Back to Journals » Eye and Brain » Volume 14
Involvement of High Mobility Group Box 1 Protein in Optic Nerve Damage in Diabetes
Authors Mohammad G , Kowluru RA
Received 6 December 2021
Accepted for publication 16 February 2022
Published 10 May 2022 Volume 2022:14 Pages 59—69
DOI https://doi.org/10.2147/EB.S352730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Ghulam Mohammad,1 Renu A Kowluru1,2
1Department of Ophthalmology, Visual and Anatomical Sciences, Wayne State University, Detroit, MI, 48201, USA; 2Kresge Eye Institute, Wayne State University, Detroit, MI, 48201, USA
Correspondence: Ghulam Mohammad, Tel +1 313-577-0744, Email [email protected]
Introduction: Diabetic patients routinely have high levels of high mobility group box 1 (HMGB1) protein in their plasma, vitreous and ocular membranes, which is strongly correlated with subclinical chronic inflammation in the eye. Our previous work has suggested that high HMGB1 in diabetes plays a role in retinal inflammation and angiogenesis, but its role in the optic nerve damage is unclear. Therefore, our goal is to examine the role of HMGB1 in optic nerve damage in diabetes.
Methods: Gene expression of HMGB1 was quantified in the optic nerve from streptozotocin-induced diabetic mice by qRT-PCR, and their protein expressions by Western blot analysis and immunofluorescence staining. Using immunohistochemical technique, expression of reactive astrogliosis (indicator of neuroinflammation) and nerve demyelination/damage were determined by quantifying glial fibrillary acid protein (GFAP) and myelin basic protein (MBP), respectively. The role of HMGB1 in the optic nerve damage and alteration visual pathways was confirmed in mice receiving glycyrrhizin, a HMGB1 inhibitor. Similar parameters were measured in the optic nerve from human donors with diabetes.
Results: Compared to normal mice, diabetic mice exhibited increased levels of HMGB1, higher GFAP expression, and decreased MBP in the optic nerve. Double immunofluorescence microscopy revealed that diabetes induced increased HMGB1 immunoreactivities were significantly colocalized with GFAP in the optic nerve. Glycyrrhizin supplementation effectively reduced HMGB1 and maintained normal axonal myelination and visual conduction. Results from mice optic nerve confirmed the results obtained from human donors with diabetes.
Discussions: Thus, diabetes-induced HMGB1 upregulation promotes optic nerve demyelination and inflammation. The regulation of HMGB1 activation has potential to protect optic nerve damage and the abnormalities of visual pathways in diabetic patients.
Keywords: diabetes, visual function, optic nerve, inflammation, HMGB1
Introduction
Diabetes, besides damaging the vascular system of the retina, also damages the tissue surrounding the retina.1–3 Optic nerve transmits visual information including brightness, color perception and contrast sensitivity in the form of electrical impulses from the eye to the brain, and is formed by the axons of retinal ganglion cells belonging to the central nervous system. In diabetes, optic nerve damage is seen in both retinopathy and neuropathy, and in advanced stages of diabetic retinopathy, clinical manifestations of optic nerve damage, such as apillopathy, atrophy and neovascularization of optic disc can be observed.4–6 However, recently several clinical studies have demonstrated defects in color perception, contrast sensitivity, retinal nerve fiber layer (RNFL), pattern electroretinograms (pERGs) and visual evoked potentials (VEP), reflecting ganglion cell and optic nerve dysfunction, in the absence of vascular retinopathy. The magnitude of the defects increases with the duration of diabetes, suggesting that the optic nerve damage is an early event in visual dysfunctions in diabetes.7–14 Experimental models have shown that optic nerve conduction and its integrity are damaged within few weeks after induction of diabetes without showing any signs of retinopathy.15,16
Optic nerve myelination is fundamental for electrical pulse conductance, and destruction of the myelin sheaths can lead to delay in visual conduction or vision loss.17 Myelin basic proteins (MBPs) are a family of positively charged proteins that contribute to formation and compaction of myelin sheath, and MBPs are disarranged and disorganized in diabetes.15 Animal models have shown that the optic nerve has reduced axon numbers, impaired axonal transport, and swollen neurites in diabetes, and their microglia/astrocytes have increased immunoreactivity for ionized calcium binding adaptor molecule 1 (Iba-1)/glial fibrillary acid protein (GFAP) as early as six weeks after streptozotocin administration,15,16,18–21 suggesting that inflammation could be one of the key factors in the optic nerve damage in diabetes. However, the pathogenesis underlying optic nerve damage in diabetes remains unclear.
Early systemic inflammatory signaling after an injury can be induced by a large group of circulating molecules, collectively referred as damage-associated molecular patterns (DAMPs). These endogenous molecules are released from the damaged cells and act as danger signals to promote and exacerbate the inflammatory responses.22 One of the prototypic DAMP molecules, a chromatin-binding protein, High mobility group box 1 (HMGB1), is considered as a critical factor in mediating inflammation under cellular stress or cell loss.23 HMGB1 is a multifunctional protein, exerting various biologic effects depending on its cellular localization; while in the nucleus, it regulates biological functions including transcriptional regulation and nucleosome stabilization, and once released in the extracellular space, it promotes inflammation.24,25 In the pathogenesis of ocular diseases, HMGB1 has been shown to participate in inflammatory and immune-related processes.26 Previously, we have shown that intravitreal administration of HMGB1 in rat increases retinal vascular permeability and activates nuclear factor-kappa-light-chain-enhancer of activated B cells (NF-κB).27 Glycyrrhizin, a HMGB1 inhibitor which inhibits its expression, protects retina from diabetes-induced increased inflammation, oxidative stress and vascular permeability.27–32 HMGB1 also damages retinal neuronal functions in diabetes and inhibition of HMGB1 prevents retinopathy and neuropathy.33 The role of HMGB1 in the optic nerve damage in diabetes is, however, not clear.
The aim of this study was to investigate the role of HMGB1 in the optic nerve damage in diabetes. Using optic nerve from mice, 12 weeks after streptozotocin-induced diabetes, we investigated the role of HMGB1 in its damage and visual impairments. The results were confirmed in the optic nerve from human donors with >15 years of diabetes.
Materials and Methods
Mice
Diabetes was induced in 7–9-week-old male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) by intraperitoneal injection of streptozotocin (55 mg/kg BW) for four consecutive days (n = 62; 5–6 mice/cage housed). The mice presenting blood glucose >250 mg/dL, 2 days after the last injection, were considered diabetic (Diab).34,35 Immediately after establishment of diabetes, a group of mice (n = 24) were randomly assigned to be treated with freshly prepared glycyrrhizin in their drinking water (GZA, 150 mg/kg/day; Cat. No. G2137; Sigma-Aldrich, St. Louis, MO) every day,27,31 and their water consumption was measured weekly for the first month, and then twice a week to ensure that mice were consuming the correct dose of drug. Based on mouse water consumption, glycyrrhizin did not appear to change the taste of the water. Twelve weeks after induction of diabetes, the animals were sacrificed, and their optic nerve (5–10 mm intraorbital) was collected. Age-matched normal mice were used as controls (Norm, n = 30). GZA supplementation did not affect the blood glucose levels and body weight of these diabetic mice, and the values obtained at the initiation, and at 3 months were similar in the two groups of diabetic mice (P < 0.05 versus normal and P > 0.05 versus diabetes; Table 1). The treatment of animals conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the Wayne State University’s Institutional Animal Care and Use Committee.
 |
Table 1 Effect of GZA on Body Weight and Glucose of Diabetic Mice |
Human Donor
Human postmortem eye globes with small portion of the optic nerve (2–8 mm intraorbital), enucleated between 6 and 11 hours postmortem, were supplied on ice by the Eversight Eye Bank, Ann Arbor, MI, USA. Diabetic donors, 54 to 75 years of age with a history of diabetes for 15 or more years. The optic nerve was removed, dissected, and was used for histology and biochemical assay. Age- and sex-matched nondiabetic donors (ND) were used as controls.34 The eye globes were coded by the Eye Bank, and did not contain any patient identification; this met the criteria for ‘exemption’ from Wayne State University’s Institutional Review Board.
Immunofluorescence Staining
The optic nerve was enucleated and subjected to post-fixation with 4% paraformaldehyde (PFA) at 4°C, washed with PBS and incubated in 30% sucrose solution (in PBS) for 24 h at 4°C for cryoprotection, and then were embedded in optimal cutting temperature medium. Ten-micron-thick sections (cross section for human optic nerve and longitudinal for mice) were prepared using a cryostat, and after washing with PBS, the sections were fixed with 4% PFA. They were then blocked with 5% (w/v) bovine serum albumin (BSA) and 0.2% Triton X-100 for 1 h, and incubated with primary antibodies overnight at 4°C. The primary antibodies used were mouse anti-MBP (1:200, Cat. No. sc-271524, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-HMGB1 (1:500, Cat. No. ab18256, Abcam, Cambridge, MA) and mouse anti-GFAP (1:200, Cat. No. sc-33673, Santa Cruz Biotechnology) in 2.5% (w/v) BSA. After washing three times with PBS, the slides were incubated with Alexa Fluor-488 (green) conjugated anti-rabbit (Cat. No. Molecular Probes-Life Technologies, Grand Island, NE, 1:500 dilution) or Texas red-conjugated anti-mouse (Cat. No. Vector Laboratories, Burlingame, CA; 1:500 dilution) corresponding secondary antibodies in the dark for 2 h. The sections were mounted with DAPI (4′,6-diamidino-2-phenylindole; blue) containing mounting media (Vector Laboratories, Burlingame, CA) to visualized nucleus and were examined using Zeiss ApoTome fluorescence microscope (Carl Zeiss, Inc., Chicago, IL).36 At least eight random images were recorded from each independent preparation. The ‘arithmetic mean intensity’ (AMI), and Pearson coefficient were calculated using Zeiss software module. Relative percentage change was calculated considering the values obtained from normal mice, as 100%.
Gene Transcripts
Total RNA was extracted from optic nerves (intraorbital region) using TRIZOL reagent (Invitrogen, Carlsbad, CA), and prepared cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR (qRT-PCR) reaction was performed using SYBR green master mix (Applied Biosystems) using the primers listed in Table 2. The specific products were confirmed by SYBR green single melt curve analysis with ABI750 Real Time PCR System.37 Data were normalized to β-actin (human) or 18S (mice), and the change (x-fold) in gene expression relative to normal was calculated by the delta delta Ct method.38
 |
Table 2 Primer Sequence |
Western Blotting
Optic nerve (intraorbital region) was homogenized in RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40%, and 0.1% SDS), supplemented with 1% complete EDTA-free protease inhibitor cocktail, and centrifuged at 15,000 × g for 15 min at 4°C as described previously.39 Supernatant (20μg protein) was separated on a 4–20% gradient acrylamide gel (Bio-Rad, Hercules, CA) and transferred onto a nitrocellulose membrane. After blocking at room temperature in 5% non-fat milk for 1 h, the blots were then incubated with primary antibodies against HMGB1 (1:1000 dilution; Cat No. ab18256, Abcam), NF-κB p65 (1:500 dilution; Cat No. sc-8008, Santa Cruz Biotechnology) overnight at 4°C. After incubation with the corresponding horseradish peroxidase-conjugated secondary antibodies (1:2000, Sigma-Aldrich) at RT for 1 h, the bands were visualized by West Femto chemiluminescent substrate (Thermo-scientific, Rockford, IL), and densitometry analysis was performed with the ImageJ software (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, Maryland, http://rsb.info.nih.gov/ij/, 1997–2009).27 Relative protein expression was quantified by normalizing the band intensity to that of β-actin (1:2000 dilution; Cat No. A-5316, Sigma-Aldrich), and the percentage change was calculated considering the values obtained from normal mice, as 100%.
Visual Evoked Potential
Mice were dark-adapted overnight, and anesthetized by intraperitoneal injection of Ketamine (67mg/kg)- Xylazine (10mg/kg). Their pupils were dilated with a drop of tropicamide, followed by administering one drop of 0.4% oxybuprocaine hydrochloride. Needle electrodes were placed subcutaneously at the base of the tail and at the snout to serve as ground and reference electrodes, respectively. The active electrode was inserted subcutaneously at the midline at the back of the head. Two contact-lens light-emitting diodes (LEDs) were placed over the two eyes of the animal to serve as light stimulators. The animals were placed on a heated platform to keep warm, and the VEP recordings were carried out using the Celeris ERG system (Diagnosys LLC, MA, USA).40 Each eye was separately exposed to white light flashes of 0.05 cd.s/m2, swept 100 times per trial. Percentage change was calculated considering the values obtained from normal mice, as 100%.
Statistical Analysis
Data are presented as mean ± SD. The nonparametric Mann–Whitney U-test was used to compare means from two independent groups. When comparing three independent groups, the Kruskal–Wallis test was performed. P-value less than 0.05 indicated statistical significance. GraphPad Prism version 8.0 software (GraphPad Software, Inc., San Diego, CA) for Windows was used for statistical analysis.
Results
Mice
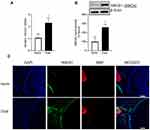
Compared to normal mice, gene transcripts of HMGB1 were elevated by over twofold in the optic nerve of diabetic mice (*P < 0.05, Figure 1A), and its protein expression was elevated by about threefold (Figure 1B). To evaluate the localization of HMGB1, optic nerve cryosections were analyzed by immunofluorescence staining; diabetic mice had an overall increase in HMGB1 fluorescence intensity, compared to normal mice (Figure 1C).
Retinal neurodegeneration in diabetes is shown to result in reactive gliosis41,42 and increased GFAP expression has been reported in neuroinflammatory diseases.43,44 We examined the expression of GFAP using immunofluorescence technique. Figure 2A shows a significant upregulation of GFAP expression in the optic nerve from diabetic mice compared to normal mice. To understand the cellular source of HMGB1 increase in the optic nerve, its colocalization with GFAP were quantified, and Figure 2B shows increased colocalization of HMGB1 and GFAP in diabetes, compared with normal, suggesting the role of HMGB1 in reactive glial cells in diabetes.
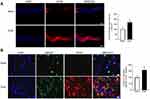
Glycyrrhizin is a natural inhibitor of HMGB1 and is reported with neuroprotective effects through oral administration;45,46 glycyrrhizin was used to determine the effects of HMGB1-inhibition on diabetes-induced HMGB1 and NF-kB expression. Figure 3A shows that glycyrrhizin administration in diabetic mice significantly decreased HMGB1 protein expression in the optic nerve. In the same animals, glycyrrhizin supplementation prevented increase in NF-kB p65, a master regulator of cytokine productions, and attenuated decrease in MBP fluorescence intensity (Figure 3B and C). Glycyrrhizin also improved diabetes-induced optic nerve structural distortion (Figure 3C).
To determine the role of HMGB1 in propagation of visual signals in diabetes, VEPs recording was performed. The VEPs recorded in diabetic mice were significantly reduced in amplitude (N1) compared with those measured in control animals. Regulation of HMGB1 by glycyrrhizin prevented diabetes-induced down regulation of VEPs’ waves amplitude; VEP waves amplitude (N1) in diabetic mice receiving glycyrrhizin was not significantly different from those obtained from normal mice (Figure 3D).
Optic Nerve from Human Donors
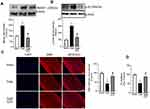
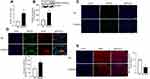
Consistent with our in vivo results, expression of HMGB1 (gene and protein) was increased in the optic nerve from human donors with diabetes, compared to their age-matched non-diabetic donors (Figure 4A and B), and HMGB1-immunoreactivity was increased (Figure 4C). Furthermore, colocalization of HMGB1 with GFAP increased (Figure 4D), and MBP expression was also decreased in the optic nerve from human donors with diabetes, compared to their age-matched nondiabetic donors (Figure 4E).
Discussion
Upregulation of HMGB1, one of the prototypic DAMP molecules, is implicated in many inflammatory diseases including traumatic brain injury, Alzheimer’s, multiple sclerosis, endophthalmitis and glaucoma.26,47,48 Our previous work has shown HMGB1 expression is upregulated in the vitreous fluid and epiretinal membranes from patients with proliferative diabetic retinopathy, and is correlated with the levels of inflammatory biomarkers.28,49,50 In addition, HMGB1 expression is also upregulated in the retinas of diabetic rodents.27,29–31 This is the first report demonstrating the role of this DAMP in the optic nerve damage in diabetes; here we show that HMGB1 and GFAP are significantly upregulated in the optic nerve of diabetic mice, and MBP is downregulated. Furthermore, administration of an inhibitor of HMGB1, glycyrrhizin, significantly ameliorates diabetes-induced increase in HMGB1 and NF-κB and decrease in MBP, and protects VEP abnormalities in the optic nerve. Taken together, these findings imply that HMGB1 upregulation has a pathogenic role in the optic nerve damage in diabetes.
Neurodegenerative changes in the visual pathway are common in diabetes, and diabetic patients have visual field defects in the absence of clinically detectable retinopathy. Multiple clinical studies have shown that VEP, which provides measurements of demyelination and axonal damage in the visual system, is altered in the absence of retinopathy in diabetic patients.7,9,51,52 Similarly, RNFL, considered as a sensitive indicator for the assessment of optic nerve health and injury,53–55 often progressively degenerates before the onset of diabetic retinopathy,7,8,56 and inflammation is considered as one of the key components of diabetes induced ocular complications contributing in chronic neurodegeneration and neurological impairments in the eye.57,58 Gene profiling studies have shown that diabetes alters expression of many genes associated with inflammation in the retina and glial cells,59 and inflammatory cytokine’s are elevated soon after induction of diabetes in rodents.60 Both clinical and experimental models have shown that inflammation is one of the crucial factors in the optic nerve damage in diabetes.15,19,20,61–64 HMGB1 acts as a cytokine by signaling via the receptor for advanced glycation end-products or toll-like receptors.24,65,66 In diabetes, elevated HMGB1 is associated with increased retinal inflammation and angiogenesis,27–31,49,50 and by promoting inflammation, it is also implicated in neuronal dysfunction.33 HMGB1 is actively released from the damaged neurons, microglia, and astrocytes during inflammation,47,48,67,68 and reactive astrogliosis, including an increased GFAP expression, has been reported in different retinal pathologies and animal models of glaucoma.69,70 Here, our results show that diabetes increases in extranuclear HMGB1-immunoreactivity, and upregulates GFAP expression and its colocalization with HMGB1.
Glycyrrhizin is a natural triterpene, and is shown to be effective against inflammatory diseases such as sepsis, chronic hepatitis, colitis brain injury and Alzheimer’s disease via inhibiting HMGB1.45,48 In neurological disorders, glycyrrhizin effects are mainly attributed to the attenuation of neuronal damage by inhibiting HMGB1 expression. In the retina, glycyrrhizin reduces the HMGB1 protein level and protects its neurodegeneration,27,31,71 and improves its neuronal and vascular outcomes in diabetic rodents.33,72 Supplementation of glycyrrhizin in diabetic mice also prevents loss of ganglion cells and the thinning of the retinal layers.33 Here, we show that glycyrrhizin effectively reduces HMGB1 levels in the optic nerve, and activation of NF-kB. Optic nerve axons are surrounded by myelin sheaths, and its demyelination causes disturbed signal transduction, leading to visual disorders. Axonal myelin also protects the neurofilaments from inflammatory processes and its downregulation is considered as an early specific indicator of neurological degenerative disorders.73,74 Optic nerve myelin disarrangement and ultrastructural alterations, and reduction in the number of myelinated fibers are seen within 6–8 weeks of diabetes in rodents.15,20 Here, we show that MBP is decreased in the optic nerve of diabetic mice, which can be prevented by glycyrrhizin, suggesting HMGB1-mediated structural damage to the optic nerve structural damage. In addition, glycyrrhizin also attenuates alterations in VEP waves, suggesting that HMGB1 inhibition also protects optic nerve axon demyelination. Results accrued from diabetic mice are supported by significant increase in HMGB1 and reduction in MBP in the optic nerve from diabetic human donors, further strengthening the role of HMGB1 in optic nerve structural and functional damage in diabetes. However, further investigation is required to address the pharmacokinetics of glycyrrhizin after oral administration of it, especially the tissue distribution.
In conclusion, results presented here suggest that HMGB1 upregulation plays a causative role in structural and functional damage of optic nerve in diabetes (Figure 5), and blockage of HMGB1 upregulation could be a novel therapeutic option to protect the optic nerve damage in diabetic patients.
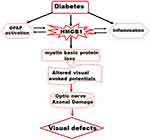 |
Figure 5 A schematic representation depicting a possible mechanism of HMGB1-derived optic nerve damage in the diabetes. |
Funding
This work presented here was supported in parts by grants from the National Institutes of Health (EY014370, EY017313, EY022230) and Thomas Foundation to Renu A Kowluru and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Visual and Anatomical Sciences.
Disclosure
GM and RK report no conflicts of interest in relation to this work.
References
1. Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes. 2015;6(1):92–108. doi:10.4239/wjd.v6.i1.92
2. Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular complications of diabetes and therapeutic approaches. Biomed Res Int. 2016;2016:3801570. doi:10.1155/2016/3801570
3. Jeganathan VS, Wang JJ, Wong TY. Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care. 2008;31(9):1905–1912. doi:10.2337/dc08-0342
4. Algan M, Ziegler O, Drouin P.[Optic neuropathy in diabetic subjects]. Diabete Metab. 1993;19(5):395–399. French.
5. Bikbova G, Oshitari T, Baba T, Yamamoto S. Neurotrophic factors for retinal ganglion cell neuropathy - with a special reference to diabetic neuropathy in the retina. Curr Diabetes Rev. 2014;10(3):166–176. doi:10.2174/1573399810666140508121927
6. Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(2):1156–1163. doi:10.1167/iovs.10-6293
7. Bao YK, Yan Y, Gordon M, McGill JB, Kass M, Rajagopal R. Visual field loss in patients with diabetes in the absence of clinically-detectable vascular retinopathy in a nationally representative survey. Invest Ophthalmol Vis Sci. 2019;60(14):4711–4716. doi:10.1167/iovs.19-28063
8. Montesano G, Ometto G, Higgins BE, et al. Evidence for structural and functional damage of the inner retina in diabetes with no diabetic retinopathy. Invest Ophthalmol Vis Sci. 2021;62(3):35. doi:10.1167/iovs.62.3.35
9. Parisi V, Uccioli L, Monticone G, et al. Electrophysiological assessment of visual function in IDDM patients. Electroencephalogr Clin Neurophysiol. 1997;104(2):171–179. doi:10.1016/S0168-5597(97)96606-5
10. Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113(19):E2655–E2664. doi:10.1073/pnas.1522014113
11. van Dijk HW, Kok PH, Garvin M, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50(7):3404–3409. doi:10.1167/iovs.08-3143
12. Verrotti A, Lobefalo L, Trotta D, et al. Visual evoked potentials in young persons with newly diagnosed diabetes: a long-term follow-up. Dev Med Child Neurol. 2000;42(4):240–244. doi:10.1017/S0012162200000414
13. Wan ZQ, Gao Y, Cui M, Zhang YJ. Association between risk factors and retinal nerve fiber layer loss in early stages of diabetic retinopathy. Int J Ophthalmol. 2021;14(2):255–262. doi:10.18240/ijo.2021.02.12
14. Zeng Y, Cao D, Yu H, et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. 2019;103(12):1747–1752. doi:10.1136/bjophthalmol-2018-313582
15. Dorfman D, Aranda ML, Rosenstein RE. Enriched environment protects the optic nerve from early diabetes-induced damage in adult rats. PLoS One. 2015;10(8):e0136637. doi:10.1371/journal.pone.0136637
16. Fernandez DC, Pasquini LA, Dorfman D, Aldana Marcos HJ, Rosenstein RE. Early distal axonopathy of the visual pathway in experimental diabetes. Am J Pathol. 2012;180(1):303–313. doi:10.1016/j.ajpath.2011.09.018
17. Pihl-Jensen G, Schmidt MF, Frederiksen JL. Multifocal visual evoked potentials in optic neuritis and multiple sclerosis: a review. Clin Neurophysiol. 2017;128(7):1234–1245. doi:10.1016/j.clinph.2017.03.047
18. Scott TM, Foote J, Peat B, Galway G. Vascular and neural changes in the rat optic nerve following induction of diabetes with streptozotocin. J Anat. 1986;144:145–152.
19. Icel E, Icel A, Ucak T, et al. The effects of lycopene on alloxan induced diabetic optic neuropathy. Cutan Ocul Toxicol. 2019;38(1):88–92. doi:10.1080/15569527.2018.1530258
20. Mendonca HR, Carvalho JNA, Abreu CA, et al. Lack of Galectin-3 attenuates neuroinflammation and protects the retina and optic nerve of diabetic mice. Brain Res. 2018;1700:126–137. doi:10.1016/j.brainres.2018.07.018
21. Zhang L, Inoue M, Dong K, Yamamoto M. Alterations in retrograde axonal transport in optic nerve of type I and type II diabetic rats. Kobe J Med Sci. 1998;44(5–6):205–215.
22. Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. doi:10.1038/s41577-019-0215-7
23. Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi:10.1016/j.mam.2014.05.001
24. Yang H, Wang H, Andersson U. Targeting Inflammation Driven by HMGB1. Front Immunol. 2020;11:484. doi:10.3389/fimmu.2020.00484
25. Xue J, Suarez JS, Minaai M, et al. HMGB1 as a therapeutic target in disease. J Cell Physiol. 2021;236(5):3406–3419. doi:10.1002/jcp.30125
26. Liu Y, Zhuang GB, Zhou XZ. HMBG1 as a driver of inflammatory and immune processes in the pathogenesis of ocular diseases. J Ophthalmol. 2018;2018:5195290. doi:10.1155/2018/5195290
27. Mohammad G, Siddiquei MM, Othman A, Al-Shabrawey M, Abu El-Asrar AM. High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Exp Eye Res. 2013;107:101–109. doi:10.1016/j.exer.2012.12.009
28. Abu El-Asrar AM, Alam K, Garcia-Ramirez M, et al. Association of HMGB1 with oxidative stress markers and regulators in PDR. Mol Vis. 2017;23:853–871.
29. Abu El-Asrar AM, Mohammad G, Nawaz MI, Siddiquei MM. High-mobility group box-1 modulates the expression of inflammatory and angiogenic signaling pathways in diabetic retina. Curr Eye Res. 2015;40(11):1141–1152. doi:10.3109/02713683.2014.982829
30. Mohammad G, Abdelaziz GM, Siddiquei MM, Ahmad A, De Hertogh G, Abu El-Asrar AM. Cross-talk between sirtuin 1 and the proinflammatory mediator high-mobility group box-1 in the regulation of blood-retinal barrier breakdown in diabetic retinopathy. Curr Eye Res. 2019;44(10):1133–1143. doi:10.1080/02713683.2019.1625406
31. Mohammad G, Alam K, Nawaz MI, Siddiquei MM, Mousa A, Abu El-Asrar AM. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J Physiol Biochem. 2015;71(3):359–372. doi:10.1007/s13105-015-0416-x
32. Nawaz MI, Mohammad G. Role of high-mobility group box-1 protein in disruption of vascular barriers and regulation of leukocyte-endothelial interactions. J Recept Signal Transduct Res. 2015;35(4):340–345. doi:10.3109/10799893.2014.984309
33. Liu L, Jiang Y, Steinle JJ. Glycyrrhizin protects the diabetic retina against permeability, neuronal, and vascular damage through anti-inflammatory mechanisms. J Clin Med. 2019;8(7):957. doi:10.3390/jcm8070957
34. Mohammad G, Duraisamy AJ, Kowluru A, Kowluru RA. Functional regulation of an oxidative stress mediator, rac1, in diabetic retinopathy. Mol Neurobiol. 2019;56(12):8643–8655. doi:10.1007/s12035-019-01696-5
35. Mohammad G, Kowluru RA. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J Cell Physiol. 2012;227(3):1052–1061. doi:10.1002/jcp.22822
36. Mohammad G, Radhakrishnan R, Kowluru RA. Hydrogen sulfide: a potential therapeutic target in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2020;61(14):35. doi:10.1167/iovs.61.14.35
37. Mohammad G, Radhakrishnan R, Kowluru RA. Epigenetic modifications compromise mitochondrial DNA quality control in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2019;60(12):3943–3951. doi:10.1167/iovs.19-27602
38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
39. Elsherbiny NM, Abdel-Mottaleb Y, Elkazaz AY, et al. Carbamazepine alleviates retinal and optic nerve neural degeneration in diabetic mice via nerve growth factor-induced PI3K/Akt/mTOR activation. Front Neurosci. 2019;13:1089. doi:10.3389/fnins.2019.01089
40. Visuvanathan S, Baker AN, Lagali PS, et al. XIAP gene therapy effects on retinal ganglion cell structure and function in a mouse model of glaucoma. Gene Ther. 2021;29(3–4):147–156. doi:10.1038/s41434-021-00281-7
41. Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41(7):1971–1980.
42. Lieth E, Barber AJ, Xu B, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn state retina research group. Diabetes. 1998;47(5):815–820. doi:10.2337/diabetes.47.5.815
43. Horstmann L, Schmid H, Heinen AP, Kurschus FC, Dick HB, Joachim SC. Inflammatory demyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J Neuroinflammation. 2013;10(1):120. doi:10.1186/1742-2094-10-120
44. Qu J, Jakobs TC. The time course of gene expression during reactive gliosis in the optic nerve. PLoS One. 2013;8(6):e67094. doi:10.1371/journal.pone.0067094
45. Paudel YN, Angelopoulou E, Semple B, Piperi C, Othman I, Shaikh MF. Potential neuroprotective effect of the HMGB1 inhibitor glycyrrhizin in neurological disorders. ACS Chem Neurosci. 2020;11(4):485–500. doi:10.1021/acschemneuro.9b00640
46. Mollica L, De Marchis F, Spitaleri A, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14(4):431–441. doi:10.1016/j.chembiol.2007.03.007
47. Kim JB, Sig Choi J, Yu YM, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26(24):6413–6421. doi:10.1523/JNEUROSCI.3815-05.2006
48. Paudel YN, Shaikh MF, Chakraborti A, et al. HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front Neurosci. 2018;12:628. doi:10.3389/fnins.2018.00628
49. Abu El-Asrar AM, Nawaz MI, Kangave D, Abouammoh M, Mohammad G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:697489. doi:10.1155/2012/697489
50. El-Asrar AM, Nawaz MI, Kangave D, et al. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. 2011;17:1829–1838.
51. Di Leo MA, Caputo S, Falsini B, et al. Nonselective loss of contrast sensitivity in visual system testing in early type I diabetes. Diabetes Care. 1992;15(5):620–625. doi:10.2337/diacare.15.5.620
52. Parisi V, Uccioli L, Parisi L, et al. Neural conduction in visual pathways in newly-diagnosed IDDM patients. Electroencephalogr Clin Neurophysiol. 1998;108(5):490–496. doi:10.1016/S0168-5597(98)00026-4
53. Lin SC, Singh K, Jampel HD, et al. Optic nerve head and retinal nerve fiber layer analysis: a report by the American Academy of Ophthalmology. Ophthalmology. 2007;114(10):1937–1949. doi:10.1016/j.ophtha.2007.07.005
54. Quigley HA, Miller NR, George T. Clinical evaluation of nerve fiber layer atrophy as an indicator of glaucomatous optic nerve damage. Arch Ophthalmol. 1980;98(9):1564–1571. doi:10.1001/archopht.1980.01020040416003
55. Soltan-Sanjari M, Parvaresh MM, Maleki A, Ghasemi-Falavarjani K, Bakhtiari P. Correlation between retinal nerve fiber layer thickness by optical coherence tomography and perimetric parameters in optic atrophy. J Ophthalmic Vis Res. 2008;3(2):91–94.
56. Lopes de Faria JM, Russ H, Costa VP. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86(7):725–728. doi:10.1136/bjo.86.7.725
57. Lutty GA. Effects of diabetes on the eye. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF81–ORSF87. doi:10.1167/iovs.13-12979
58. Rubsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19(4):942. doi:10.3390/ijms19040942
59. Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P. Expression of acute-phase response proteins in retinal Muller cells in diabetes. Invest Ophthalmol Vis Sci. 2005;46(1):349–357. doi:10.1167/iovs.04-0860
60. Forrester JV, Kuffova L, Delibegovic M. The role of inflammation in diabetic retinopathy. Front Immunol. 2020;11:583687. doi:10.3389/fimmu.2020.583687
61. Mesquida M, Drawnel F, Fauser S. The role of inflammation in diabetic eye disease. Semin Immunopathol. 2019;41(4):427–445. doi:10.1007/s00281-019-00750-7
62. Selvakumar A, Muthumeena M, Noronha OV, Bapu S. Bilateral optic neuropathy with central diabetes insipidus in a child. Indian J Ophthalmol. 2018;66(11):1642–1644. doi:10.4103/ijo.IJO_281_18
63. Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic retinopathy. J Neuroinflammation. 2015;12(1):141. doi:10.1186/s12974-015-0368-7
64. Mendonca HR, Carpi-Santos R, da Costa Calaza K, Blanco martinez AM. Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res. 2020;15(4):625–635. doi:10.4103/1673-5374.266910
65. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi:10.1038/nature00858
66. Yang H, Liu H, Zeng Q, et al. Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Mol Med. 2019;25(1):13. doi:10.1186/s10020-019-0081-6
67. Bucova M, Majernikova B, Durmanova V, et al. HMGB1 as a potential new marker of disease activity in patients with multiple sclerosis. Neurol Sci. 2020;41(3):599–604. doi:10.1007/s10072-019-04136-3
68. Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarmin: the role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun. 2015;48:1–7. doi:10.1016/j.bbi.2015.03.010
69. Garcia-Bermudez MY, Freude KK, Mouhammad ZA, van Wijngaarden P, Martin KK, Kolko M. Glial cells in glaucoma: friends, foes, and potential therapeutic targets. Front Neurol. 2021;12:624983. doi:10.3389/fneur.2021.624983
70. Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003;230:263–290.
71. Abu El-Asrar AM, Siddiquei MM, Nawaz MI, Geboes K, Mohammad G. The proinflammatory cytokine high-mobility group box-1 mediates retinal neuropathy induced by diabetes. Mediators Inflamm. 2014;2014:746415. doi:10.1155/2014/746415
72. Liu L, Jiang Y, Steinle JJ. Epac1 and glycyrrhizin both inhibit HMGB1 levels to reduce diabetes-induced neuronal and vascular damage in the mouse retina. J Clin Med. 2019;8(6):772. doi:10.3390/jcm8060772
73. Azeez IA, Olopade F, Laperchia C, et al. Regional myelin and axon damage and neuroinflammation in the adult mouse brain after long-term postnatal vanadium exposure. J Neuropathol Exp Neurol. 2016;75(9):843–854. doi:10.1093/jnen/nlw058
74. Stassart RM, Mobius W, Nave KA, Edgar JM. The axon-myelin unit in development and degenerative disease. Front Neurosci. 2018;12:467. doi:10.3389/fnins.2018.00467
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.