Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
Investigation of PON1 activity and MDA levels in patients with epilepsy not receiving antiepileptic treatment
Authors Dönmezdil N, Cevik MU, Ozdemir HH, Taşin M
Received 29 December 2015
Accepted for publication 8 March 2016
Published 22 April 2016 Volume 2016:12 Pages 1013—1017
DOI https://doi.org/10.2147/NDT.S103336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Nilüfer Dönmezdil, Mehmet Uğur Çevik, Hasan Hüseyin Özdemir, Muhterem Taşin
Department of Neurology, Dicle University, Diyarbakır, Turkey
Purpose: There are many studies dedicated to researching the etiopathogenesis of epilepsy. In such research, oxidative and antioxidant indicators of etiopathogenesis have also been examined under the scope. Drawing on a group of patients with epilepsy who were receiving no treatment, we have tried to evaluate whether or not an increase in oxidative indicators is linked directly with the disorder, independent of epileptic medicaments.
Methods: Thirty people in good health and 30 newly diagnosed with epilepsy and who received ambulatory treatment in the polyclinic of the Neurology Department took part in the study. The tests relating to serum malondialdehyde (MDA) levels and paraoxonase 1 (PON1) activity were carried out in the biochemistry laboratory.
Results: Even though the levels of MDA in the patient group (14.34±3.59 nmol/mL) were found to be high compared to those of the control group, which consisted of people in good health (13.53±3.56 nmol/mL), there was no statistically significant difference. PON1 activity in the serum taken from people in the patient group (0.65±0.17) was lower in comparison to that observed in the serum of the control group (0.71±0.17 U/L). Nonetheless, it was not so low as to have significance from a statistical point of view.
Conclusion: We conclude that such a high level of oxidative parameters should have been related to the disease and that statistically significant findings that emerged in some other studies could have been related to an antiepileptic treatment.
Keywords: epilepsy, paraoxonase 1, malondialdehyde, oxidative stress, epilepsy, biochemical marker
Introduction
Epilepsy is one of the most common chronic diseases in developed and developing countries, affecting ~1% of the population.1 Many genetic, radiological, and biochemical researches have been performed to determine the etiology of this disease. Impaired gamma-amino butyric acid (GABA) inhibition, impaired activation of neurons, and an increase in excitatory neurotransmitters are responsible for the pathophysiology of epilepsy.2–6 Production of free radicals has a role in the regulation of biological function and damage to cell structures, as well as in the pathogenesis of neurodegenerative diseases, such as Parkinson’s disease, stroke, epilepsy, and dementias, in the central nervous system.2,3 Oxidative and nitrosative stress are regarded as possible mechanisms in the pathogenesis of epilepsy.4 Oxidative markers have therefore become popular in recent years, and many of them have been studied.5
The paraoxonase (PON) gene family is localized in the long arm of chromosome 7, and includes PON1, PON2, and PON3 genes, which are adjacent to each other. PON is a 43–45 kDa glycoprotein which is synthesized mainly by the liver. PON1 is a Ca2+-dependent enzyme that is transported with high-density lipoproteins (HDLs) in circulation.6 It is reported that the antioxidant effect of PON1 and PON3 protects HDLs and low-density lipoproteins (LDLs) from oxidation.7,8 The antioxidant effect is probably due to the hydrolysis of activated phospholipids and/or lipid peroxide products. It is known that diet, pregnancy, and hormones can affect serum PON1 levels.9,10
Malondialdehyde (MDA) is a mutagenic, carcinogenic, and genotoxic compound that is the end product of lipid peroxidation and is the main secondary oxidation product of polyunsaturated fatty acids. It is known that the levels of this compound increase in several diseases that are related to free-radical damage. MDA is the most commonly used marker of oxidative damage.11,12 Brain tissue is particularly susceptible to free-radical damage for several reasons (eg, high oxygen consumption or high concentrations of phospholipids can increase peroxidation and prevent regeneration of neurons), and the extent of damage is proportional to the the amount of free radicals formed.
The results of some studies show that oxidative stress can play a role in the etiopathogenesis of epilepsy, and there are some studies which also report that this stress is generated by antiepileptic drugs (AEDs).13,14 Our main aim in this study was to investigate the relationship of oxidative stress to PON1 activity and MDA levels by measuring these parameters in epileptic patients who were not using AEDs and have had nonprovoked seizures.
Materials and methods
This study was started on June 10, 2013, and this study was approved by the Dicle University Faculty of Medicine Ethics Committee (approval number 293). Patients aged between 16 and 65 years who applied to the neurology clinic of the Dicle University Faculty of Medicine (DUTF) hospital with a prediagnosis of epilepsy were evaluated. Classification of epileptic seizures was made according to the epilepsy classification of the International League against Epilepsy (ILAE), which was published in 1981. The results of routine biochemistry, electroencephalogram, and cranial magnetic resonance imaging (MRI) were requested from the patients who were selected for this study, in order to exclude patients with provoked epileptic seizures and select patients with only nonprovoked epileptic seizure diagnosis. Patients with significant pathology in cranial MRIs (sinus vein thrombosis, encephalitis, intracranial tumors) were excluded to prevent erroneous assessment of oxidative markers. In addition, patients with known presence of neurological disease, hypertension, diabetes mellitus, mental retardation, pregnancy, cardiogenic syncope, and psychogenic nonepileptic seizures; use of antioxidant drugs and agents; and those with a diagnosis of provoked epilepsy were also excluded from this study. As a result, 30 newly diagnosed patients were included in the study.
A total of 30 volunteers who did not have any other neurological or chronic diseases according to the anamnesis and who were similar in age and sex to the members of the patient group were included in this study as a control group. Informed consent forms were signed by the individuals in both the patient and control groups.
From the control group and patients in the interictal period, 5 cc of blood was drawn from the antecubital vein into the biochemical tubes. This blood sample was centrifuged for 5 minutes at 5,000 rpm with a Rotafix 32-A brand device, and serum was obtained and stored at −80°C in a refrigerator at the Department of Biochemistry, DUTF. After the stored blood was defrosted, it was homogenized by vortexing using a M16 Vortex mixer.
Paraoxonase enzyme activity measurement
The activity of PON1, which is a lipophilic, hydrophobic, antioxidant enzyme connected to HDL-cholesterol, was detected by using a commercial Reel Assay brand kit according to manufacturer’s instructions. In this method, hydrolysis of the paraoxon (O, O-diethyl-O-p-nitrophenyl phosphate) substrate by PON1 enzyme generates a colored p-nitrophenol product. PON1 enzyme activity was then measured photometrically with an Architect C16000 autoanalyzer. The rate of paraoxon hydrolysis was measured by monitoring the increase of absorbance at 412 nm, and the enzyme activity was expressed as U/L.15
Malondialdehyde level measurement
MDA levels were determined using a Northwest MDA assay kit according to manufacturer’s instructions. MDA forms a pink complex in aerobic conditions as a result of incubation with thiobarbituric acid (TBA) at pH 3.4 and 95°C. This colored complex was read at 532 nm by spectrophotometry, and the absorbance was measured. The MDA concentration in the sample was determined by comparing the measured absorbance value to a MDA standard curve.16
Statistical analysis
All the data were analyzed using the Statistical Package for Social Sciences (SPSS) program for Windows 16.0 (SPSS Inc., Chicago, IL, USA). For comparing the results of the control and patient groups, independent t-tests and chi-square tests were performed. To find independent associations of age, variables with a P-value ≤0.05 from the bivariate correlation analysis were selected for multiple linear regression analysis. Results with P-values <0.05 were considered statistically significant.
Results
The average age of our patients was 24.6±8.15 years, and that of the control group was 23±4.83 years. There was no statistically significant difference between the two groups with respect to age. The patient group consisted of 14 females (46.7%) and 16 males (53.7%) patients, and the control group consisted of 13 females (43.3%) and 17 males (56.7%) individuals. There was no statistically significant difference between the two groups in terms of sex.
There was a statistically significant difference between the patient and control groups with respect to education level and marital status. There was no statistically significant difference between the two groups with respect to living place, living status, disease history, and disease history in the family (P>0.05) (Table 1).
  | Table 1 Demographic characteristics of control group and patients |
Epilepsy classification was made in accordance with the Classification of Epileptic Seizures of the ILAE, which was published in 1981. In 43.3% (n=13) and 56.7% of patients (n=17), partial and generalized seizures were detected, respectively. The distribution of partial seizures was found to be simple partial in 30.8% (n=4) and complex partial in 69.2% (n=9) of the patients. The distribution of generalized seizures was found to be tonic in 26.5% (n=4), atonic in 5.9% (n=1), and tonic–clonic in 70.6% (n=12) of the patients. In the patients included in our study, absence, myoclonic and clonic seizures were not observed.
PON1 activity and MDA levels, both of which were investigated in our study, were statistically insignificant between the patient and control groups (P>0.05) (Table 2).
  | Table 2 Relationship between PON1 activity and MDA levels in the patient and control groups |
In the patient group, no statistically significant difference was noted for the relationship of PON1 activity to MDA levels and seizure types (P>0.05) (Table 3). There was no correlation between MDA levels and PON1 activity and age.
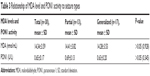  | Table 3 Relationship of MDA level and PON1 activity to seizure types |
Discussion
When studies related to epilepsy and oxidative stress were analyzed, it was found in some cases that oxidative stress parameters were high in epileptic patients, and on the other hand, in some studies, it was considered that the use of AEDs increased the levels of these parameters.17,18
In epilepsy patients, various oxidative markers and antioxidant defense mechanisms were studied, such as PON1, MDA, arylesterase, oxidative stress, and antioxidant capacity.18 Varoğlu et al conducted a study on 61 epilepsy patients who were taking antiepileptic treatment.13 When they compared PON, arylesterase, 8-hydroxyl guanine, and oxidized LDL levels in the patient and control groups, the levels of oxidative stress markers were high in all the antiepileptic treatment groups, whereas the PON levels were found to be significantly low. But as there was no patient group that had not undergone treatment for epilepsy in this study, it cannot be known whether this change in the levels of oxidative markers was due to disease or drugs.13 The results of a study by Çevik et al showed that MDA levels in an epileptic patient group were significantly higher than those of the control group, and PON1 activity was found to be significantly low in the patient group. These results were thought to be related to AED use.19
In a study conducted by Tong et al on rats,20 the animals were divided into two groups. Saline injection was given to the control group and 500 mg/kg of valproic acid injections (once a day) were administered to the other group. For observing the changes in rats, oxidative stress markers, such as TBA and hydrolipid peroxidase levels, were measured at 2, 4, 7, 10, and 14 days. To measure the levels of TBA, a MDA assay kit was used and the levels of MDA and TBA were increased. As a result of this study, on the 14th day of valproic acid injections, MDA levels were found to be significantly higher in the test group than in the saline-injected group.20
AEDs, at least in part, impair antioxidant systems. Some of them, especially from the older generation of AEDs (such as carbamazepine and valproic acid), may trigger oxygen-dependent tissue injury by several mechanisms.21 Increased activities of superoxide dismutase and catalase and decreased activities of glutathione peroxidase and glutathione reductase were observed in numerous studies of drug-naive patients with epilepsy.22 Also, it has been proposed that the long-term use of certain AEDs increases free-radical formation and causes oxidative damage in neurons.23,24
Limitations
Our study has several limitations. One of them is that the number of patients was insufficient; therefore, the statistical analysis may not be satisfactory. On the other hand, we did not evaluate the relationship of MDA levels and PON1 activity with the frequency and duration of seizures.
Conclusion
In conclusion, no statistically significant difference in the MDA levels between the patient and control groups was observed in our study. There were no differences in MDA levels and PON1 activity when comparing between the control group and the patient group that did not have antiepileptic treatment. Also, there was no correlation between MDA levels and PON1 activity and age in the patient group. Some articles in the literature reported that the levels of oxidative markers increased in epileptic patients. In the majority of studies, the impact of antiepileptic treatment on oxidative markers was ignored because the increase in oxidative markers might be related to the damage done to the liver by the antiepileptic treatment. We think that it might be helpful to include nonmedicated epilepsy patients in our future studies, because adding these patients can make the understanding of the effects of changes in oxidative markers on the pathophysiology of epilepsy easy.
Disclosure
The authors report no conflicts of interest in this work.
References
Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16(2):165–170. | ||
Aguiar CC, Almeida AB, Araújo PV, et al. Oxidative stress and epilepsy: literature review. Oxid Med Cell Longev. 2012;2012:795259. | ||
Kong Q, Lin CL. Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci. 2010;67(11):1817–1829. | ||
Chang S-J, Yu B-C. Mitochondrial matters of the brain: mitochondrial dysfunction and oxidative status in epilepsy. J Bioenerg Biomembr. 2010;42(6):457–459. | ||
Peker E, Oktar S, Ari M, et al. Nitric oxide, lipid peroxidation, and antioxidant enzyme levels in epileptic children using valproic acid. Brain Res. 2009;1297:194–197. | ||
Mackness MI, Mackness B, Durrington PN, Connelly PW, Hegele RA. Paraoxonase: biochemistry, genetics and relationship to plasma lipoproteins. Curr Opin Lipidol. 1996;7(2):69–76. | ||
Navab M, Hama-Levy S, Van Lenten BJ, et al. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest. 1997;99(8):2005. | ||
Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286(1):152–154. | ||
Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci. 2004;107(5):435–448. | ||
Balci Ekmekçi Ö, Donma O, Ekmekçi H. Paraoxonase. Cerrahpaşa J Med. 2004;35:78–82. | ||
Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J Chromatogr B. 2005;827(1):76–82. | ||
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. | ||
Varoğlu AO, Yildirim A, Aygul R, Gundogdu OL, Sahin YN. Effects of valproate, carbamazepine, and levetiracetam on the antioxidant and oxidant systems in epileptic patients and their clinical importance. Clin Neuropharmacol. 2010;33(3):155–157. | ||
Schulpis KH, Lazaropoulou C, Regoutas S, et al. Valproic acid monotherapy induces DNA oxidative damage. Toxicology. 2006;217(2):228–232. | ||
Furlong CE, Richter R, Seidel S, Motulsky A. Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am J Hum Genet. 1988;43(3):230. | ||
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. | ||
Naziroğlu M, Yürekli VA. Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol. 2013;33(5):589–599. | ||
Menon B, Ramalingam K, Kumar RV. Low plasma antioxidant status in patients with epilepsy and the role of antiepileptic drugs on oxidative stress. Ann Indian Acad Neurol. 2014;17(4):398. | ||
Çevik MU, Varol S, Yücel Y, et al. Serum paraoxonase-1 activities and malondialdehyde levels in patients with epilepsy. Dicle Med J. 2012;39(4):557–560. | ||
Tong V, Teng XW, Chang TK, Abbott FS. Valproic acid I: time course of lipid peroxidation biomarkers, liver toxicity, and valproic acid metabolite levels in rats. Toxicol Sci. 2005;86(2):427–435. | ||
Karikas GA, Schulpis KH, Bartzeliotou A, et al. Early effects of sodium valproate monotherapy on serum paraoxonase/arylesterase activities. Scand J Clin Lab Invest. 2009;69(1):31–35. | ||
Martinc B, Grabnar I, Vovk T. The role of reactive species in epileptogenesis and influence of antiepileptic drug therapy on oxidative stress. Curr Neuropharmacol. 2012;10(4):328–343. | ||
Yiiksel A, Cengiz M, Seven M, Ulutin T. Erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and serum lipid peroxidation in epileptic children with valproate and carbamazepine monotherapy. J Basic Clin Physiol Pharmacol. 2000;11(1):73–81. | ||
Yüksel A, Cengiz M, Seven M, Ulutin T. Changes in the antioxidant system in epileptic children receiving antiepileptic drugs: two-year prospective studies. J Child Neurol. 2001;16(8):603–606. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
