Back to Journals » Patient Preference and Adherence » Volume 15
Is E-Version Transition of the Medication Adherence Scale Feasible for CKD Management? A Pilot Study
Authors Chen HF, Lei N, Xu YM, Luo L, Zhang XL, Lao BN, Tang F, Fu LZ, Liu XS, Wu YF
Received 4 June 2021
Accepted for publication 21 July 2021
Published 17 August 2021 Volume 2021:15 Pages 1785—1793
DOI https://doi.org/10.2147/PPA.S323393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Hui-Fen Chen,1 Nuo Lei,1 Yan-Min Xu,1 Li Luo,1 Xian-Long Zhang,1 Bei-Ni Lao,1 Fang Tang,2 Li-Zhe Fu,2 Xu-Sheng Liu,3 Yi-Fan Wu3
1The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 2Chronic Disease Management Outpatient Clinic, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou, People’s Republic of China; 3Renal Division, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou, People’s Republic of China
Correspondence: Yi-Fan Wu Email [email protected]
Background: To transfer a paper-version Chinese and Western medication adherence scale for CKD into an electronic scale, and evaluate its validity, internal consistency and clinical implementation, and assess whether the transition is feasible in clinic.
Methods: We built an e-version Chinese and Western medication adherence scale based on the Wen-JuanXing platform. CKD subjects’ responses were applied to test the scale’s validity and internal consistency. We retested some of the participants two weeks later randomly. We also tested the clinical application.
Results: Of the 434 recruited patients, 228 responded. In exploratory factor analysis (EFA), the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy = 0.8 and Bartlett’s approx. Chi-Square = 1340.0 (df = 105, p < 0.001). We extracted four common factors which could explain 61.47% of the variance. However, Item 15 “Have you changed a traditional Chinese medicine prescription yourself within the past month?” had factor loading = 0.3 and measure of sampling adequacy (MSA) = 0.5, meaning we could not enter it into the factor analysis. The internal consistency reliability for medication adherence was 0.9, with a Guttman split-half coefficient = 0.5 and a Spearman–Brown coefficient = 0.6. Cronbach’s α was 0.9, 0.4 and 0.5 for the knowledge, belief and behavior domains, respectively. The correlation coefficient r of the test–retest reliability was − 0.8 and was − 0.8, 0.4, − 0.3 in the knowledge, belief and behavior domains, respectively. Patients with comorbidities were more likely to respond. We detected no other significant differences in the clinical profiles between respondents and non-respondents.
Conclusion: The e-version Chinese and Western medication adherence scales have undesirable construct validity and internal consistency. Thus, caution is needed in transitioning the paper-version scale into an e-version.
Keywords: medication adherence, renal insufficiency, chronic, surveys and questionnaires
Introduction
Chronic kidney disease (CKD) affects 9.1% of adults worldwide and 10.8% of adults in China.1,2 Costs related to CKD and end-stage renal disease (the terminal manifestation of CKD) exert an enormous burden on both individuals and healthcare systems, making it a growing public health problem worldwide. Furthermore, its chronic, progressive and non-communicable characteristics are usually associated with adverse metabolic effects, anemia, comorbidities and psychological problems, such as anxiety disorders.3 Medical treatment is generally lifelong, and ideal medication adherence, defined as “the extent to which the patient’s behaviour matches agreed recommendation from the prescriber”,4 is a crucial factor in thwarting CKD progression. A systematic review and meta-analysis reveal that patients with advanced CKD, misconceptions about medication, lack of perceived self-efficacy in medication use, polypharmacy, loss of confidence in the physician, poor social support and lower education levels perform lower medication adherence.5 Complex disease characteristics, frequent follow-up, various dosage forms, pre-existing conditions, side effects, financial disparities, and Chinese medicine prescriptions (eg, oral solutions, decoctions, as well as their taste and temperature) present new challenges to medication adherence in CKD.
As such, reliable medication adherence assessment tools are needed, including direct measures (eg, drug assays of blood and/or urine), indirect measures (eg, pill counting, electronic monitoring and measures based on big data) and patient-reported outcome measures (PROMs) (eg, the Morisky, Green and Levine scale (MGL scale), Hill-Bone Compliance Scale, and Medication Adherence Rating Scale (MARS)).6 PROMs are used by many researchers due to their advantages over other methods, including simplicity, cost-effectiveness, applicability across settings, and the ability to provide immediate feedback at the point of care and detect potential factors influencing adherence.7 However, PROMs may be sub-optimal in evaluating herbal medication and CKD-specific medication adherence. Additionally, they are limited to a single aspect of behavior or cognitive modification.
Hence, we developed a paper-version Chinese and Western medication adherence scale for CKD (version-1) in 2017 on the basis of the MGL scale, Knowledge-Attitude-Belief Practice (KABP) theory and items analysis theory.8 This provided physicians with feedback on medication adherence for people with chronic diseases taking medication long term. Considering the shortcomings of version-1, we devised a Chinese and Western medication adherence scale for CKD patients (version-2) in 2019, based on the Samejima’s GRM from Item Response Theory (IRT).9 The version-2 scale, which tests have shown has desirable reliability and validity, accounts for herbal medication and CKD medication characteristics, and has been applied in many clinical settings.
Great progress has been made in telemedicine, and this has been accentuated by the COVID-19 epidemic. Traditional paper-version scales have disadvantages, such as inadequate reliability, poor distribution, laborious data collection and entry, and costly resources. As such, the electronic administration of a scale is appealing in medication adherence assessment, as it may reduce administrative work and improve extendibility. Based on the advantages of e-version scales, we have transferred a paper-version medication adherence scale into an e-version. We improve our existing CKD-specific medication adherence questionnaire based on smartphone use, to explore 1) the validity and reliability of an e-version medication adherence questionnaire, and 2) medication adherence response rate facilitating or hindering factors so that we could determine whether the transition is feasible in clinic.
Materials and Methods
Study Setting
In October 2020, we conducted a single-center, exploratory study at the Guangdong Provincial Hospital of Chinese Medicine’s Renal Chronic Disease Management Department. The study was approved by the Guangdong Provincial Hospital of Chinese Medicine’s Ethics Committee (B2016-93) and conducted in accordance with the Declaration of Helsinki.
Study Participants
All patients meeting the following criteria were eligible for inclusion in the pilot study: 1) diagnosed as non-dialysis stage 1–5 CKD according to the 2012 KDIGO guidelines;10 2) taking a Chinese medicine prescription; 3) able to sign informed consent; 4) judged mentally and physically able to participate by our medical team staff; 5) had regularly visited the Guangdong Hospital of Chinese Medicine’s Chronic Disease Management Department (in the Renal Division); 6) able to use WeChat via smartphone. Two researchers notified the eligible patients using the Hospital Information System (HIS) and chronic disease management system. Eligible patients would give a written signed informed consent.
Sample sizes were estimated as at least 150 patients for this 15-item Likert Scale based on a recommended 5:1 patient-item ratio11 and a 50% estimated response rate for the electronic scale.12 For test–retest, given that Park et al13 reported that 40% of studies set a sample-item ratio from 1:1 to 1:4 in order to test the external reliability, we calculated the required sample size as at least 15 patients based on a 1:1 ratio.
Study Procedures
The 15-item Likert Scale which consists of 15 items and 3 dimensions, was input, and possible omitted or incorrect responses were checked in Wen-JuanXing by two trained interviewers just prior to the first version of the questionnaire. Questions were same as paper-version scale. It contains both positively worded (PW) and reverse worded (RW) items (item 10, 11), with ordinal scores of 5-1 or 1–5 points, as sequential options for A–E. The reverse worded scores were pre-designed when typing the e-version. To guide each patient, text clarifying the study details were written in advance. Eligible patients received an electronic invitation around noon on October 13, 14 and 15, 2020, via a smartphone-based WeChat application, by four interviewers. Patients who agreed to participate started the survey by clicking the attached website link or quick response (QR) code (2-dimensional bar code) independently. Additional virtual guidance related to the administration of the survey was offered via WeChat or phone by individual team members, if necessary. The test–retest was performed among randomly selected patients from those who had responded to the questionnaire in the first round by the same group of interviewers two weeks later.13
Information including the name, HIS code, date, survey time, and score, was collected by Wen-JuanXing. All requested data was treated confidentially and used internally for research purposes only. Demographic characteristics (age, sex, marital status, education level, employment status and comorbidity) and relevant indicators of renal function (serum creatinine (SCr) level, estimated glomerular filtration rate (eGFR), and CKD stage) were acquired in the HIS system and chronic disease system (Figure 1).
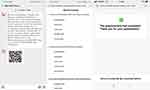 |
Figure 1 Issuing and design of the electronic Chinese and western medication adherence questionnaire. |
Statistical Analysis
We conducted statistical analysis with IBM SPSS version 23.0. Values were considered statistically significant if P < 0.05 upon two-tailed analysis. Descriptive statistics were expressed using means (M), standard deviation (SD), medians, and interquartile ranges (IQR) for quantitative variables. We employed frequency and percentages for qualitative variables.
Validity is the accuracy of measuring the degree of psychological or behavioral traits. It includes content validity (logical validity), criterion-related validity (empirical validity), and construct validity. We conducted an exploratory factor analysis (EFA) to evaluate the construct validity.
Internal consistency means that all items contribute positively towards measuring the same construct. To evaluate internal consistency, we used Cronbach’s α, split-half reliability, and test–retest reliability in reliability analysis. For test–retest reliability (coefficient of stability), a Pearson correlation coefficient of >0.7 indicated a high correlation with total scores.
Clinical Application
To evaluate the feasibility of the e-questionnaire, we compared the clinical profiles of those patients who had responded and those who had not. We analyzed continuous variables with an independent Student’s t-test or a Mann–Whitney U-test, while binary data were handled with a Chi-Square test. We used a Mann–Whitney U-test to compare ordinal data.
Results
Study Participants
We screened 474 patients in the HIS and chronic disease system; two were on Western prescription medication only, twenty-nine were missing demographic data, one was below 18 years of age, and eight were undergoing dialysis. Finally, 434 patients were enrolled and provided e-version scale. We received valid information (e-version questionnaires and additional clinical documents) for 228 copies, corresponding to a 52.5% response rate. The participants who responded spent a mean time of 348.7 (164.0, 375.8) seconds completing the questionnaire with an average total score of 55.3 (49.3, 61.0) points. There was no other missing data in any of the input information.
Basic information and the available data from the assigned patients can be found in Table 1. The median participant age was 49.0 years, with a 25th percentile below 37.0 and a 75th percentile above 61.0. 51.2% were males. 85.3% were married, 39.4% worked full time and 36.9% were retired. 25.3% had completed junior high school, and 30.2% had completed senior high school/junior technical school. For relevant indicators of renal function, the median SCr and eGFR levels of the participants were 102.5 (79.8, 160.3) μmol/L and 63.3 (35.3,91.1) mL/min/1.73m2, and there were 108 stage 1 (24.9%), 109 stage 2 (25.1%), 141 stage 3 (32.5%), 46 stage 4 (10.6%), and 30 stage 5 (6.9%) CKD patients. Additionally, 52.1% of the participants had chronic diseases other than CKD, and 42.4% had hypertension.
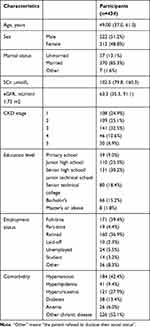 |
Table 1 Clinical Profiles of the 434 Patients Invited to Participate |
Validity
Development and refinement in versions 1 and 2 maintained the scale’s acceptable content validity.5,6 Table 2 provides results of the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy (0.8), and detected an approx. Chi-Square of 1340.0. This means it is applicable for the EFA. We extracted four distinct factors in Principle Component Analysis (PCA). This model could explain 61.5% of the total variance (Table 3). The factor loading on all items was >0.4, except for Item 15. Item 15 also had a Measure of Sampling Adequacy (MSA) of 0.5 (Table 4). Thus, Item 15 was unfit for the factor analysis process. Therefore, the construct validity of the Chinese and Western Adherence Scale’s e-version still needs improvement.
 |
Table 2 Kaiser–Meyer–Olkin and Bartlett’s Test of Sphericity |
 |
Table 3 Total Variance Explained Using Exploratory Factor Analysis |
 |
Table 4 Measure of Sampling Adequacy and Factor Loading |
Internal Consistency
The internal consistency of medication adherence was desirable with a Cronbach’s α of 0.9, but was inadequate with a Guttman split-half coefficient of 0.5 and a Spearman–Brown coefficient of 0.6. The Cronbach’s α was 0.9, 0.4 and 0.5 in the knowledge, belief and behavior domains, respectively. For test–retest reliability, results of the collected 35 copies highlighted a correlation coefficient r for the test–retest reliability of −0.8, and coefficients of −0.8, 0.4, −0.3 for the knowledge, belief and behavior domains, respectively (Table 5).
 |
Table 5 Results of Internal Consistency |
The Relationship Between Patient Characteristics and Scale Response
Of the 434 recruited patients, 228 responded while the other 206 were non-respondents. Patients with comorbidities (Hypertension: Pearson x2=17.5, P < 0.001, Hyperlipidemia: Pearson x2=414.7, P < 0.001, Hyperuricaemia: Pearson x2=129.2, P < 0.001, Diabetes: Pearson x2=347.0, P < 0.001, Anemia: Pearson x2=506.1, P < 0.001, other chronic disease: Pearson x2=56.2, P < 0.001) were more likely to respond. The comparison of other clinical profiles, including age (z =−0.4, P = 0.7), sex (Pearson χ2 = 0.6, P = 0.4), marital status (z = −1.1, P=0.3), SCr (z = −1.1, P = 0.3), eGFR (z =−1.0, P = 0.3), education level (z = −0.4, P=0.7) and working status (z = −1.5, P=0.1) between the respondent and non-respondent group indicated no significant differences (Table 6).
 |
Table 6 Association Between Patient Characteristics and e-Questionnaire Response |
Discussion
After being transferred into the e-version, EFA revealed that the CKD Chinese and Western Medication Adherence Scale is four-dimensional, unlike the paper-version. The MSA and factor loadings indicated that Item 15 needed to be deleted. This indicated poor construct validity in the e-version scale. The Cronbach’s α were 0.9 and 0.9 in medication adherence and medication knowledge, and 0.4 and 0.5 in belief and behavior. Split-half coefficients were worse than Cronbach’s α. The Pearson correlation coefficients were −0.8 in medication adherence and −0.8, 0.4, −0.3 in knowledge, belief and behavior, respectively. This indicated that the e-version scale had undesirable internal consistency reliability, both overall, and among its subscales. In clinical application, individuals with comorbidities were found to be more likely to respond. Based on the validity and reliability analysis, we implemented second factor analysis. Common factor analysis removed Item 15, and extracted four components which explained 65.3% of the variance. The Cronbach’s Alpha was the same as in the original e-version (0.9), but with a higher Spearman–Brown coefficient (0.7) and Guttman coefficient (0.7). However, the Pearson correlation coefficient showed little improvement.
These results were confusing because the paper-version had a Cronbach’s α of 0.84 for medication adherence and 0.9, 0.6, 0.7 for the knowledge, belief and behavior subscales, respectively,6 the latter three having superior quality and balance. It also produced a Pearson correlation coefficient of 0.9.6 It meant that there was a research gap between paper-version and e-version transition. We considered five potential reasons for these undesirable results in e-version scale: 1) there may have been a ceiling or floor effect in some of the items. We found that at least 15% of the participants chose 5 points on Items 1–6 (Knowledge Part) and 13–15 (Behavior Part), and chose 1 point on Item 7 (Knowledge Part). This explained why the knowledge part outperformed the others in validity and reliability. However, this begs the question: why was this not a problem in the paper-version study. This could mean that our participants were regular hospital visitors, and had received CKD medicine education in clinic. As such, they would have been familiar with fundamentals of medicine and would have been supervised by clinic staff. 2)Other potential reasons for the phenomenon may be connected to that fact that most participants had comorbidities. As such, they had attached great importance to these conditions and had exceptional adherence to treatment processes. This demonstrates the achievements in CKD management, in terms of improving medication adherence. On the negative side, our participants were overconfident. 3) Another potential reason for the undesirable results is that technical issues may have impaired response patience, rates and accuracy. Answers to the e-version questionnaire were limited by internet speeds and device limitations. The layout of the questionnaire requested that users slide to navigate the scale forwards and backwards, and font size might have visually influenced questionnaire administration. 4) Environmental factors also may have impaired response concentration. Some participants finished the survey carelessly, without the supervision of medical personnel, or interrupted by external factors, especially when they proceeded to the last item.14 Moreover, for the convenience of the electronic version, completion time was limited to 24 hours, which was too long to reveal actual completion times. 5) Also, inter-patient difference impaired reliability. Course of disease, acquisition of medical fundamentals, degree of recognition and self-management ability varied from participant to participant. Comorbidities encouraged patients to pay more attention to their medication, and investigate their own medication statuses.
The electronic version of the questionnaire had advantages such as its convenience, unlimited time and setting, and rapid and accurate data administration. Plus, it was paperless. However, this study revealed that adapting a paper-version medication adherence scale into an e-version was challenging. Considering the difficulties, we offer several suggestions: For electronic devices, an offline e-version questionnaire less dependent on internet access might prevent network latency. For example, the payment processor Alipay can generate offline QR codes and unique identities for each user as seed data so that their customers can complete transactions even when their devices are operating with a poor signal. Moreover, remote monitoring of time limits could prevent intermittent and/or careless completion. For e-version questionnaires, personalized design is necessary to improve visual perception. Further improvement in content elevates acceptable reliability and validity, which is more suitable to participants. For users, disease management education needs improvement to narrow inter-patient knowledge disparities. In sum, more research on other commonly used PROMs tools for evaluating medication adherence is needed to narrow the transition gap between paper-version and e-version scales.
Limitations
This study has several limitations. Our population had accepted CKD self-management education, which may limit this study’s generalizability to other CKD populations. Moreover, we did not compare the medication adherence questionnaire to either a paper medication adherence questionnaire, or a different computer-administered adherence questionnaire within the same study population. Furthermore, because of data deficiency, we did not analyze the correlation between the medication adherence questionnaire and other biochemical indexes.
Conclusion
We evaluated the e-version Chinese and Western medication adherence scale for CKD, and obtained unacceptable reliability and validity. Methods to monitor scale completion are essential in e-version scale implementation. Caution is needed in transitioning paper-version scales into e-versions. Further research into this would provide the basis for further improvement of computer-administered medication adherence questionnaires.
Author Contributions
Hui-fen Chen: conception and design, execution, collection and analysis of data, draft and interpretation; Nuo Lei: acquisition of data and substantially revised; Yan-min Xu: acquisition of data and substantially revised; Li Luo & Xian-long Zhang: conception and design, collection and assembly of data, critically reviewed the article; Bei-ni Lao: data analysis and interpretation, critically reviewed the article. Fang Tang & Li-zhe Fu: execution and critically reviewed the article; Xu-sheng Liu & Yi-fan Wu: conception and design, administrative support and critically reviewed the article. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by Science and Technology Planning Project of Guangdong Province, China: Establishment and Evaluation of the Medication Adherence Scale of Traditional Chinese and Western Medicine for Patients with Chronic Kidney Disease under Grant number 2014A020221087 and The National Key Research and Development Program of China: Establishment and Evaluation an Exposed Omics based Prediction Model on CKD Risk and Benefit Factors (Project No. 2019YFE0196300).
Disclosure
The authors have no conflicts of interest for this work to declare. All authors have completed the ICMJE uniform disclosure form. Financial disclosure: none reported.
References
1. Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733.
2. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi:10.1016/S0140-6736(12)60033-6
3. Huang CW, Wee PH, Low LL, et al. Prevalence and risk factors for elevated anxiety symptoms and anxiety disorders in chronic kidney disease: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2021;69:27–40. doi:10.1016/j.genhosppsych.2020.12.003
4. Yeam CT, Chia S, Tan H, Kwan YH, Fong W, Seng J. A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos Int. 2018;29(12):2623–2637. doi:10.1007/s00198-018-4759-3
5. Seng J, Tan JY, Yeam CT, Htay H, Foo W. Factors affecting medication adherence among pre-dialysis chronic kidney disease patients: a systematic review and meta-analysis of literature. Int Urol Nephrol. 2020;52(5):903–916. doi:10.1007/s11255-020-02452-8
6. Kwan YH, Weng SD, Loh D, et al. Measurement properties of existing patient-reported outcome measures on medication adherence: systematic review. J Med Internet Res. 2020;22(10):e19179. doi:10.2196/19179
7. Voils CI, Hoyle RH, Thorpe CT, Maciejewski ML, Yancy WJ. Improving the measurement of self-reported medication nonadherence. J Clin Epidemiol. 2011;64(3):250–254. doi:10.1016/j.jclinepi.2010.07.014
8. Tan J, Luo L, Zhang M, et al. A Chinese and Western medication adherence scale in patients with chronic kidney disease. Patient Prefer Adherence. 2019;13:1487–1495. doi:10.2147/PPA.S207693
9. Huang Q, Luo L, Xia BQ, et al. Refinement and evaluation of a Chinese and Western medication adherence scale for patients with chronic kidney disease: item response theory analyses. Patient Prefer Adherence. 2020;14:2243–2252. doi:10.2147/PPA.S269255
10. [No authors listed].Erratum: Kidney Disease: improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59. Kidney Int Suppl. 2017;7(3):e1. doi:10.1016/j.kisu.2017.10.001
11. Cedillo-Couvert EA, Ricardo AC, Chen J, et al. Self-reported medication adherence and CKD progression. Kidney Int Rep. 2018;3(3):645–651. doi:10.1016/j.ekir.2018.01.007
12. Hsu KL, Fink JC, Ginsberg JS, et al. Self-reported medication adherence and adverse patient safety events in CKD. Am J Kidney Dis. 2015;66(4):621–629. doi:10.1053/j.ajkd.2015.03.026
13. Park MS, Kang KJ, Jang SJ, Lee JY, Chang SJ. Evaluating test-retest reliability in patient-reported outcome measures for older people: a systematic review. Int J Nurs Stud. 2018;79:58–69. doi:10.1016/j.ijnurstu.2017.11.003
14. Luo L, Zhang M, Chen HF, et al. Validity, reliability, and application of the electronic version of a chronic kidney disease patient awareness questionnaire: a pilot study. Postgrad Med. 2021;133(1):48–56. doi:10.1080/00325481.2020.1801029
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
