Back to Journals » Orthopedic Research and Reviews » Volume 11
Is Dextrose Prolotherapy Superior To Corticosteroid Injection In Patients With Chronic Lateral Epicondylitis?: A Randomized Clinical Trial
Authors Bayat M , Raeissadat SA, Mortazavian Babaki M , Rahimi-Dehgolan S
Received 7 June 2019
Accepted for publication 8 October 2019
Published 5 November 2019 Volume 2019:11 Pages 167—175
DOI https://doi.org/10.2147/ORR.S218698
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Clark Hung
Masume Bayat,1 Seyed Ahmad Raeissadat,2 Maryam Mortazavian Babaki,3 Shahram Rahimi-Dehgolan4
1Physical Medicine and Rehabilitation Department of Mahdiyeh Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Clinical Development Research Center of Shahid Modarres Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 3Physical Medicine and Rehabilitation Department & Research Center, Shohada-E-Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 4Physical Medicine and Rehabilitation Department of Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Maryam Mortazavian Babaki
No. 1989934148, Shohada-e-Tajrish Hospital, Tehran, Iran
Tel +982122731112
Fax +982122724210
Email [email protected]
Purpose: To compare the efficacy of dextrose prolotherapy versus steroid injection in the treatment of patients with chronic lateral epicondylitis.
Methods: Thirty subjects with chronic lateral epicondylitis were randomly assigned into two groups of hypertonic dextrose or methylprednisolone injection. Participants were assessed through Quick DASH and VAS scores, once before injection, and then after 1- and 3-months follow-up. Two patients were excluded due to not completing the follow-up timepoints.
Results: In both groups VAS scores revealed significant improvement during the first month follow-up [mean difference (MD) = 1.9±3.3, versus 1.5±1.9 for the prolotherapy and steroid groups, respectively]. This declining trajectory continued at the third month visit in the prolotherapy group and MD reached 4.4±2.9, while it did not change remarkably in the steroid group (MD=1.9±3.4). In fact, comparing VAS scores between the 1st- and 3rd-month time points did not reveal a significant improvement in the steroid group (p=0.6). Also, the Quick DASH index showed a similar pattern and improved remarkably in both groups during the first visit. However, only the efficacy in the prolotherapy group persisted after 3-month follow-up (MD = 9.5±21.6, p=0.044). One month after injections no preference between the two interventions was observed (p=0.74 for VAS and 0.14 for Quick DASH score). However, the 3rd-month follow-up revealed a meaningful superiority (p=0.03 for VAS and p=0.01 for Quick DASH score) favoring the prolotherapy method.
Conclusion: Both methods were proven to be effective in the short-term treatment of chronic lateral epicondylitis, but dextrose prolotherapy seems to be slightly more efficacious than steroid injection over a longer period.
Clinical trial registration: Iranian Registry of Clinical Trials Database: IRCT20170311033000N3.
Keywords: regenerative medicine, tennis elbow, methylprednisolone, prolotherapy
Introduction
Lateral epicondylitis, also called tennis elbow syndrome, is known to be the most common condition of elbow pain with a prevalence of 1–2% among th enormal population aged 30–65 years, and up to 40% among certain subgroups such as professional tennis players.1–4 Highly repetitive activities might be the most important cause of lateral epicondylitis.5 Lateral epicondylitis can affect the daily activities of individuals and in severe cases it can impose a relatively high financial burden on the sufferers.1,3 Chronic lateral epicondylitis was considered in cases lasting more than 3 months as opposed to early or subacute lateral epicondylitis.5 There are several non-surgical options for the treatment, but the current literature has not provided any conclusive evidence regarding the non-surgical methods.2
Non-surgical therapies include anti-inflammatory drugs, prefabricated splints, eccentric forearm-dorsiflexors exercise, injections, and lastly the physical agent modalities such as ultrasound (US), extracorporeal shockwave therapy (ESWT), low-level LASER.2,6 There are multiple types of injections including autologous blood, platelet-rich plasma, botulinum toxin, ozone-oxygen solution, hyaluronic acid, dextrose prolotherapy, and steroid. The last two options have been conventionally more available and are the main issue of this investigation.1,7–12
From the histopathologic point of view, microscopic injuries to the forearm common extensor tendons such as the extensor carpi radialis brevis have been established in patients with lateral epicondylitis. However, the lack of inflammatory cells confirms that it is not an acute inflammatory condition. The current therapeutic options should be oriented to correct the mentioned pathology.1–3,10 Prolotherapy is a traditional injection method which has been recently categorized as a regenerative treatment. Conventionally, hypertonic dextrose (10–20%) has been used in prolotherapy. It can result in a stimulated local inflammation and helps the restoration of the injured tissue. Based on previous research, it seems that prolotherapy can stimulate the healing process, reduce pain, and improve function in chronic musculoskeletal problems such as lateral epicondylitis. However, the exact mechanism of action is not yet fully understood.13–17 The strength of existing evidence in favor of prolotherapy is considered as level B recommendations.8,18
On the other hand, steroid injection has been known as the most rapid treatment for early epicondylitis. However, the present literature is not enough to support its effectiveness for chronic cases. In fact, although it was beneficial for short-term pain relief of acute conditions, the mid- and long-term follow-up did not support the use of steroids.6,19–21 This study aimed to evaluate the efficacy of steroid injection versus dextrose prolotherapy in patients with lateral epicondylitis.
Methods
Design And Participants
Eighty-six patients with confirmed diagnosis of lateral epicondylitis presenting to our center during August to October 2018 were evaluated to enroll in this randomized clinical trial (RCT). The diagnosis was made clinically based on symptoms, point tenderness, and pain elicited by Cozen’s test. Subjects aged 18–55 years who had had symptoms for longer than 3 months were included. Our exclusion criteria included (a) any history of local trauma, surgery, or prior injection about the lateral epicondyle during the last 3 months; (b) the presence of any concomitant cervical radiculopathy in the same limb; and (c) systemic comorbidities such as diabetes, rheumatologic disorders, etc.
Interventions
Among the mentioned population, 30 eligible participants were randomly assigned into two categories using computer-based randomization software. In the first category, 14 subjects received a local injection of 3 mL solution containing 1 mL methylprednisolone 40 mg/mL and 2 mL lidocaine 1%. The other group with 16 participants underwent dextrose prolotherapy method; in fact, they received an injection of 3 mL solution containing 2.5 mL dextrose 20% and 1 mL lidocaine 2%. The dextrose concentration in the final solution was about 16%. The present study was a double-blinded RCT in which participants and the physician who was responsible for assessing outcomes were completely unaware of the patients' group. All injections were performed using a 23-gauge needle under sterile condition and by an expert physiatrist (MB) who had 10 years' of experience in the musculoskeletal injection field. The patients were placed in a lateral-decubitus position. Participants received injections at the point of maximal tenderness using a peppering technique spreading in a clockwise manner to achieve a wider zone of delivery. Then patients were asked to use ice massage for 5–10 min on the injection site. They were also advised to consume acetaminophen 500 mg orally during the first 48 hr after injections. Using non-steroidal anti-inflammatory drugs was not allowed during the follow-up period. Eventually, subjects were instructed how to correctly wear their splint (tennis-elbow band) and to do gentle stretching exercises of the common extensors on a regular basis of three sessions per week. After two weeks, eccentric loaded exercises were started twice a day for five weeks.
Outcome Measures
VAS is a visual graphic-rating scale of 0–10 in which 0 indicates no pain and 10 shows the worst pain ever experienced. The validity and reliability of self-rating scales like VAS were previously well described.22,23 The elbow disability scale, as the primary outcome measure, was assessed using the Quick DASH (disability of arm, shoulder & hand) questionnaire containing 11 questions with five choices for each question. The final score can range between 0 (best condition) and 100 (worst condition).24 Also, the patients’ satisfaction was asked after a 3-month follow-up ranging from 0 to 5 (from 0 = dissatisfaction to 5 = very satisfied). Furthermore, all subjects were monitored if they had any side effects from the injection such as ecchymosis, redness, increasing pain, or reduced range of motion.
Registry And Analysis
In accordance with the Declaration of Helsinki, after providing necessary data, a written informed consent was obtained for all patients. The study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1396.646). Also, it was registered in the Iranian Registry of Clinical Trials Database (IRCT20170311033000N3).
The sample size estimated based on prior similar articles was calculated to be 30 patients, considering a power of 80% and a probable drop rate of 10%.3
Data were analyzed using SPSS version 22.0 (Statistical Package for Social Sciences, Inc., Chicago, Illinois, USA). The Shapiro–Wilk test was used to test the normal distribution of variables. Descriptive results were expressed using frequency, percentage, mean and standard deviation. Chi-squared method and Student’s t-test were applied for comparing categorical and quantitative variables, respectively. P-values less than 0.05 were considered to be statistically significant.
Results
Among 30 eligible patients included in this trial, 28 completed the study. Two subjects in the prolotherapy group discontinued for personal reasons (Figure 1). The mean age was 46.2 years in the prolotherapy group and 50.7 in the steroid group (p=0.1). Seventeen participants were female (60%). The majority (75%) of cases were attributed to the dominant upper limb. The mean duration of symptoms was 5.7 and 10.3 months for the prolotherapy and steroid groups, respectively (p=0.053). Only about 7% of patients were heavy workers, and the rest were housewives or low-load workers (p=0.183). The demographic characteristics and the baseline values of clinical variables have been demonstrated in Table 1. Pain intensity using VAS and functional status via Quick DASH were evaluated before the intervention as the baseline outcomes. No significant difference was observed between the two groups regarding the initial VAS and DASH scores (Table 1).
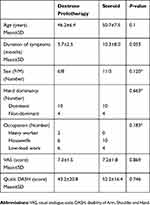 |
Table 1 Demographics And Baseline Characteristics |
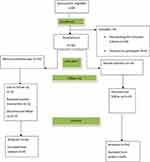 |
Figure 1 Flowchart of the study population. |
Comparing to the baseline level, mean VAS score decreased significantly in both groups (Table 2) at the first follow-up [mean difference (MD) = 1.9±3.3, p=0.045 and MD = 1.5±1.9, p=0.012 for the prolotherapy and steroid groups, respectively). Later at the 3rd month, this improvement remained significant (MD = 4.4±2.9, p < 0.001 and MD = 1.9±3.4, p=0.043 in the prolotherapy and steroid groups, respectively). Also, comparing the 1st- and 3rd- month VAS mean values revealed a significant decrease for the prolotherapy group (MD = 2.5±2.6, p < 0.005), while this reduction was not remarkable in the steroid group (p=0.606) (Figure 2). In a similar manner, comparing the baseline level, the Quick DASH score improved significantly in both groups at the first follow-up time-point (MD = 18.9±24.8, p=0.014 and MD = 17.3±10.7, p < 0.001 for prolotherapy and steroid groups, respectively), as well as at the second visit (p=0.001 for both groups). Again despite the changes in the prolotherapy group (MD = 9.5±21.6, p=0.044), the improvement between the 1st and 2nd visits was not statistically significant in the steroid group (p = 0.954) (Figure 3).
 |
Table 2 Comparison Of Efficacy Within The Two Groups Based On Changes From The Baseline |
 |
Figure 2 The therapeutic trajectory for VAS changes within the two groups. |
 |
Figure 3 The therapeutic trajectory for Quick DASH changes within the two groups. |
One month after injection, there was no remarkable difference between the two interventions (p=0.74 for VAS and p = 0.14 for Quick DASH). However, the 2nd follow-up revealed a meaningful superiority (p=0.01) favoring the prolotherapy group (Table 3). Eventually, the success rate was defined as at least 50% reduction of VAS score compared to the baseline values. The values of both groups were similar at the first follow-up (21.4% for prolotherapy versus 28.6% for steroid injection). However, after 3 months it increased to 58.3% in the prolotherapy group, whereas the success rate remained 42.9% in the steroid group.
 |
Table 3 Comparison Of Efficacy Between The Two Groups Based On Their Clinical Improvement |
Among our subjects, eight patients (57.1%) in the prolotherapy category and one patient (7.1%) in the steroid group were totally satisfied by the treatment (p=0.025). In the prolotherapy group, none of the patients mentioned any adverse events. However, one subject in the steroid group reported a transient redness and decreased range of movement, and two patients mentioned post-injection pain (Table 4).
 |
Table 4 Comparison Of Satisfaction, Side Effects, And Success Rate Between Two Groups |
Discussion
This investigation showed that both corticosteroid injection and dextrose prolotherapy efficiently improved pain and function in patients with chronic lateral epicondylitis. In the prolotherapy group, this improvement persisted even after 1-month follow-up and the results after one injection were still improvable, whereas in the parallel group, steroid only provided a short-term improvement. This finding proved that dextrose prolotherapy had better and longer effects in treating chronic tennis elbow; however, the impact of exercise and splinting as the basic treatment should not be ignored in the improvement of patients. Prior research has achieved a level 1B of evidence for the efficacy of prolotherapy.9,25 Among the few studies assessing prolotherapy effectiveness in tennis elbow, we have discussed some of the most important ones.3,26,27 Primarily, in 2008 Scarpone et al. evaluated the efficacy of prolotherapy (dextrose 11%) in refractory tennis elbow. They demonstrated improvement in pain and isometric strength scores compared to the control group in which normal saline was injected. The effect was maintained at long-term follow-up.26 In the current study we used higher concentrations of dextrose (16%). Our findings showed improvement in both the prolotherapy and steroid groups at the 3rd-month follow-up. However, prolotherapy proved to have significantly better and longer effects. This finding is consistent with a recent study that has suggested inferior long-term efficacy of steroid than other treatments for chronic lateral epicondylitis.28 In 2011 Crayannopoulos et al. compared prolotherapy (phenol 1.2%, glycerin 12.5%, and dextrose 12.5% in sterile water) versus methylprednisolone 40 mg/mL in a double-blinded RCT. After 6-months follow-up, they detected a significant improvement in the functional status (based on DASH) of both groups, but VAS scores did not show significant changes in the steroid group. Finally, their conclusion did not support any superiority of prolotherapy to steroid. However, they stated that it might be due to lack of statistical power.27 In our trial, we observed a remarkable difference between the prolotherapy and steroid efficacy in favor of phototherapy for chronic cases of tennis elbow with sufficient statistical power. Rabago et al., in 2014, in a three-arm RCT evaluated 26 patients with chronic lateral epicondylitis comparing dextrose prolotherapy, dextrose–morrhuate sodium and conservative treatment of wait-and-watch. Morrhuate sodium is known as an irritant substance reserved for clinically more severe cases of chronic lateral epicondylitis.26 The results revealed a significant improvement in Patient-Rated Tennis Elbow Evaluation (PRTEE) questionnaire score for both prolotherapy groups. However, the grip strength improved only in the dextrose prolotherapy group.26 In their study, a higher concentration of dextrose was used in comparison to the previous studies.3,27 Generally, our findings were in line with the earlier researches. We have used only one injection, which is less invasive than multiple-injection trials.3,26,27
In 2014 Sims et al., in a systematic review, assessed the efficacy of non-surgical treatments of lateral epicondylitis including various types of injections, bracing, and physical agent modalities such as ESWT and low-level LASER. Regarding the effectiveness of local steroid injection, they reported a short-term improvement in pain and function, but the results did not support the long-term benefits of steroid.29–32 They also evaluated and reviewed the efficacy of prolotherapy method in three studies.3,27,33 Only one of them compared the prolotherapy with steroid injection, exactly similar to our investigation.27 However, that study was inconclusive due to the high amount of loss to follow-up (29%). Similarly, Krogh et al. in 2013 evaluated several RCTs and finally concluded that in contrast to steroid, prolotherapy was significantly better than placebo.3,15,16,34 Lastly, in 2018, Dwivedi et al. reviewed articles working on utility of prolotherapy in upper extremity. Their study proved the beneficial effects of prolotherapy for upper extremity pathology such as hand osteoarthritis, lateral epicondylitis, and rotator cuff disease as it is safe and cost-effective.35 In the present RCT we detected a short-term efficacy for local steroid injection. However, prolotherapy revealed longer and higher therapeutic effects.
Limitations
The major limitation of this RCT was small sample size. However, compared to previous studies3,26,27 that was acceptable. As the other drawback, we did not use any objective measurement such as grip strength, pressure pain threshold or imaging modalities such as US. These outcome measuring tools could accurately confirm the improvement. Another point which may affect the result was considerable difference in symptom chronicity between the two groups. However, it was not statistically significant and could be due to outlier data of one patient, which was about 24 months in the steroid group. We didn’t exclude him due to the small sample size and it doesn’t seem to affect the end result significantly. On the other hand, it should be emphasized that the double-blinded RCT design, validated patient-oriented outcome measure, and minimal data loss were our strengths. In the future, larger RCTs with longer duration of follow-up are needed.
Conclusions
This study proved a significant improvement in both the prolotherapy and steroid injection groups during one-month follow-up. However, in the prolotherapy group, this improvement persisted even after 3 months, while in the parallel group, steroid only provided a short-term improvement. To summarize, dextrose prolotherapy had better and longer effects in treating chronic tennis elbow.
Data Sharing Statement
The authors do not intend to share substantial data of this study, but they are ready to share the de-identified datasheet file of other study-related documents, at any specific time of any period, on the related demand.
Acknowledgments
This study is an extension of the thesis written by Dr. Maryam Mortazavian Babaki at the School of Medicine, Shahid Beheshti University of Medical Sciences.
Disclosure
This study had no funding source and the authors report no conflicts of interest in this work.
References
1. Kahlenberg C, Knesek M, Terry M. New developments in the use of biologics and other modalities in the management of lateral epicondylitis. Biomed Res Int. 2015;2015:1–10. doi:10.1155/2015/439309
2. Sims S, Miller K, Elfar J, Hammert W. Non-surgical treatment of lateral epicondylitis: a aystematic review of randomized controlled trials. HAND. 2014;9(4):419–446. doi:10.1007/s11552-014-9642-x
3. Scarpone M, Rabago D, Zgierska A, Arbogast G, Snell E. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18(3):248–254. doi:10.1097/jsm.0b013e318170fc87
4. Shiri R, Viikari-Juntura E, Varonen H, Heliovaara M. Prevalence and determinants of lateral and medial epicondylitis: a population study. Am J Epidemiol. 2006;164(11):1065–1074. doi:10.1093/aje/kwj325
5. Gabel G. Acute and chronic tendinopathies at the elbow. Curr Opin Rheumatol. 1999;11(2):138–143. doi:10.1097/00002281-199903000-00010
6. Bellapianta J, Swartz F, Lisella J, Czajka J, Neff R, Uhl R. Randomized prospective evaluation of injection techniques for the treatment of lateral epicondylitis. Orthopedics. 2011. doi:10.3928/01477447-20110922-13
7. Louw F. The occasional prolotherapy for lateral epicondylosis(tennis elbow). Can J Rural Med. 2014;19(1):31–33.
8. Korthals-de Bos I, Smidt N, van Tulder M, et al. Cost effectiveness of interventions for lateral epicondylitis. Pharmacoeconomics. 2004;22(3):185–195. doi:10.2165/00019053-200422030-00004
9. Reeves K, Sit R, Rabago D. Dextrose prolotherapy. Phys Med Rehabil Clin N Am. 2016;27(4):783–823. doi:10.1016/j.pmr.2016.06.001
10. Raeissadat S, Rayegani S, Hassanabadi H, Rahimi R, Sedighipour L, Rostami K. Is Platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014;6(1). doi:10.1186/2052-1847-6-12
11. Raeissadat S, Abdollahzadeh M. Comparison of the effects of ozone versus steroid injection, in treatment of patients with tennis elbow. Ann Phys Rehabil Med. 2018;61:e168. doi:10.1016/j.rehab.2018.05.382
12. Raeissadat SA, Babaee M, Rayegani SM, et al. An overview of platelet products (PRP, PRGF, PRF, etc.) in the Iranian studies. Futur Sci OA. 2017;3(4):FSO231. doi:10.4155/fsoa-2017-0045
13. Hauser R, Lackner J, Steilen-Matias D, Harris D. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights. 2016;9:
14. Trescot A. Everything old is new again: new developments in prolotherapy. Tech Reg Anesth Pain Med. 2015;19(1–2):14–18. doi:10.1053/j.trap.2016.09.003
15. Yelland M, Sweeting K, Lyftogt J, Ng S, Scuffham P, Evans K. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2009;45(5):421–428. doi:10.1136/bjsm.2009.057968
16. Ahn KH, Kim HS, Lee WK, et al. The effect of the prolotherapy on the injured Achilles tendon in a rat model. J Korean Acad Rehabil Med. 2002;26:332–336.
17. Krogh TP, Bartels EM, Ellingsen T, et al. Comparative effectiveness of injection therapies in lateral epicondylitis: a systematic review and network meta-analysis of randomized controlled trials. Ann Rheum Dis. 2013;72(suppl3). doi:10.1136/annrheumdis-2012-eular.1755
18. Rabago D, Best TM, Zgierska AE, Zeisig E, Ryan M, Crane D. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med. 2009;43(7):471–481. doi:10.1136/bjsm.2008.052761
19. Olaussen M, Holmedal O, Lindbaek M, Brage S, Solvang H. Treating lateral epicondylitis with corticosteroid injections or non-electrotherapeutical physiotherapy: a systematic review. BMJ Open. 2013;3:10. doi:10.1136/bmjopen-2013-003564
20. Wolf JM, Ozer K, Scott F, Gordon MJ, Williams AE. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36(8):1269–1272. doi:10.1016/j.jhsa.2011.05.014
21. Mardani-Kivi M, Karimi-Mobarakeh M, Karimi A, et al. The effects of corticosteroid injection versus local anesthetic injection in the treatment of lateral epicondylitis: a randomized single-blinded clinical trial. Arch Orthop Trauma Surg. 2013;133(6):757–763. doi:10.1007/s00402-013-1721-x
22. Boonstra AM, Preuper HRS, Reneman MF, Posthumus JB, Stewart RE. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res. 2008;31(2):165–169. doi:10.1097/mrr.0b013e3282fc0f93
23. Turchin D, Beaton D, Richards R. 8 validity of observer-based aggregated scoring systems as descriptors of elbow function. J Shoulder Elbow Surg. 1998;7(3):305. doi:10.1016/s1058-2746(98)90068-4
24. Clement ND, Duckworth AD, Jenkins PJ, Mceachan JE. Interpretation of the QuickDASH score after open carpal tunnel decompression: threshold values associated with patient satisfaction. J Hand Surg Eur Vol. 2016;41(6):624–631. doi:10.1177/1753193415622341
25. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Med. 2004;17(1):59–67. doi:10.3122/jabfm.17.1.59
26. Rabago D, Lee KS, Ryan M, et al. Hypertonic dextrose and morrhuate sodium injections (Prolotherapy) for lateral epicondylosis (Tennis Elbow). Am J Phys Med Rehabil. 2013;92(7):587–596. doi:10.1097/phm.0b013e31827d695f
27. Carayannopoulos A, Borg-Stein J, Sokolof J, Meleger A, Rosenberg D. Prolotherapy versus corticosteroid injections for the treatment of lateral epicondylosis: a randomized controlled trial. PM&R. 2011;3(8):706–715. doi:10.1016/j.pmrj.2011.05.011
28. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376(9754):1751–1767. doi:10.1016/s0140-6736(10)61160-9
29. Bisset L, Beller E, Jull G, Brooks P, Darnell R, Vicenzino B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ. 2006;333(7575):939. doi:10.1136/bmj.38961.584653.ae
30. Bisset L, Smidt N, Windt DAVD, et al. Conservative treatments for tennis elbow do subgroups of patients respond differently? Rheumatology. 2007;46(10):1601–1605. doi:10.1093/rheumatology/kem192
31. Smidt N, Windt DAVD, Assendelft WJ, Devillé WL, Bos IBK-D, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657–662. doi:10.1016/s0140-6736(02)07811-x
32. Newcomer KL, Laskowski ER, Idank DM, Mclean TJ, Egan KS. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11(4):214–222. doi:10.1097/00042752-200110000-00002
33. Zeisig E, Fahlstrom M, Ohberg L, Alfredson H. Pain relief after intratendinous injections in patients with tennis elbow: results of a randomised study. Br J Sports Med. 2008;42(4):267–271. doi:10.1136/bjsm.2007.042762
34. Lindenhovius A, Henket M, Gilligan BP, Lozano-Calderon S, Jupiter JB, Ring D. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg Am. 2008;33(6):909–919. doi:10.1016/j.jhsa.2008.02.004
35. Dwivedi S, Sobel AD, Dasilva MF, Akelman E. Utility of prolotherapy for upper extremity pathology. J Hand Surg Am. 2019;44(3):236–239. doi:10.1016/j.jhsa.2018.05.021
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
