Back to Journals » Journal of Inflammation Research » Volume 16
Irisin Ameliorates PM2.5-Induced Acute Lung Injury by Regulation of Autophagy Through AMPK/mTOR Pathway
Authors Ma J, Han Z, Jiao R, Yuan G, Ma C, Yan X, Meng A
Received 20 September 2022
Accepted for publication 2 March 2023
Published 11 March 2023 Volume 2023:16 Pages 1045—1057
DOI https://doi.org/10.2147/JIR.S390497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Jiao Ma,1,* Zhuoxiao Han,1,* Rui Jiao,1 Guanli Yuan,1 Cuiqing Ma,2 Xixin Yan,1 Aihong Meng1
1Department of Respiratory and Critical Care Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050000, People’s Republic of China; 2Key Laboratory of Immune Mechanism and Intervention on Serious Disease in Hebei Province, Shijiazhuang, Hebei, 050000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Aihong Meng, Department of Respiratory and Critical Care Medicine, The Second Hospital of Hebei Medical University, No. 215, Heping West Road, Shijiazhuang, Hebei, 050000, People’s Republic of China, Email [email protected]
Background: PM2.5 exposure is one of the major inducements of various respiratory diseases and related mortality. Meanwhile, irisin, a metabolism and thermogenesis-related hormone, is found to be protective against acute lung injury induced by LPS, which indicates its therapeutic function in lung injury. However, the function and underlying mechanism of irisin in PM2.5-induced acute lung injury (ALI) are still unclear. This study is aimed to discover the potential mechanisms of irisin in PM2.5-induced acute lung injury.
Methods: Atg5 deficient mice and cells were established to clarify the relationship between irisin and autophagy in PM2.5-induced ALI. We also used Ad-mCherry-GFP-LC3B as a monitor of autophagy flux to claim the effects of irisin on autophagy. Western blotting and qPCR were used to reveal the molecular mechanism.
Results: As a result, PM2.5 exposure induced lung injury whereas mitigated by irisin. Moreover, PM2.5 hampered autophagy flux, characterized by accumulation of p62, and autophagosomes, as well as blocked autolysosomes. Irisin improved the disturbed autophagy flux, which was abrogated by deficiency of Atg5. Additionally, we demonstrated that irisin activated AMPK and inhibited mTOR, which indicated the enhanced autophagy. Moreover, blockage of AMPK by compound C terminated irisin’s induction of autophagy in cultured MH-S cells.
Conclusion: Our findings reveal that irisin performs protective effects against PM2.5-induced ALI by activating autophagy through AMPK/mTOR signaling pathway.
Keywords: irisin, PM2.5, acute lung injury, autophagy
Introduction
Fine particulate matter with an aerodynamic diameter equal or less than 2.5 microns, as well known as PM2.5 is regarded as a critical air pollutant, which carries a wide range of toxic substances.1,2 Long-term exposure to PM2.5 has been shown to be related to numerous respiratory diseases, ranging from chronic obstructive pulmonary disease (COPD), asthma, lung injury to lung cancer.3,4 Worse still, PM2.5 exposure increases the risk of mortality of patients with chronic pulmonary disease and presents a huge challenge to human health.5 Thus, there are urgent needs to discover the internal mechanism of respiratory injury caused by PM2.5 and also the potential treatment for it.
Autophagy, a highly conserved “self-eating” process, plays a vital role in maintaining the homeostasis of intercellular cells through degradation of damaged organelles or dysfunctional proteins by lysosomes,6 which also acts a critical role in pulmonary diseases. PM2.5 is identified to involve in autophagy from different perspectives of mechanism in lung injury.7–9
Irisin, a fragment of fibronectin type III domain-containing protein 5 (FNDC5), is a novel recognized exercise-induced myokine and mainly secreted from heart and muscles.10,11 It has been elucidated that irisin mainly influences energy metabolism and metabolic homeostasis by facilitating the conversion of white adipose tissue to brown one.10,12 Moreover, in acute lung injury (ALI) caused by lipopolysaccharide, irisin could attenuate inflammatory cytokine expression,13,14 indicating that irisin might be protective against ALI. As we already know, irisin could act as an autophagy regulator in various pathological status.15,16 For example, it could repair unbalanced autophagy through activation of AMPK-ULK1 signaling pathway in cardiac hypertrophy mice model.17
Currently, little is known about the role of irisin in ALI induced by PM2.5. In order to provide a valuable therapeutic alteration for PM2.5-induced respiratory diseases, the aim of this investigation is to verify the relation of irisin in PM2.5-induced ALI as well as the underlying mechanisms of the process.
Materials and Methods
PM2.5 Sample Collection and Preparation
Briefly, atmospheric PM2.5 was collected by a HVAIR high-volume air sampler (Thermo Fisher Scientific) from November to December 2021 at the monitor site provided by the Shijiazhuang Environmental Monitoring center (Shijiazhuang, China) when PM2.5 > 115 µg/m3. The collected glass filters were cut into rectangular strips (1 cm × 2 cm), immersed in deionized water, sonicated (5x30 min) using an ultrasonic cleaner (KQ-400KDB). Then, the resultant solution was filtered by eight-layer sterile gauze. Each PM2.5 suspension was freeze-dried under vacuum, weighed, and stored at −20°C.
Animals
Wild-type (WT) and Atg5 knockout (Atg5-KO) male C57BL/6J mice (6–8 weeks old, 22±2 g) were kindly provided by the Key Laboratory of Immune mechanism and Intervention on Serious Disease in Hebei Province and housed in the standard environment (23±2°C, 55±5% humidity, 12 h/12 h light/dark cycle) a week before the study. All of the experimental protocols were approved by the Animal Care and Use Committee of the Second Hospital of Hebei Medical University (2022-AE008) China, and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal Groups and Model of ALI
Mice were randomly allocated to the following five groups (n=5/group): WT-saline group, WT-PM2.5 group, WT-PM2.5+Irisin group, Atg5-KO group, and PM2.5+Irisin+Atg5-KO group. WT refers to wild-type mice, and Atg5-KO refers to Atg5 genetic knockout mice. In order to create acute lung injury model,7,18,19 mice in the PM2.5 (2 mg/mL) group were intratracheally instilled with sonicated PM2.5 sterile saline suspension (100 ug PM in 50 ul saline) per day for 2 days. Meanwhile, the mice in the saline group received the same volume of sterile saline. Mice in the irisin group (Ann Arbor, MI, USA, dissolved by normal saline)13 were administrated with 0.5 ug/g body weight of irisin 1h before PM2.5 challenge by intraperitoneal injection, while the other three groups were injected with the same volume of normal saline. Twenty-four hours after the last administration of PM2.5, all mice were anesthetized by pentobarbital at a dose of 60 mg/kg (P0225, EKEAR Bio, Shanghai, China). After then, the mice were sacrificed by rapid cervical dislocation. Bronchoalveolar lavage fluid (BALF) and the lung tissue were harvested for further examination.
BALF Collection
The right lobe of the mouse was instilled with 0.5mL sterile normal saline for thrice, assuring more than 50% fluid was obtained. The collected fluid was centrifuged at 252 g for 10 min at 4°C. And then the supernatant was stored at −80°C for later ELISA test according to the direction (IL-1β, IL-18, TNF-α) (Neobioscience Biotech Co., Ltd, Shanghai, China). The percent of macrophage in the BALF was counted by microscopic.
Hematoxylin & Eosin (H&E) and Immunofluorescence Staining
Lung tissues were fixed in 10% formalin, dehydrated with gradient alcohol, embedded in paraffin and sectioned into 5 μm slices. The sliced slides were stained with H&E according to the manufacturer’s instruction and assessed the general pathological changes in the lungs by inverted optical microscopy. The expression of FNDC5 (Bioss, Beijing, China) in lung tissues was determined by immunofluorescence staining and then visualized the images and detected fluorescence intensity by fluorescence microscope (Zeiss Inc., Germany).
Cell Culture and Viability Assay
Mouse alveolar macrophage cells (MH-S cells) were purchased from ATCC (USA) and maintained in medium with 10% fetal bovine serum (Gibco, USA). The plated cells in a 96-well plate were treated with different doses of PM2.5 suspension (0, 12.5 ug/mL, 25 ug/mL, 50 ug/mL, 100 ug/mL) for different periods (0, 2 h, 4 h, 8 h, 12 h, 24 h). The cytotoxicity of PM2.5 to MH-S was measured according to the instruction of CCK-8 kit (Glpbio Technology, Montclair, USA). Irisin (20 nM) or AMPK inhibitor compound C (Glpbio Technology, Montclair, USA) (10 uM) was treated 30 mins before PM2.5 administration.14,17 Then, levels of IL-1β, IL-18, and TNF-α in culture cells were determined by commercially mouse specific kits according to the manufacturer’s instructions.
Transmission Electron Microscopy (TEM) and Ad-mCherry-GFP-LC3B Transfection
MH-S cells receiving different treatments were embedded in 3% glutaraldehyde and examined by transmission electron microscopy (H-600, Hitachi, Japan). The cells were transfected with Ad-mCherry-GFP-LC3B (Beyotime, Beijing, China) adenovirus at an MOI of 20 for 24 h at 37°C treated with trial conditions as indicated. Finally, the number of fluorescent puncta was observed by a confocal microscope (Carl Zeiss, Gottingen, Germany).
Atg5 siRNA Transfection
Atg5 siRNA and control nonspecific siRNA as mock group (RiboBio, Guangzhou, China), using RFect transfection Reagent (BAIDAI, Changzhou, China), were adopted to silence the expression of Atg5 in MH-S cells. The cells were starved for 24 hours and transfected with Atg5 siRNA or nonspecific following the instructions for another 24 h. Then, treated the cells with PM2.5 (100 ug/mL) or in combination with irisin (20 nM) for 8 h. Transfection efficiency of silencing protein expression was identified by Western blotting at 48 h.
Western Blotting
Total proteins in lung tissues and iced cell lysates were extracted after tissues or cells were lysed by a RIPA buffer. The protein concentrations were then detected by the BCA Protein Assay Kit. And then, the total proteins were loaded into an 10% or 12% SDS-PAGE gel, transferred on a PVDF membrane. Next, the membranes were, respectively, incubated with primary antibodies for p-AMPK (1:1000, CST, USA), AMPK (1:1000, CST, USA), p-mTOR (1:1000, CST, USA), mTOR (1:1000, CST, USA), LC3-II (1:1000, CST, USA), P62 (1:500, Proteintech, USA) and fluorescent secondary antibody (1:10,000, CST, USA), then screened and visualized using an Odyssey Imaging System (Gene Co., Ltd, Hong Kong). The protein levels were normalized to β-actin (1:10,000, Proteintech, USA). Finally, the intensity of the band was quantified using Image J software.
Quantitative Real-Time PCR (qPCR)
Total RNA from cells or lung tissues was extracted by TriQuick Reagent (Solarbio, Beijing, China). Reverse transcription was performed with Reverse Transcription Reagents (GeneCopoeia, USA) and subsequently synthesized into cDNA. The DNA targets were amplified with primers (Table 1) by qPCR (GeneCopoeia, USA). The mRNA levels were calculated with the 2−ΔΔCt method and normalized to GAPDH. Table 1 demonstrates the primer information.
 |
Table 1 Sequence of Primers Used in qRT-PCR |
Statistical Analysis
Data were derived from at least three separate trials. All data were performed using SPSS 22.0 (IBM, Armonk, NY) and presented as mean ± standard deviation. Comparison means of different groups were calculated by one-way analysis of variance (ANOVA). Comparisons between two groups were evaluated using the two sample Student’s t-test. The statistical significance was set at p < 0.05.
Results
Irisin Alleviates Acute Lung Injury Caused by PM2.5 Through Autophagy and Reduces Inflammatory Cytokines in vivo
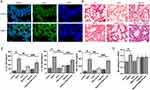
First of all, to investigate the anti-inflammatory capacity of irisin in PM2.5-induced ALI, FNDC5 expression in the lung tissue was determined by immunofluorescence. We identified that FNDC5 was abundant in PM2.5 exposed group compared with the control one (Figure 1A). Besides, the histological changes and the inflammatory cytokine level in BALF were analyzed. PM2.5 exposure group exhibited damaged and thickened alveolar walls, with mass infiltration of inflammatory cells around alveolar space. Meanwhile, irisin pretreatment improved the infiltration of inflammatory cells induced by PM2.5 (Figure 1B). In addition, PM2.5 significantly increased the secretion of IL-1β, IL-18, and TNF-α in BALF, and these effects were remarkably reduced by irisin (Figure 1C). Furthermore, we established Atg5-KO mice to inhibit autophagy and further claimed the role of irisin and autophagy in PM2.5-induced ALI. Atg5-KO alone aggravated the pathological damage compared with the control group and reversed the protective effect of irisin in PM2.5-induced ALI (Figure 1B and C). In addition, there were more macrophage cells in alveolar in PM2.5 group than the control one (Figure 1D). Taken together, these results suggested that irisin protects pulmonary from injury induced by PM2.5 through inducing autophagy.
Irisin Increases Autophagy Related Protein and mRNA Expression in vivo
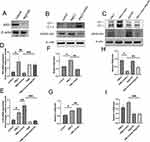
After then, we try to clarify if irisin improve PM2.5-induced ALI via inducing autophagy. We identified that PM2.5 exposure upgraded the level of SQSTM1/P62 (Figure 2B, D and F), a critical autophagy-related protein (ATG), which is the marker of autophagy activity.20 High expression of P62 might be the consequence of over-stimulated or blocked autophagy flux.21,22 Meanwhile, the expression of LC3II, associated with autophagosome development and also employed to monitor autophagy activity,21,23 was elevated in PM2.5 exposure group compared to control one (Figure 2B, E and G). Furthermore, treatment of irisin (20 nM) enhanced the amount of LC3II and reduced the level of P62 significantly, which demonstrated that irisin recovers the autophagy blocked by PM2.5 (Figure 2B–I). Moreover, the function of irisin was abrogated by Atg5-KO group, a kind of autophagy-blockage transgenic mice (Figure 2A, C, E, 2H and I). Therefore, it is clear that irisin enhances the autophagy which has been inhibited by PM2.5 in vivo.
Irisin Heightens Autophagy Related Protein and mRNA Expression and Lightens Inflammatory Cytokines in vitro
We have confirmed that irisin may remit inflammation and raise autophagy in vivo. Then, we use MH-S cells, a murine alveolar macrophage cell, which accounting for more than a half of the cells in the alveolar and playing a demonstrative role in the pathogenesis of ALI,24 to explore how irisin regulates autophagy under PM2.5 exposure.
Firstly, CCK-8 kit was used to calculate the cell cytotoxicity of PM2.5 at different intervention concentration and period to determine the final administrative dose and time. Combined with previous research and our CCK-8 results,25 PM2.5 at the concentration of 100ug/mL for 8h was selected as treatment scheme in vitro experiment (Figure 3J).
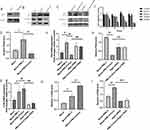
Similarly, irisin pre-treatment lessened the expression of P62 and enhanced the level of LC3II compared with PM2.5 exposure group (Figure 3B–I). After that, Atg5 deficient MH-S cells model via siRNA was built up to verify the relationship between irisin and autophagy (Figure 3A). The effects of irisin were also abated by Atg5 knockdown (Figure 3C, E, H and I). Ad-GFP-LC3B fusion protein puncta were rather higher in irisin pre-treatment group than PM2.5 group, which revealed that irisin enhanced autophagy activity in MH-S cells (Figure 4A and B). Moreover, Atg5 siRNA could reduce the number of LC3B puncta induced by irisin (Figure 4A and B). Furthermore, irisin remarkably relieved the secretion of inflammatory cytokines, indicating the anti-inflammatory capacity of irisin on MH-S cells (Figure 4C).
Taken together, the results above demonstrate that irisin recovers autophagy of MH-S cells from blockage caused by PM2.5 and inhibited inflammatory response.
Irisin Renovates Impaired Autophagy Flux in vitro
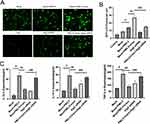
Next, transmission electron microscopy (TEM) and Ad-mCherry-GFP-LC3B vector were employed to testify the effects of irisin on autophagy flux visually. Autophagosome has been recognized as a typical double-layer membrane autophagic vacuoles including organelle remnants and autolysosome is circled by a single line containing electron-dense substances.26 Appropriate ratio of autophagosome to autolysosome insures the stability of autophagy flux.27 TEM analysis was used to observe and detect intercellular autophagosomes and autolysosomes, as shown PM2.5 exposure induced more autophagosomes but fewer autolysosomes (Figure 5A and D). The imbalance between autophagosomes and autolysosomes indicated disturbed autophagy flux, whereas the number of autophagosomes and autolysosomes were both greatly raised by irisin pre-treatment compared with PM2.5 alone in MH-S cells, suggesting that the disturbed autophagy flux triggered by PM2.5 was renovated by irisin.
Next, Ad-mCherry-GFP-LC3B vector was used as an additional monitor of autophagy flux. Depending on the theory that mCherry fluorescence is stable in the acidic lysosomal compartment, and GFP fluorescence is quenched in the low PH condition. Thus, autophagy flux can be identified by distinguishing yellow autophagosomes from red autolysosomes.28,29 According to the data, MH-S cells exposed to PM2.5 further yielded significant accumulation of yellow puncta but few red ones, revealing increased autophagosomes and decreased autolysosomes (Figure 5B and E), which suggested that autophagy flux was disturbed by PM2.5, whereas irisin supplementation significantly elevated both yellow and red puncta number. As results shown, irisin improved the disturbed autophagy flux triggered by PM2.5 in MH-S cells.
Irisin Promoted Autophagy Flux via AMPK/mTOR Signaling Pathways in vitro
Since we have demonstrated that irisin attenuates PM2.5 induced ALI through repairing the impaired autophagy flux, the underlying mechanism should be probed. Among numerous signaling pathways who participate in the management of autophagy, AMPK (Adenosine monophosphate-activated protein kinase)/mTOR (mammalian target of rapamycin) pathway is considered to be important.30–32 Hence, the expression level of AMPK/mTOR pathway was quantified by Western blotting. As the data illustrated PM2.5 administration increased the expression of phosphorylated AMPK but decreased that of mTOR (Figure 5C and F). Irisin enhanced the trends of AMPK/mTOR pathway in MH-S cells under PM2.5 exposure. Irisin also upregulated the expression of LC3II and downregulated p62 level (Figure 5C and F). These results indicated that irisin activated autophagy through AMPK /mTOR pathway in MH-S cells treated by PM2.5.
After that, AMPK inhibitor compound C (10 µM) was adopted. As shown Compound C impeded the activity of irisin in autophagy flux and AMPK/mTOR pathway (Figure 5C and F).
All in all, these results demonstrated that irisin repaired the blocked autophagy flux, which was triggered by PM2.5, via AMPK/mTOR pathways probably (Figure 6).
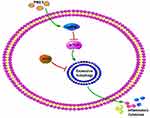 |
Figure 6 The mechanism diagram of irisin in PM2.5-induced acute lung injury. |
Discussion
PM2.5 exposure-related mortality has become a huge threat to human health. Our previous studies have demonstrated that PM2.5 exposure led to lung injury.2,33–35 Irisin, a myokine and adipokine, released by skeletal muscle is proved to be a potential therapy for ALI.13,14 However, the relationship between irisin and PM2.5-induced lung injury is still unknown. Here, in order to fill the blank in the research field, a PM2.5-induced ALI mice model and MH-S cell model were established to investigate the possible role and potential mechanism of irisin in the procedure. Eventually, our results demonstrated that irisin ameliorated PM2.5-induced ALI via repairing autophagy flux.
After lung ischemia-reperfusion injury, irisin enters into alveolar cells and the irisin level in BALF is much higher than that in the serum.36 The above studies indicate that FNDC5 might be recruited into the lung tissue from circulation under pathological condition. In PM2.5-induced ALI model, we observe that there is more FNDC5 in PM2.5 exposed group compared with the control one by immunofluorescence. PM2.5 induces acute lung injury characterized by infiltration of inflammatory cells and alveolar wall thickening from the aspect of morphological alternation, which was consistent with our previous studies,37,38 whereas irisin supplementation not only mitigated the pathological injury but also inhibited the inflammatory response in BALF.
Autophagy-related proteins are the critical players in the procedure of autophagy, and ATG5, in charge of autophagy vesicle formation, is indispensable.39,40 Knocking out ATG5 can lead to suppression of autophagy.41 Atg5-KO mouse model was established to evaluate the interplay of irisin and autophagy in PM2.5-induced ALI. Results showed that Atg5-KO hasten lung injury and abrogated the protective impact of irisin in PM2.5-induced ALI.
Irisin improves PM2.5-induced ALI from the perspective of macroscopic morphology. Then, biochemical markers are used to uncover the relationship between irisin and autophagy in PM2.5-induced ALI. SQSTM1/P62 serves as a marker of autophagy, as well as LC3. PM2.5 has been unveiled to induce the lung injury by blocking autophagy flux.22,42 Similarly, this study disclosed that PM2.5 exposure did enhance the expression of P62 protein not only in mice lung tissues but also in MH-S cells. Yet accumulation of P62 might due to the impaired autophagy.20,43,44 In addition, LC3II (microtubule associated protein 1 light chain 3 LC3), another essential universal contributor to autophagy, remains on the mature autophagosome until it integrates with the lysosomes.45 The elevated LC3II has become the key criterion for evaluation of autophagy level. Our previous study identified that PM2.5 elevated the expression of LC3II protein in MH-S cells and mice lung tissues. In this study, we verify that PM2.5 exposure considerably raised the protein and mRNA level of P62 and LC3II. We speculated that the over expression of P62 in PM2.5-induced lung injury was associated with the blocked flux, whereas irisin could notably lessen the expression of P62 and further elevate the level of LC3II triggered by PM2.5, which suggested that irisin can regenerate the disturbed autophagy flux induced by PM2.5 through clearance of accumulated P62 and further activation of LC3II. Furthermore, the above protective effect of irisin was abrogate by Atg5-KO mice model, demonstrating that the protective impact of irisin in PM2.5-induced ALI was associated with autophagy.
Alveolar macrophages accounting for more than a half of lung immune cells are situated on the inner surface of the lung, which participate in numerous lung diseases and are essential for maintaining airway homeostasis by clearing debris and apoptotic cells and stopping pulmonary alveolar from severe injury and dysfunction.46,47 Our previous study has confirmed that MH-S cells were involved in the autophagy process of PM2.5-induced lung injury.35 Thus, to imitate ALI in mice model, MH-S cells originated from the pulmonary of mice were adopted to our in vitro study. The protein and mRNA level of P62 and LC3II in PM2.5 exposure group were also increased, and irisin lessen P62 expression but abolished by Atg5 siRNA. Therefore, the results from the in vitro trial further are confirmed in the in vivo trial. Furthermore, TEM and Ad-mCherry-GFP vector were used to observe autophagy flux.7,17,19,25 In smokers’ alveolar macrophages, the increased number of autophagosomes and decreased autolysosomes might be the indication of suppressed autophagy flux.48 In our study, both TEM and Ad-mCherry-GFP vector exhibited that PM2.5 exposure aggregated more autophagosomes but few autolysosomes where the cytosolic materials are broken down and digested, which was in accordance with previous studies49 and suggested that the autophagy flux was disturbed. Irisin administration repaired the disturbed autophagy flux by elevating the number of autolysosomes and reducing the level of P62 in PM2.5 exposure MH-S cells.
At last, the mechanism of irisin stopped lung injury from PM2.5 administration was further investigated. AMPK/mTOR signaling pathway is recognized as predominant driv ers among various autophagy-related pathways. AMPK positively regulates autophagy under low energy status by dual regulation of mTOR and ULK1,32,50 which inhibits the activity of mTOR inducing the activation of a set autophagy-regulating proteins and the formation of autophagosomes and autolysosomes. Since a study uncovered that PM2.5 triggered autophagy in human lung epithelial cells via the activation of phosphorylation of AMPK and irisin also stimulated AMPK activity,51 our investigation also unveiled that PM2.5 exposure stimulated the activity of AMPK and prohibited the level of mTOR. Besides, pre-treatment of irisin further facilitated the stimulation of AMPK and inhibition of mTOR. However, AMPK inhibitor, compound C, eventually eliminated irisin induced activation of the AMPK and reduction of P62. Accordingly, these results suggested that irisin activated autophagy and restored impaired autophagy flux via AMPK/mTOR signaling pathway.
Conclusion
In summary, we disclose that irisin significantly attenuates PM2.5-induced ALI by lessening the release of inflammatory cytokines through regulation of disturbed autophagy flux. The possible mechanism involved in the whole story is the activation of AMPK/mTOR pathway. Taken together, our study unveils the beneficial effect and potential mechanism of irisin in PM2.5-induced ALI, which might shed promising light on the therapeutic approaches towards lung injury caused by environmental pollution.
Abbreviations
COPD, chronic obstructive pulmonary disease; ALI, acute lung injury; WT, wild-type; BALF, bronchoalveolar lavage fluid; TEM, transmission electron microscopy.
Data Sharing Statement
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Ethics Approval
All of the experimental protocols were approved by the Animal Care and Use Committee of the Second Hospital of Hebei Medical University (2022-AE008) China, and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Acknowledgments
This experiment was carried out at the Hebei Key Laboratory of Vascular Homeostasis and Hebei Collaborative Innovation Center for Cardio-Cerebrovascular Disease, Shijiazhuang, 050000, Hebei, China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Science Foundation of Hebei Province (H2019206263), the Key R&D Program of Hebei Province (19277760D), and the Hebei Province Applied Basic Research Program (15967753D).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Gautam S, Yadav A, Tsai CJ, Kumar P. A review on recent progress in observations, sources, classification and regulations of PM(2.5) in Asian environments. Environ Sci Pollut Res Int. 2016;23(21):21165–21175. doi:10.1007/s11356-016-7515-2
2. Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH. Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am J Epidemiol. 2017;186(8):961–969. doi:10.1093/aje/kwx166
3. Leclercq B, Platel A, Antherieu S, et al. Genetic and epigenetic alterations in normal and sensitive COPD-diseased human bronchial epithelial cells repeatedly exposed to air pollution-derived PM(2.5). Environ Pollut. 2017;230:163–177. doi:10.1016/j.envpol.2017.06.028
4. Zhou Y, Ma J, Wang B, et al. Long-term effect of personal PM(2.5) exposure on lung function: a panel study in China. J Hazard Mater. 2020;393:122457. doi:10.1016/j.jhazmat.2020.122457
5. Chen H, Goldberg MS, Villeneuve PJ. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Rev Environ Health. 2008;23(4):243–297. doi:10.1515/reveh.2008.23.4.243
6. Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–364. doi:10.1038/s41580-018-0003-4
7. Pei C, Wang F, Huang D, et al. Astragaloside IV protects from PM2.5-induced lung injury by regulating autophagy via inhibition of PI3K/Akt/mTOR signaling in vivo and in vitro. J Inflamm Res. 2021;14:4707–4721. doi:10.2147/JIR.S312167
8. Wang Z, Wu Y, Pei C, et al. Astragaloside IV pre-treatment attenuates PM2.5-induced lung injury in rats: impact on autophagy, apoptosis and inflammation. Phytomedicine. 2022;96:153912. doi:10.1016/j.phymed.2021.153912
9. Wang Y, Tang M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci Total Environ. 2020;710:136397. doi:10.1016/j.scitotenv.2019.136397
10. Korta P, Pocheć E, Mazur-Biały A. Irisin as a multifunctional protein: implications for health and certain diseases. Medicina. 2019;55(8):485. doi:10.3390/medicina55080485
11. Pesce M, Ballerini P, Paolucci T, Puca I, Farzaei MH, Patruno A. Irisin and autophagy: first update. Int J Mol Sci. 2020;21(20):7587. doi:10.3390/ijms21207587
12. Mazur-Bialy AI, Pocheć E, Zarawski M. Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. 2017;18(4):701. doi:10.3390/ijms18040701
13. Ma LY, Liu JM, Du GL, Dang XB. Irisin attenuates lipopolysaccharide-induced acute lung injury by downregulating inflammatory cytokine expression through miR-199a-mediated Rad23b overexpression. Exp Cell Res. 2021;404(2):112593. doi:10.1016/j.yexcr.2021.112593
14. Shao L, Meng D, Yang F, Song H, Tang D. Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem Biophys Res Commun. 2017;487(2):194–200. doi:10.1016/j.bbrc.2017.04.020
15. Li R, Wang X, Wu S, et al. Irisin ameliorates angiotensin II-induced cardiomyocyte apoptosis through autophagy. J Cell Physiol. 2019;234(10):17578–17588. doi:10.1002/jcp.28382
16. Wang FS, Kuo CW, Ko JY, et al. Irisin mitigates oxidative stress, chondrocyte dysfunction and osteoarthritis development through regulating mitochondrial integrity and autophagy. Antioxidants. 2020;9(9). doi:10.3390/antiox9090810
17. Li RL, Wu SS, Wu Y, et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J Mol Cell Cardiol. 2018;121:242–255. doi:10.1016/j.yjmcc.2018.07.250
18. Chen ZH, Wu YF, Wang PL, et al. Autophagy is essential for ultrafine particle-induced inflammation and mucus hyperproduction in airway epithelium. Autophagy. 2016;12(2):297–311. doi:10.1080/15548627.2015.1124224
19. Wu Y, Xiao W, Pei C, et al. Astragaloside IV alleviates PM2.5-induced lung injury in rats by modulating TLR4/MyD88/NF-κB signalling pathway. Int Immunopharmacol. 2021;91:107290. doi:10.1016/j.intimp.2020.107290
20. Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. Febs j. 2015;282(24):4672–4678. doi:10.1111/febs.13540
21. Satyavarapu EM, Das R, Mandal C, Mukhopadhyay A, Mandal C. Autophagy-independent induction of LC3B through oxidative stress reveals its non-canonical role in anoikis of ovarian cancer cells. Cell Death Dis. 2018;9(10):934. doi:10.1038/s41419-018-0989-8
22. Wei Y, Cao XN, Tang XL, et al. Urban fine particulate matter (PM2.5) exposure destroys blood-testis barrier (BTB) integrity through excessive ROS-mediated autophagy. Toxicol Mech Methods. 2018;28(4):302–319. doi:10.1080/15376516.2017.1410743
23. Schaaf MB, Keulers TG, Vooijs MA, Rouschop KM. LC3/GABARAP family proteins: autophagy-(un)related functions. FASEB j. 2016;30(12):3961–3978. doi:10.1096/fj.201600698R
24. Svedberg FR, Brown SL, Krauss MZ, et al. The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nat Immunol. 2019;20(5):571–580. doi:10.1038/s41590-019-0352-y
25. Liu T, Wu B, Wang Y, et al. Particulate matter 2.5 induces autophagy via inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin kinase signaling pathway in human bronchial epithelial cells. Mol Med Rep. 2015;12(2):1914–1922. doi:10.3892/mmr.2015.3577
26. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi:10.1016/j.cell.2010.01.028
27. Zhang L, Xu J, Han YF, et al. Detection of autophagic flux in primary cerebral cortical neurons after oxygen glucose deprivation/reperfusion (OGD/R) using various methods. J Chem Neuroanat. 2021;117:101999. doi:10.1016/j.jchemneu.2021.101999
28. He X, Yuan W, Li Z, Hou Y, Liu F, Feng J. 6-Hydroxydopamine induces autophagic flux dysfunction by impairing transcription factor EB activation and lysosomal function in dopaminergic neurons and SH-SY5Y cells. Toxicol Lett. 2018;283:58–68. doi:10.1016/j.toxlet.2017.11.017
29. Yu P, Zhang C, Gao CY, et al. Anti-proliferation of triple-negative breast cancer cells with physagulide P: ROS/JNK signaling pathway induces apoptosis and autophagic cell death. Oncotarget. 2017;8(38):64032–64049. doi:10.18632/oncotarget.19299
30. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi:10.1146/annurev-genet-102808-114910
31. He L, Zhang J, Zhao J, et al. Autophagy: the last defense against cellular nutritional stress. Adv Nutr. 2018;9(4):493–504. doi:10.1093/advances/nmy011
32. Mao K, Klionsky DJ. AMPK activates autophagy by phosphorylating ULK1. Circ Res. 2011;108(7):787–788. doi:10.1161/RES.0b013e3182194c29
33. Lu X, Zhang H, Wang M, et al. Novel insights into the role of BRD4 in fine particulate matter induced airway hyperresponsiveness. Ecotoxicol Environ Saf. 2021;221:112440. doi:10.1016/j.ecoenv.2021.112440
34. Wang M, Hou S, Lu X, Li J, Li R, Yan X. Interleukin-37 inhibits inflammation activation and disease severity of PM2.5-induced airway hyperresponsiveness. Ecotoxicol Environ Saf. 2021;227:112890. doi:10.1016/j.ecoenv.2021.112890
35. Yuan G, Liu Y, Wang Z, et al. PM2.5 activated NLRP3 inflammasome and IL-1beta release in MH-S cells by facilitating autophagy via activating Wnt5a. Int J Immunopathol Pharmacol. 2022;36:3946320221137464. doi:10.1177/03946320221137464
36. Chen K, Xu Z, Liu Y, et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9:418. doi:10.1126/scitranslmed.aao6298
37. Feng S, Duan E, Shi X, et al. Hydrogen ameliorates lung injury in a rat model of subacute exposure to concentrated ambient PM2.5 via Aryl hydrocarbon receptor. Int Immunopharmacol. 2019;77:105939. doi:10.1016/j.intimp.2019.105939
38. Zhao Y, Zhang H, Yang X, Zhang Y, Feng S, Yan X. Fine particulate matter (PM(2.5)) enhances airway hyperresponsiveness (AHR) by inducing necroptosis in BALB/c mice. Environ Toxicol Pharmacol. 2019;68:155–163. doi:10.1016/j.etap.2019.03.013
39. Arakawa S, Honda S, Yamaguchi H, Shimizu S. Molecular mechanisms and physiological roles of Atg5/Atg7-independent alternative autophagy. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(6):378–385. doi:10.2183/pjab.93.023
40. Ye X, Zhou XJ, Zhang H. Exploring the role of autophagy-related gene 5 (ATG5) yields important insights into autophagy in autoimmune/autoinflammatory diseases. Front Immunol. 2018;9:2334. doi:10.3389/fimmu.2018.02334
41. Guo H, Chitiprolu M, Roncevic L, et al. Atg5 disassociates the V(1)V(0)-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev Cell. 2017;43(6):716–730.e717. doi:10.1016/j.devcel.2017.11.018
42. Li Y, Fu S, Li E, et al. Modulation of autophagy in the protective effect of resveratrol on PM2.5-induced pulmonary oxidative injury in mice. Phytother Res. 2018;32(12):2480–2486. doi:10.1002/ptr.6187
43. Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10(12):2208–2222. doi:10.4161/15548627.2014.981787
44. Zhang C, Huang C, Xia H, Xu H, Tang Q, Bi F. Autophagic sequestration of SQSTM1 disrupts the aggresome formation of ubiquitinated proteins during proteasome inhibition. Cell Death Dis. 2022;13(7):615. doi:10.1038/s41419-022-05061-8
45. Vishnupriya S, Priya Dharshini LC, Sakthivel KM, Rasmi RR. Autophagy markers as mediators of lung injury-implication for therapeutic intervention. Life Sci. 2020;260:118308. doi:10.1016/j.lfs.2020.118308
46. Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–449. doi:10.1016/j.immuni.2016.02.024
47. Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM. Tissue-resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity. 2017;47(5):913–927.e916. doi:10.1016/j.immuni.2017.10.006
48. Monick MM, Powers LS, Walters K, et al. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol. 2010;185(9):5425–5435. doi:10.4049/jimmunol.1001603
49. Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22(11):733–750. doi:10.1038/s41580-021-00392-4
50. Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32(1):2–11. doi:10.1128/MCB.06159-11
51. Li Q, Jia S, Xu L, Li B, Chen N. Metformin-induced autophagy and irisin improves INS-1 cell function and survival in high-glucose environment via AMPK/SIRT1/PGC-1α signal pathway. Food Sci Nutr. 2019;7(5):1695–1703. doi:10.1002/fsn3.1006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.