health Records and Information Privacy Act 2002 (NSW) as a low/negligible risk study in compliance with the declaration of Helsinki. Patient data were extracted and stored on a password-protected shared drive, which could only be accessed by the personnel named in this application. Unique identification numbers were used to protect patient identity when storing the data in the study database. Patient demographics, symptomatology and indications for embolization were obtained from electronic medical records. Pre-procedural and follow-up computed tomography (CT) scans were accessed for each patient, lesion dimensions were recorded, and change in tumor volume was calculated. Follow-up data were recorded from the electronic medical record. At our institution, selective angioembolization of renal angiomyolipomas is performed by several interventional radiologists. They are routinely performed through a femoral puncture using a 5 or 6 Fr catheter and microcatheter. Perioperative antibiotics are given, and sedation or general anesthesia is administered by an anesthetist. Embolization was achieved with PVA particles, microcoils, ethanol (with or without lipiodol) or a combination of these. Post-embolization angiography is performed in all patients and any loss to the surrounding renal parenchyma is noted. In our cohort, all patients were seen before and after embolization and all had repeat imaging in the first year post-embolization. Overall success was defined as no bleeding or need for re-embolization post-SAE. Technical success was defined as complete and immediate embolization of all arterial feeders and complete devascularization of the AML with no bleeding or need for re-embolization.
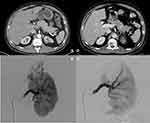
Two independent radiologists reviewed images before and after embolization to determine the characteristics of each AML including size, fat content, presence of intralesional aneurysm, whether complete embolization was achieved and any damage to the renal parenchyma (Figure 1). Once each radiologist had independently reviewed the cases, they reviewed images and results together and any differences in reporting were re-evaluated to determine final interpretation.
Results
26 patients with 30 AMLs were included (Table 1). The mean age was 47.2 (range 31–74) years. There were 20 female patients and 6 males. 7 patients were known to have TSC. 12 patients (46.2%) presented symptomatically with pain being the most common symptom followed by bleeding and hematuria, and 14 patients (53.8%) were asymptomatic at the time of diagnosis (Table 2). AML size was calculated in both maximal diameter and volume with a mean maximal diameter of 72.9mm (range 23–217) and a mean volume of 178.2cc (range 3.5–1281.6). AMLs were also characterized on their fat content (<25%, 25–50%, 50–75% and >75% fat content), vascularity and the presence of aneurysm (n = 14). SAE was performed using polyvinyl alcohol particles (PVA), alcohol/lipiodol, microcoils or a combination of these (Supplementary Data 1).
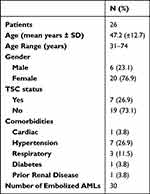 |
Table 1 Patient Demographics |
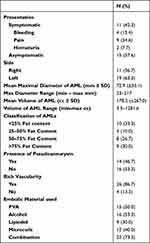 |
Table 2 AML Characteristics |
Overall success was seen in 25 patients (83.3%). Three patients required re-embolization, two secondary to bleeding and one to achieve complete embolization of a partially treated AML. One patient had minor bleeding post-SAE but was managed conservatively and one patient underwent an emergency nephrectomy due to failure of SAE and ongoing blood loss (Table 3). Technical success and complete devascularization of AML was seen in 50% of AMLs embolized. Complete embolization was not achieved in 15 patients due to the following; difficulty reaching all accessory arterial branches (n = 8), arterial spasm (n = 6) and interventionalists’ discretion with the main supplying artery being embolized (n = 1). Complications were seen in 15 patients including post-embolic syndrome (n = 9), segmental renal parenchyma devascularization (n = 3), acute bleeding (n = 2), and delayed bleeding (n = 1).
 |
Table 3 Overall/Technical Success Rate and Complications |
Post-procedural outcomes showed a statistically significant reduction in both mean maximal diameter and volume of AMLs (p = 0.003 and <0.001 respectively). There was no difference seen in patients’ creatinine post-embolization in the immediate or long-term follow-up (Table 4 and Supplementary Data 2). Outcomes for specific AML characteristics such as low and high fat content, presence of aneurysm, vascularity and complete or incomplete embolization were also investigated and we found significant decrease in size across all groups (Table 5). Long-term follow-up showed mean reduction in AML diameter was 23% at 3 months, 30% at 12 months, and 42% at ≥24 months post-SAE (Table 6). Similar reduction in volume was also seen with a 41% reduction in AML volume noted at 3 months follow-up (Table 6 and Figure 2).
 |
Table 4 Outcomes of Selective Angioembolization for AMLs |
 |
Table 5 AML Characteristics and Response to Treatment |
 |
Table 6 Long-Term Outcomes |
 |
Figure 2 Mean change in volume of angiomyolipoma post selective angioembolization with trend line. |
Of the five patients who presented with acute bleeding, three had fat content of <50%, and two less than 25%. Three of the five had an aneurysm, and all five had rich vascularity (Appendix 1). Two of the five bleeding AMLs were incompletely embolized and these two required further management (re-embolization and nephrectomy respectively). Of the four patients who required further treatment through repeat embolization or nephrectomy, three had aneurysms and all four initially were incompletely embolized.
Discussion
Renal AML is the most common benign renal lesion affecting approximately 0.5% of the population with a strong female predilection. Management of AMLs can comprise conservative management, SAE, thermal ablation, nephron sparing surgery, nephrectomy and mammalian target of rapamycin (mTOR) inhibitors depending on the presence of symptoms and patient’s request.9 Our results showed that SAE is an effective and safe method of managing renal AML in both symptomatic and prophylactic circumstances with significant reduction in size of AMLs seen irrespective of size, fat content, vascularity, and patient characteristics with relatively few major complications. A mean reduction of 41% in volume and 23% in diameter was seen at 3 months post-SAE. This decrease in volume and diameter continued and showed that a successful complete embolization will provide ongoing reduction in size of AMLs in the future. Complete embolization is imperative as all patients in our cohort who required re-embolization or further intervention (nephrectomy) did not achieve complete embolization (Appendix 1).
Our cohort did have complications with post-embolization syndrome (PES) presenting in 27% of our cohort. This is in line with similar reviews with a mean incidence ranging between 33 and 60%5,11,12 and is an expected sequalae of embolization. Major complications were present in five patients. Three patients required re-embolization (one for acute bleeding post-SAE), one required nephrectomy for ongoing bleeding post-SAE and one had further bleeding post-SAE but was managed conservatively. These are also in line with previous literature which shows a retreatment rate of approximately 30% of patients who underwent SAE, secondary to further bleeding or tumor growth.3,13 Further treatment in patients post-SAE is much higher in comparison to those patients who underwent primary surgical management (30% vs 1%).3 As the mean age of patients in our cohort was young at 47 years, a minimally invasive approach through SAE was considered beneficial compared to NSS or nephrectomy to decrease morbidity from treatment. In the three patients who had acute bleeding post-SAE, surgical management was used effectively in one unwell patient to manage this complication, showing that a minimally invasive approach may be the best primary approach and if unsuccessful, conversion to surgical management is an effective and usually definitive alternative.
The decision of which AML characteristics are concerning and indicate treatment has been unclear for some time. In the past, treatment has been offered for patients with symptoms such as bleeding or pain, suspected malignancy, women of child-bearing age or when the AML is >4cm in maximal diameter.3 This “4cm rule” stems from historical literature in 1986 showing the larger the AML, the more likely the bleeding risk.14 However, multiple different authors have shown that size alone should not be the sole determinant for intervention. In patients presenting with major retroperitoneal bleeding, tumors were significantly larger on average than their non-bleeding counterparts, yet 9% and 26% of tumors that were bleeding were under 4 and 6cm respectively in maximal diameter.15 Growth rate of >0.25cm/year, the presence of TSC, symptoms (pain or bleeding) have all been identified as indications for active treatment of AMLs.1,3,16 Active surveillance has been identified as a possible management option for AMLs >4cm with literature highlighting that up to 92% of untreated AMLs irrespective of size had not grown and only 5.6% required intervention.1 The risk of spontaneous and life-threatening bleeding has been recognized in approximately 2.2% of patients undergoing active surveillance.3 In our cohort, 97% of AMLs were >4 cm in size yet only 50% were symptomatic at the time of diagnosis and only 16.7% of these large AMLs presented with bleeding. Furthermore, out of the four patients in our cohort with AMLs >10cm, only two of the four presented with symptoms.
The presence of aneurysms has been hypothesized to be a significant risk factor and predictor of bleeding and complications in renal AML. A true aneurysm is defined as a 50% increase in the normal diameter of the vessel that can predispose it to complications including rupture, thrombosis or distal embolization.17 Previous literature has identified that aneurysms >5mm in size have a strong correlation with rupture, moreso than size itself.13 Yamakado et al found that the traditional cut-off of 4cm to initiate treatment had a significantly lower specificity (38%) than aneurysms ≥5mm (86%) and aneurysm size was the only significant predictor of rupture in their multiple regression analysis (p=0.001).18 In addition, in similar retrospective reviews of patients who underwent prophylactic or emergency SAE for renal AML it was found that between 63 and 67% of patients with intralesional aneurysms presented with an acute hemorrhage compared to just 7–15% without aneurysms.2,19 In our study, fourteen patients were identified with aneurysms and of the five that presented with bleeding 60% had aneurysms. It was also interesting to note that of the five patients with major complications post-SAE, 80% had aneurysms. Potential causes for why these were more difficult to manage include the presence of multiple aneurysms or more small aneurysms not identified at the time of embolization that may have grown or ruptured. Unfortunately, at the time of publication, direct size of aneurysms could not be retrospectively measured on digital subtraction angiography (DSA) uploaded to our picture archiving and communication system (PACS).
Fat poor AMLs are another area in which complications can arise. Renal AMLs have a characteristic radiological appearance based on its fat, muscle and vascular composition. A fat poor AML may be difficult to diagnose on imaging because if the fat content is extremely low, it may be considered or be a fat-containing RCC.7 This is especially true as fat poor AMLs are historically smaller lesions in females of a younger age.20 The differential diagnosis of RCC may require more patients to undergo biopsy prior to management and if features are concerning for RCC, active surveillance or SAE may not be the appropriate choice of management.21 Furthermore, the low fat content may mean that there is a larger proportion of muscle and higher vascularity in fat poor AMLs, potentially predisposing the patient to higher risks of bleeding. In our cohort, two of the five patients who presented with bleeding had AMLs with <25% fat content. Meanwhile, for those patients in our cohort with a very high fat content (>75%), two of these had poor vascularity and only one patient with a fat content >75% presented with bleeding. Despite the small sample sizes, the predictive nature of fat content may provide further risk stratification in the future with fat poor AMLs potentially at a higher risk of bleeding and potential to be a fat-containing RCC, resulting in a more active role for treatment in this patient subset.
Rich vascularity may also be a predictor of bleeding and complications in renal AML. The formation of microaneurysms and incomplete vascular walls have been identified as high risk features for rupture and bleeding.13 Xu et al determined that the probability of rupture in patients who had AMLs with rich vascularity was 5.23 times higher than that of AMLs with poor vascularity.22 Furthermore, several other groups determined similar findings, showing that poorly vascularized AMLs were much less likely to bleed.23,24 Within our cohort, no patient with a poorly vascularized AML presented with bleeding, and all five patients presenting with bleeding, had richly vascular AMLs. Determining the vascularity of an AML prior to treatment may prove to be another crucial predictor of the success or failure of active surveillance in asymptomatic AMLs and further large cohort studies would provide much needed insight into this interesting and potentially key AML characteristic.
Only one patient in this cohort received medical therapy (mTOR inhibitor) in combination with SAE for their AMLs secondary to TSC. It is difficult to ascertain the benefit of mTOR inhibitors from our single patient, however multiple reviews demonstrated benefit of mTOR inhibitors in reducing the volume of renal AML.25,26 Yet, mTOR inhibitors seem to have significant side effects with over 35% of patients having ≥ grade 3 adverse events, demonstrated in multiple studies.26–28 This suggests mTOR inhibitors may be used in combination with SAE or as a non-invasive alternative to SAE, however patients should be well informed of risks and adverse events.
Our study did have several limitations. Firstly, this was a heterogenous population group from a single institution with sporadic (n=19) and TSC (n=7) patients mixed into the same cohort which may limit the generalizability of the findings. Furthermore, SAE was retrospectively reviewed regardless of the indication whether it be for symptomatic or prophylactic management. In addition, no set follow-up period was determined prior to patient treatment. Most patients had CT follow-up within 3 months of SAE however eleven patients had their initial follow-up between 6 and 12 months post-SAE. Also, most patients had one follow-up CT post-SAE to confirm its effectiveness and long-term outcomes were only known in a minority of our cohort. Balancing the risks of multiple repeat CTs to further characterize change post initial embolization in predominantly young female patients may not be the best follow-up modality, due to increased radiation exposure. Magnetic resonance imaging (MRI) or ultrasound (US) follow-up post initial CT may help to improve long-term outcome data without repeatedly irradiating patients, however variability in accuracy between modalities may also become another challenge through this approach. In future, a specific follow-up regime at yearly or second yearly intervals may help to clarify the success of SAE over a 5 or 10-year period. Another key limitation was the inability to retrospectively measure aneurysms and microaneurysms through DSA. Confidently diagnosing and measuring aneurysms >5mm and microaneurysms on CT was occasionally difficult for our radiologists and they could not confidently measure their size or presence with certainty. DSA did allow confirmation of aneurysms, but at the time of publication we are unable to retrospectively measure their exact size. In the case of a patient with TSC including multiple AMLs requiring repeated intervention, DSA studies in which aneurysms are measured prior to SAE would be extremely beneficial in the future to determine the presence and size of aneurysms’ relative risk of bleeding.
We strongly believe the presence of intralesional aneurysms, increased vascularity of the AML, and decreased fat content are important additional predictors of bleeding and complications in patients with renal AML. Current guidelines do not reflect or represent these findings, but should be considered in future management criteria for determining whether intervention is required.
Conclusion
Renal AML is a benign tumor with potentially life-threatening complications. Management through SAE for both symptomatic and asymptomatic patients is safe and effective with the majority of patients having successful long-term outcomes. Re-embolization and surgical management for further bleeding or regrowth of tumors are potential complications. Size alone may not be the best way to predict complications in renal AML and key characteristics such as vascularity, fat content, and the presence of aneurysms are risk factors and predictors of bleeding. However, further review is required to ascertain their risk level.
Disclosure
The authors declare they have no known competing financial or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Bhatt JR, Richard PO, Kim NS, et al. Natural history of Renal Angiomyolipoma (AML): most patients with large AMLs >4cm can be offered active surveillance as an initial management strategy. Eur Urol. 2016;70(1):85–90. doi:10.1016/j.eururo.2016.01.048
2. Bardin F, Chevallier O, Bertaut A, et al. Selective arterial embolization of symptomatic and asymptomatic renal angiomyolipomas: a retrospective study of safety, outcomes and tumor size reduction. Quant Imaging Med Surg. 2017;7(1):8–23. doi:10.21037/qims.2017.01.02
3. Fernández-Pello S, Hora M, Kuusk T, et al. Management of sporadic renal angiomyolipomas: a systematic review of available evidence to guide recommendations from the European Association of Urology Renal Cell Carcinoma Guidelines Panel. Eur Urol Oncol. 2020;3(1):57–72. doi:10.1016/j.euo.2019.04.005
4. Nozadze G, Larsen SB, Heerwagen S, Juhl Jensen R, Lönn L, Røder MA. Selective arterial embolization of renal angiomyolipomas: a 10-year experience. BJUI Compass. 2022;3(1):86–92. doi:10.1002/bco2.107
5. Murray TE, Doyle F, Lee M. Transarterial embolization of angiomyolipoma: a systematic review. J Urol. 2015;194(3):635–639. doi:10.1016/j.juro.2015.04.081
6. Ramon J, Rimon U, Garniek A, et al. Renal angiomyolipoma: long-term results following selective arterial embolization. Eur Urol. 2009;55(5):1155–1162. doi:10.1016/j.eururo.2008.04.025
7. Seyam RM, Alkhudair WK, Kattan SA, Alotaibi MF, Alzahrani HM, Altaweel WM. The risks of renal angiomyolipoma: reviewing the evidence. J Kidney Cancer VHL. 2017;4(4):13–25. doi:10.15586/jkcvhl.2017.97
8. Makki A, Graumann O, Høyer S, et al. Cryoablation of renal angiomyolipoma: an evaluation of safety and efficacy. J Endourol. 2017;31(11):1117–1122. doi:10.1089/end.2017.0376
9. Vos N, Oyen R. Renal angiomyolipoma: the good, the bad, and the ugly. J Belg Soc Radiol. 2018;102(1):41. doi:10.5334/jbsr.1536
10. Urology EAo. EAU guidelines - renal cell carcinoma. 3.4.5. 2022.
11. Abouelkheir RT, El-Ksas M, Abdel Fattah S, Amer T, El-Diasty T. Efficacy and safety of selective renal arterial embolization in renal angiomyolipoma: a prospective single-center study. Egypt J Radiol Nuclear Med. 2022;53(1):165. doi:10.1186/s43055-022-00848-3
12. Prigent F-V, Guillen K, Comby P-O, et al. Selective arterial embolization of renal angiomyolipomas with a N-butyl cyanoacrylate-lipiodol mixture: efficacy, safety, short- and mid-term outcomes. J Clin Med. 2021;10(18):4062. doi:10.3390/jcm10184062
13. Wang C, Li X, Peng L, Gou X, Fan J. An update on recent developments in rupture of renal angiomyolipoma. Medicine. 2018;97(16):e0497. doi:10.1097/md.0000000000010497
14. Oesterling JE, Fishman EK, Goldman SM, Marshall FF. The management of renal angiomyolipoma. J Urol. 1986;135(6):1121–1124. doi:10.1016/s0022-5347(17)46013-7
15. Kuusk T, Biancari F, Lane B, et al. Treatment of renal angiomyolipoma: pooled analysis of individual patient data. BMC Urol. 2015;15(1):123. doi:10.1186/s12894-015-0118-2
16. Restrepo JCA, Millan DAC, Sabogal CAR, Bernal AFP, Donoso WD. New trends and evidence for the management of renal angiomyolipoma: a comprehensive narrative review of the literature. J Kidney Cancer VHL. 2022;9(1):33–41. doi:10.15586/jkcvhl.v9i1.177
17. Thompson MM, Bell PR. ABC of arterial and venous disease. Arterial aneurysms. BMJ. 2000;320(7243):1193–1196. doi:10.1136/bmj.320.7243.1193
18. Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002;225(1):78–82. doi:10.1148/radiol.2251011477
19. Chaiyasoot W, Yodying J, Limsiri T. Selective arterial embolization of renal angiomyolipoma: efficacy, tumor volume reduction and complications. Siriraj Med J. 2021;73(5):337–343. doi:10.33192/Smj.2021.44
20. Bauman TM, Potretzke AM, Wright AJ, Vetter JM, Potretzke TA, Figenshau RS. Patient and nonradiographic tumor characteristics predicting lipid-poor angiomyolipoma in small renal masses: introducing the BEARS index. Investig Clin Urol. 2017;58(4):235–240. doi:10.4111/icu.2017.58.4.235
21. Potretzke AM, Potretzke TA, Bauman TM, et al. Computed tomography and magnetic resonance findings of fat-poor angiomyolipomas. J Endourol. 2017;31(2):119–128. doi:10.1089/end.2016.0219
22. Xu X-F, Hu X-H, Zuo Q-M, Zhang J, Xu H-Y, Zhang Y. A scoring system based on clinical features for the prediction of sporadic renal angiomyolipoma rupture and hemorrhage. Medicine. 2020;99(20). doi:10.1097/MD.0000000000020167
23. Rimon U, Duvdevani M, Garniek A, et al. Large renal angiomyolipomas: digital subtraction angiographic grading and presentation with bleeding. Clin Radiol. 2006;61(6):520–526. doi:10.1016/j.crad.2006.02.003
24. Andersen PE, Thorlund MG, Wennevik GE, Pedersen RL, Lund L. Interventional treatment of renal angiomyolipoma: immediate results and clinical and radiological follow-up of 4.5 years. Acta Radiologica Open. 2015;4(7):2058460115592442. doi:10.1177/2058460115592442
25. Li M, Zhou Y, Chen C, et al. Efficacy and safety of mTOR inhibitors (rapamycin and its analogues) for tuberous sclerosis complex: a meta-analysis. Orphanet J Rare Dis. 2019;14(1):39. doi:10.1186/s13023-019-1012-x
26. Wang W, Guo G, Shi G, et al. A multi-centric study assessing safety and efficacy of everolimus in adult Chinese patients with tuberous sclerosis complex associated renal angiomyolipomas. Original Research. Front Oncol. 2022;12. doi:10.3389/fonc.2022.871723
27. Geynisman DM, Kadow BT, Shuch BM, et al. Sporadic angiomyolipomas growth kinetics while on everolimus: results of a phase II trial. J Urol. 2020;204(3):531–537. doi:10.1097/JU.0000000000001065
28. Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus long-term use in patients with tuberous sclerosis complex: four-year update of the EXIST-2 study. PLoS One. 2017;12(8):e0180939. doi:10.1371/journal.pone.0180939
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.