Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Investigation of variants in estrogen receptor genes and perinatal depression
Authors Tan EC , Lim HW, Chua TE , Tan HS, Lee TMY, Chen HY
Received 26 December 2017
Accepted for publication 9 February 2018
Published 29 March 2018 Volume 2018:14 Pages 919—925
DOI https://doi.org/10.2147/NDT.S160424
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Ene-Choo Tan,1,2 Hwee-Woon Lim,1 Tze-Ern Chua,2,3 Hui-San Tan,1 Theresa MY Lee,2,3 Helen Y Chen2,3
1KK Research Centre, KK Women’s and Children’s Hospital, Singapore, Singapore; 2Paediatrics Academic Clinical Programme, SingHealth Duke-NUS Medical School, Singapore, Singapore; 3Department of Psychological Medicine, KK Women’s and Children’s Hospital, Singapore, Singapore
Objectives: Depressive symptoms are common during pregnancy and after childbirth. Estrogen levels fluctuate greatly during the course of pregnancy and may contribute to mood instability. The first aim of this case–control study was to investigate whether variants in the two estrogen receptor genes might contribute to the genetic susceptibility to pregnancy-related depression using controls that were screened for postnatal depression. The second aim was to uncover new variants in the two estrogen receptor genes.
Patients and methods: Our study sample comprised 554 control subjects who had Edinburgh Postnatal Depression Scale (EPDS) scores below 7 at postnatal screening, and 159 patients with clinically diagnosed pregnancy-related depression. They were genotyped for four single-nucleotide polymorphisms (SNPs) and a dinucleotide repeat in the two genes: estrogen receptor α (ESR1) and estrogen receptor β (ESR2). Fifty-six cases with personal and/or family history of depression of psychiatric disorders were selected for resequencing of the two genes.
Results: There was no statistically significant association with perinatal depression for all five variants. However, there was a trend toward higher frequencies of the genotypes associated with higher risk of depression for rs2077647 and rs4986938 in the case group. From resequencing, two novel ESR1 variants were identified from two different patients.
Conclusion: Our study that used screened controls with low EPDS scores and cases with clinically diagnosed pregnancy-related depression could not replicate the association with depression for any of the SNPs for both genotype and allele frequencies. Two novel SNPs were identified and could be further investigated in a larger sample set.
Keywords: childbirth, depression, hormone, single-nucleotide polymorphism, postpartum
Introduction
Depression is the most common type of mental illness, affecting about 10% of the population worldwide, with prevalence reported to range from 1% to 19% in different countries.1 The Singapore Mental Health Survey found that the lifetime prevalence of major depressive disorder in Singapore adult residents was 5.8%.2 In the perinatal population, a local study found the prevalence of both major and minor depression to be 12% during pregnancy, and 7% during the postpartum period; while 4.3% had major depression alone at both time points.3 The prevalence of depression is even higher in the elderly and those with chronic conditions such as cancers and other physical illnesses.4–6
There are consistent data from multiple populations indicating that the risk of developing depression for women is higher and even nearly twice as high compared to men.7–10 For many women, the depressive episodes coincide with periods of hormonal changes such as during pregnancy, after childbirth, or menopause. The major hormone that undergoes such fluctuations in level during these periods is estrogen, and studies have also found that estrogen therapy is effective in the treatment of postnatal depression.11,12 It has also been shown in animal studies that estrogen deprivation led to the decreased expression of serotonin receptors, which could be restored by the administration of estrogen.13 Estrogens act on target tissues by binding to estrogen receptors. Estrogen–estrogen receptor complexes bind to estrogen response elements of nuclear DNA and cause activation of estrogen-responsive genes. Both estrogen receptor α (ESR1) and estrogen receptor β (ESR2) are expressed in many areas of the brain, such as the amygdala14,15 which is part of the limbic system involved in emotional memories and mental states.16–18
The effect of estrogens on mood is likely mediated through the serotonin pathway, the major system implicated in mood disorders.19–21 There are some reports that specific estrogen receptor gene variants are associated with major depression.22,23 As women are at increased risk for depression during pregnancy and the immediate postpartum period, we hypothesized that estrogen receptor gene variants might be even more important in perinatal depression, and hence investigated the association of five common ESR1 and ESR2 polymorphisms with having a diagnosis of depression related to pregnancy and childbirth. Another aim of our study was to uncover new genetic variants in the estrogen receptor genes.
Patients and methods
Study participants
The study protocol for this prospective study was approved by the SingHealth Institutional Review Board. A total of 747 women were recruited with written informed consent from the outpatient clinic of the hospital. All were self-reported to be of Chinese descent. The case group consisted of 162 patients who met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria for clinical depression related to pregnancy/childbirth as assessed by a board-certified psychiatrist. All eligible patients on active follow-up during the period of active recruitment from 2010 to 2013 were also included. Women who had a history of substance abuse, a coexisting psychiatric disorder, or a previous diagnosis of a neurological disorder were excluded. The control group comprised 558 consecutive obstetric patients who scored 7 or below on the Edinburgh Postnatal Depression Scale (EPDS) during postnatal assessment following a recent delivery. This lower cutoff limit was chosen, instead of the conventional cutoff value of 9 or less, to ensure controls were truly not depressed, in keeping with the cutoff point identified by Chee et al.3
Saliva samples were collected with either Oragene (DNA Genotek Inc., Ottawa, ON, Canada) or Norgen (Norgen Biotek Corp., Thorold, ON, Canada) saliva collection kits. Genomic DNA was extracted according to the manufacturers’ recommendations. DNA samples were not available for four controls and three cases either because saliva samples were not successfully collected or because there was a technical problem with the genomic DNA extraction, leaving a sample size of 713 (159 cases and 554 controls) for genetic analysis.
ESR1 and ESR2 resequencing
DNA samples from 56 cases were selected for the resequencing of ESR1 and ESR2. These cases were patients who were diagnosed with clinical depression for more than one pregnancy, had a family history of mental illness, or had reported more severe psychological and physical symptoms during premenstrual/menstrual periods according to a questionnaire they completed.
For the resequencing of the two genes, 16 pairs of primers were designed using Primer 3 plus and Primer Express 3.0 software based on reference sequences for NM_000125.3 for ESR1 and NM_0.001437.2 for ESR2 (list of primers available upon request). The targeted regions included all eight ESR1 or nine ESR2 exons and at least 100 bases of the intronic sequences at each exon–intron junction. Amplified products were sequenced using the ABI BigDye Terminator (v3.1) and the 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The resulting sequences were compared with the respective reference sequences to identify base changes.
Genotyping
Four single-nucleotide polymorphisms (SNPs) were selected based on previous reports of association with depression in other populations. Three SNPs (ESR1 rs2234693 and rs9340799; and ESR2 rs4986938) were genotyped using TaqMan assays (Assay ID C_11462726_10, C_3163590_10, and C_3163591_10 respectively) (Applied Biosystems, Foster City, CA, USA). The reaction mix of 12 μL contained 25 ng genomic DNA, 0.25× stock genotyping assay, and 1× TaqMan genotyping PCR master mix. Amplification and hybridization were performed using the Applied Biosystems StepOnePlus Real-Time PCR System according to the manufacturer’s recommendation. Results were analyzed using StepOne Software v2.3.
The remaining SNP ESR1 rs2077647 (c.30 T>C) was genotyped by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) according to the published protocol.24 PCR products were incubated overnight with BamHI (New England Biolabs, Ipswich, MA, USA) at 37°C and the restricted products resolved on 2% agarose gels. Genotypes were manually called according to the presence or absence of bands of expected sizes.
The number of TA repeats for ESR1 was determined by Sanger sequencing. Genomic DNA was amplified by PCR with fluorescently labeled forward primer and unlabeled reverse primer.25 The amplicons were size-separated by automated capillary electrophoresis on the ABI 3130 Genetic Analyzer. Alleles were called using GeneMapper (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
All analysis was performed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA), with statistical significance set at 0.05. Differences in the distribution of genotypes between cases and controls were evaluated using a chi-square (χ2) test. Individual SNPs were also analyzed under the dominant, codominant, and recessive models. The haplotypes for the ESR1 polymorphisms for each individual were statistically inferred using an expectation-maximization procedure using S-PLUS (SolutionMetrics, Sydney, Australia).
Results
Demographic data
Demographic information for the 713 subjects is presented in Table 1. Controls and cases had a similar distribution in the highest education level attained and occupation held. There was a statistically significant difference in housing type with a higher proportion of cases living in private apartments and landed homes. There was also a highly significant difference in mean age, which was 33.99±5.98 years for cases and 31.29±5.71 years for controls (F=35.797, p=0.000).
  | Table 1 Demographic characteristics of controls and cases |
Resequencing of ESR1 and ESR2
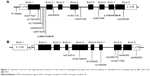
There were 12 variants for ESR1 from the 56 samples (Figure 1A). Nine were substitutions, two were tetranucleotide insertions, and one was a single-nucleotide insertion (Table 2A). Four variants were in exons and the remaining eight were in introns. All except two intronic single-nucleotide variants had been reported previously. The two novel variants that were not present in any existing population databases were in intron 1 (NM_000125.3:c.452+111G>C) and intron 7 (NM_000125.3:c.1553+97G>A). Both were singletons that were observed only once in two different patients.
For ESR2, eight variants were found, and all had been previously reported (Table 2B). All were single-nucleotide substitutions. Five of the eight variants were in exons, one in the 3′ untranslated region, and two in introns (Figure 1B).
ESR1 and ESR2 SNPs
Genotyping for the four SNPs (ESR1 rs2077647, rs2234693, and rs9340799; and ESR2 rs4986938) was successful for >96% of samples, the remainder failed due to no amplification or no-calls. Genotype distributions for all four were in Hardy–Weinberg equilibrium. There was no statistically significant difference in both genotype and allele frequencies between control and case groups for any of the four SNPs, and also no dominant or recessive effects. The genotype frequency for rs2077647 from sequencing of the 56 cases (Table 2A) was similar to the result of PCR-RFLP for both control and case groups (Table 3).
Haplotype analysis for the three ESR1 SNPs did not reveal any statistically significant association with the diagnosis of perinatal depression. There was significant linkage disequilibrium between rs2077647 and rs2234693 (D′=−0.870145503; p=0.000999001), between rs2077647 and rs9340799 (D′=0.936017103; p=0.001998002), and between rs2234693 and rs9340799 (D′=−0.969436481; p=0.000999001).
ESR1 TA repeat
For this TA repeat polymorphism, 21 alleles ranging from 10 to 30 repeats were found. The distribution of the different alleles for controls and cases is shown in Figure 2. Both groups showed a similar bimodal distribution with two peaks at 16 repeats and 22 repeats. Additional analysis was performed by categorizing the alleles as either short (S)≤18 repeats or long (L)≥19 repeats26,27 with L as the susceptibility allele. However, there was also no statistically significant difference in the distribution between cases and controls (Table 3), although there was a trend of cases having higher frequencies of the longer repeats.
  | Figure 2 Distribution of estrogen receptor α (ESR1) TA repeat alleles for controls and cases. |
Discussion
For the SNPs selected for this study, the ESR1 rs9340799 AA genotype is known to modify the enhancer activity of the gene,28 and has been associated with the risk of depression in menopausal women.29 The ESR1 rs2234693 TT genotype has been shown to be associated with the risk of lifetime depression that is modified by postmenopausal hormone use,22 while the ESR2 rs4986938 AA genotype was reported to be linked to higher prevalence of lifetime major depression in the Nurses Health Study.22 However, no association between these previously associated SNPs in the ESR1 and ESR2 genes and perinatal depression could be detected in our analysis. This could be due to having different exposure to environmental factors such as negative life events or additional hormonal use.22,30
For postpartum depression, a previous study from Canada by Pinsonneault et al25 found an association of both the ESR1 rs2077647 G allele and the long TA repeat with EPDS scores in 156 postpartum women and also postpartum depression in a group of 84 subjects. We did not find a similar association between EPDS scores and the TA repeat in our control subjects who included only those who had scores below 7, and were thus at very low risk of postpartum depression. Our study design is different from the Canadian study in that we only enrolled women with EPDS scores of 7 or below as controls, and our cases were all clinically diagnosed by psychiatrists. Pinsonneault et al25 used an unselected population of postpartum women, and defined postpartum depression by a cutoff score on the Montgomery–Asberg Depression Rating Scale with no confirmation of diagnosis by a psychiatrist. In any case, this TA repeat has not been found to be associated with the level of sex hormones including 17β-estradiol.31 Another study from China also did not find an association of any genotype (SS/SL/LL) of the TA repeat with first-onset major depression in adolescent girls.27
From the resequencing results, allele frequencies of the known ESR1 variants were mostly similar to those of Asian populations from population databases except for rs6914438 (χ2=7.419; p=0.006) and rs3841686 (χ2=3.935; p=0.047). Likewise for ESR2, the frequencies were similar to those previously reported except for rs4986938 (χ2=4.444; p=0.035). However, it should be noted that our sample size was only 56 and all were patients with confirmed pregnancy-related depression while the frequencies from either the 1000 Genome Project or Exome Aggregation Consortium data were obtained from unselected populations. In any case, these SNPs might be good candidates for interrogation in a larger sample set.
The multifactorial etiology of depression has been well established. One possible reason for the lack of association with genetic factors could be that in our patient population there were often other factors contributing to perinatal depression, such as marital conflicts, lack of social support, conflicts with in-laws, financial problems, and work-related stress.32 In an affluent city-state like Singapore, these psychosocial stressors can play a more prominent causative role in the development of perinatal depression. Perhaps, future studies in our population could look at epigenetic changes which might correlate better with environmental psychosocial stressors. Another reason for our findings may be that postnatal depression is also related to other hormones such as cortisol, progesterone, and oxytocin which were not studied.33,34 Yet another possible contributory factor is sleep.35 All of these factors are affected by pregnancy and their mood effects might have obscured relationships between depression and our target genes.
There are several limitations of our study. One is that the case–control design might be susceptible to population stratification. We have tried to address that by including only subjects who self-reported as Chinese, but we did not genotype ancestry information markers to confirm. Also, although the cases were evaluated by psychiatrists, the EPDS screening for controls was only done at one time point (during the postnatal follow-up visit) for control subjects. Two demographic variables showed statistically significant differences between cases and controls. A higher proportion of cases lived in more expensive private housing which might reflect better awareness of mental health issues and higher tendency to seek treatment. There was also a significant difference in mean age as our controls were new mothers and were younger than cases that included patients who had been on follow-up from perinatal depression for past pregnancies.
Conclusion
Our study comparing cases with confirmed diagnosis and controls all of whom had low EPDS scores did not find a statistically significant association of any of the genetic variants with perinatal depression. It is thus unlikely that these variants play a major role in the etiology of perinatal depression in this population. However, sequencing of selected cases uncovered two novel single-nucleotide variants which could be investigated in larger studies for a potential link to depression.
Acknowledgments
This work was supported by project grants NMRCEDG1006, NMRC/CG/006/2013, and NMRC/CG/M003/2017 administered by the Singapore Ministry of Health’s National Medical Research Council. The authors thank all the study participants, and the staff from Department of Psychological Medicine who assisted with the EPDS screening and the collection of saliva samples.
Disclosure
The authors report no conflicts of interest in this work.
References
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. | ||
Chong SA, Abdin E, Vaingankar JA, et al. A population-based survey of mental disorders in Singapore. Ann Acad Med Singapore. 2012;41(2):49–66. | ||
Chee CY, Lee DT, Chong YS, Tan LK, Ng TP, Fones CS. Confinement and other psychosocial factors in perinatal depression: a transcultural study in Singapore. J Affect Disord. 2005;89(1–3):157–166. | ||
Garrido MM, Prigerson HG, Neupane S, Penrod JD, Johnson CE, Boockvar KS. Mental illness and mental healthcare receipt among hospitalized veterans with serious physical illnesses. J Palliat Med. 2016;20(3):247–252. | ||
Mahendran R, Lim HA, Tan JY, Kua EH, Griva K. The prevalence and predictors of subsyndromal anxiety and depression in adult Asian cancer patients across the first year of diagnosis. Asia Pac J Clin Oncol. 2016;12(4):476–489. | ||
Olver JS, Hopwood MJ. Depression and physical illness. Med J Aust. 2013;199(6 Suppl):S9–S12. | ||
Hodes GE, Walker DM, Labonté B, Nestler EJ, Russo SJ. Understanding the epigenetic basis of sex differences in depression. J Neurosci Res. 2017;95(1–2):692–702. | ||
Picco L, Subramaniam M, Abdin E, Vaingankar JA, Chong SA. Gender differences in major depressive disorder: findings from the Singapore Mental Health Study. Singapore Med J. 2017;58(11):649–655. | ||
Bierut LJ, Heath AC, Bucholz KK, et al. Major depressive disorder in a community-based twin sample: are there different genetic and environmental contributions for men and women? Arch Gen Psychiatry. 1999;56(6):557–563. | ||
Meng X, Brunet A, Turecki G, Liu A, D’Arcy C, Caron J. Risk factor modifications and depression incidence: a 4-year longitudinal Canadian cohort of the Montreal Catchment Area Study. BMJ Open. 2017;7(6):e015156. | ||
Dennis CL, Ross LE, Herxheimer A. Oestrogens and progestins for preventing and treating postpartum depression. Cochrane Database Syst Rev. 2008;(4):CD001690. | ||
Studd J. Hormone therapy for reproductive depression in women. Post Reprod Health. 2014;20(4):132–137. | ||
Aggarwal M, Puri V, Puri S. Effects of estrogen on the serotonergic system and calcitonin gene-related peptide in trigeminal ganglia of rats. Ann Neurosci. 2012;19(4):151–157. | ||
Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19(3):197–209. | ||
Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151(2):121–158. | ||
Aust S, Stasch J, Jentschke S, et al. Differential effects of early life stress on hippocampus and amygdala volume as a function of emotional abilities. Hippocampus. 2014;24(9):1094–1101. | ||
Goldstein-Piekarski AN, Korgaonkar MS, Green E, et al. Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proc Natl Acad Sci U S A. 2016;113(42):11955–11960. | ||
Ye M, Qing P, Zhang K, Liu G. Altered network efficiency in major depressive disorder. BMC Psychiatry. 2016;16(1):450. | ||
Watson CS, Alyea RA, Cunningham KA, Jeng YJ. Estrogens of multiple classes and their role in mental health disease mechanisms. Int J Womens Health. 2010;2:153–166. | ||
Rybaczyk LA, Bashaw MJ, Pathak DR, Moody SM, Gilders RM, Holzschu DL. An overlooked connection: serotonergic mediation of estrogen-related physiology and pathology. BMC Womens Health. 2005;5:12. | ||
Wharton W, Gleason CE, Olson SR, Carlsson CM, Asthana S. Neurobiological underpinnings of the estrogen – mood relationship. Curr Psychiatry Rev. 2012;8(3):247–256. | ||
Keyes K, Agnew-Blais J, Roberts AL, et al. The role of allelic variation in estrogen receptor genes and major depression in the Nurses Health Study. Soc Psychiatry Psychiatr Epidemiol. 2015;50(12):1893–1904. | ||
Ryan J, Ancelin ML. Polymorphisms of estrogen receptors and risk of depression: therapeutic implications. Drugs. 2012;72(13):1725–1738. | ||
Yan Z, Tan W, Dan Y, et al. Estrogen receptor alpha gene polymorphisms and risk of HBV-related acute liver failure in the Chinese population. BMC Medical Genetics. 2012;13(1):49. | ||
Pinsonneault JK, Sullivan D, Sadee W, Soares CN, Hampson E, Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression – a pilot study. Arch Womens Ment Health. 2013;16(6):499–509. | ||
Albagha OM, Pettersson U, Stewart A, et al. Association of oestrogen receptor alpha gene polymorphisms with postmenopausal bone loss, bone mass, and quantitative ultrasound properties of bone. J Med Genet. 2005;42(3):240–246. | ||
Geng YG, Su QR, Su LY, et al. Comparison of the polymorphisms of androgen receptor gene and estrogen alpha and beta gene between adolescent females with first-onset major depressive disorder and controls. Int J Neurosci. 2007;117(4):539–547. | ||
Maruyama H, Toji H, Harrington CR, et al. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57(2):236–240. | ||
Różycka A, Słopień R, Słopień A, et al. The MAOA, COMT, MTHFR and ESR1 gene polymorphisms are associated with the risk of depression in menopausal women. Maturitas. 2016;84:42–54. | ||
Zhang J, Chen L, Ma J, et al. Interaction of estrogen receptor β and negative life events in susceptibility to major depressive disorder in a Chinese Han female population. J Affect Disord. 2017;208:628–633. | ||
Westberg L, Baghaei F, Rosmond R, et al. Polymorphisms of the androgen receptor gene and the estrogen receptor beta gene are associated with androgen levels in women. J Clin Endocrinol Metab. 2001;86(6):2562–2568. | ||
Lee TM, Bautista D, Chen HY. Understanding how postnatal depression screening and early intervention work in the real world – a Singaporean perspective. Ann Acad Med Singapore. 2016;45(10):466–470. | ||
Kammerer M, Taylor A, Glover V. The HPA axis and perinatal depression: a hypothesis. Arch Womens Ment Health. 2006;9(4):187–196. | ||
Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36(9):1886–1893. | ||
Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, Wisner KL. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130(3):378–384. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.