Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Investigation of Hyperlipidemia Associated with Increased Levels of Oxidized Low-Density Lipoproteins and 8-Hydroxy-2´-Deoxyguanosine
Authors Babakr A , Mukhtar M , Althubiti M, Al-Amodi H , Almaimani R, Nour Eldin MM , Elzubeir Abdalla M , Nasif W
Received 8 November 2022
Accepted for publication 24 January 2023
Published 14 February 2023 Volume 2023:16 Pages 447—455
DOI https://doi.org/10.2147/DMSO.S396676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Abdullatif Babakr,1 Mohamed Mukhtar,1 Mohammad Althubiti,1 Hiba Al-Amodi,1 Riyad Almaimani,1 Mohamed Mahmoud Nour Eldin,1 Mohamed Elzubeir Abdalla,1 Wesam Nasif1,2
1Department of Medical Biochemistry, Faculty of Medicine, Umm Al-Qura University, Makkah, Kingdom of Saudi Arabia; 2Molecular Biology Department, Genetic Engineering and Biotechnology Research Institute, Sadat City University, Sadat City, Egypt
Correspondence: Abdullatif Babakr, Department of Medical Biochemistry, Faculty of medicine, Umm Al-Qura University, Abdia, Makkah, Kingdom of Saudi Arabia, Tel +966-25270000/4322, Fax +96625270000/4319, Email [email protected]
Background: Hyperlipidemia is a common risk factor for atherosclerosis, heart diseases, and other pathological conditions. The factors leading to the oxidation of native low-density lipoproteins remain of valuable importance for a better understanding of the mechanisms leading to these pathologies. The aim of the present study was to evaluate the association between lipid status and the levels of oxidized low-density lipoproteins and 8-hydroxy-2´-deoxyguanosine.
Methods: One hundred and fourteen participants were enrolled. Lipid profile parameters were measured and used individually to categorize subjects into two groups of normal and hyperlipidemic cases according to the international reference ranges. Oxidized low-density lipoproteins and 8-hydroxy-2´-deoxyguanosine were then compared in normal and high lipid profile groups. The obtained results were then statistically analyzed.
Results: 8-Hydroxy-2´-deoxyguanosine was found to be positively correlated with hypercholesterolemia, hypertriglyceridemia, and high levels of low-density lipoproteins (r = 0.53, 0.41, and 0.60), respectively (p< 0.001). A positive correlation was observed also between the levels of oxidized low-density lipoproteins and the same lipid profile parameters (r = 0.42, 0.31, and 0.45), respectively (p< 0.001).
Conclusion: The present study suggests that disturbance in lipid profile may result in increased levels of oxidized low-density lipoproteins and oxidative stress in the study group; however, a larger sample is needed to confirm the present findings.
Keywords: oxidized LDL, 8-OH-dG, dyslipidemia, DNA damage
Introduction
Hyperlipidemia is considered in many previous studies as a common risk factor in heart diseases and various pathological conditions.1–3 The association between dyslipidemia and cardiovascular disease is well defined in many previous works.4,5 Oxidized low-density lipoproteins (Ox-LDL) were suggested to be atherogenic particles and play a major role in atherosclerosis and many other degenerative diseases.6,7 Many risk factors are suggested to be involved in the oxidation of native LDL, including the poor antioxidant defense of the body which may lead to a situation of oxidative stress;8 this status was suggested to be responsible for oxidative damage of biomolecules leading to serious scenarios including DNA damage.9
The equilibrium between oxidants and antioxidants in the body when altered, results in disturbance in cell structure, cell membranes, biomolecules such as proteins, lipids, and strand breakage of DNA10.
The interaction of the hydroxyl radical, one of the important reactive oxygen species, with the nucleobases found in the DNA strand, such as guanine, result in the formation of 8-OH-dG. Many diseases and pathology scenarios, such as cardiovascular or chronic obstructive pulmonary diseases (COPD), have been associated with increased levels of 8-OH-dG. In previous studies it has been established as a sensitive biomarker for the assessment of oxidative DNA modification.11 8-OH-dG levels also increase due to other factors such as smoking, aging, or exposure to physical, chemical, and biological substances.12 Wide range of 8-OH-dG cut-off values was reported in different studies.12–14
Several groups have developed techniques and methods for measuring oxidized LDL, which have been used in many clinical studies.8,13 Accumulating evidence supports the use of oxidized LDL as a useful biomarker in diagnoses and prognosis. It is important to understand the limitations of the different procedures used for oxidized LDL measurement, together with the establishment of cut-off values for justification of using this important biomarker in routine clinical settings.
Low-density lipoproteins (LDL) are the principal carriers of cholesterol in the blood, and as such, the oxidative modification of these particles is a biomarker and pathological risk factor in cardiovascular diseases (CVD), obesity, type-2 diabetes mellitus, and other metabolic disorders.15 More focus on and better understanding of the mechanisms by which normal LDL particles get oxidized and DNA damage occur may represent the key for best monitoring of many pathological scenarios especially degenerative diseases caused or mediated by these molecules.16
This study was conducted to evaluate the association between disturbance in lipid profile parameters and the levels of oxidized LDL and 8-hydroxy-2´-deoxyguanosine (8-OH-dG) as a biomarker of DNA damage.
Subjects and Methods
One hundred and fourteen subjects were recruited in the present study which was conducted in Makkah Al-Mukarramah, in the Kingdom of Saudi Arabia. Subjects with known diabetes mellitus and/or cardiovascular diseases (CVD) were excluded. Blood samples were collected from the participants who fulfilled the inclusion criteria. Ethical consent was obtained from all participants. The study protocols comply with the Declaration of Helsinki and were approved by the Biomedical Ethics Committee, Faculty of Medicine, Umm Al-Qura University, Makkah Al-Mukarramah, Kingdom of Saudi Arabia.
Participants were requested to fill the structured questionnaire, information of sex, age, and lifestyle including cigarette smoking were collected, then anthropometric measurements and clinical investigations were done for all the subjects. Blood samples were collected after 12 hours of fasting, no medications were used during the time of blood collection particularly lipid-lowering drugs, and we used the standard collection procedures for assessment of total cholesterol, triglycerides, HDL, LDL, 8-OH-dG, and oxidized LDL.
Routine lipid profile analysis was done in a full automated chemistry analyzer (Humastar-80), from Human Diagnostics, Wiesbaden, Germany. The protocols were followed as stated by the manufacturer’s guidance using the kits and controls of Human Diagnostics, Wiesbaden, Germany. Normal levels of lipid profile were considered as cholesterol (up to 200 mg/dL), triglycerides (up to 200 mg/dL), LDL (up to 135 mg/dL), and HDL (more than 35 mg/dL).
Measurement of oxidized low-density lipoproteins was performed using Mercodia Oxidized LDL Competitive ELISA. The procedure was described in the assay kit manufactured by Mercodia AB, Uppsala, Sweden (CV < 8%). This protocol uses Holvoet et al's monoclonal antibody 4E6.17 This procedure uses a competitive ELISA technique where the 4E6 antibody is directed against the apoB-100 of LDL conformational change. A fixed amount of oxidized LDL was bound to the well. Oxidized LDL in the samples competes with this amount of oxidized LDL for the binding to the specific antibodies 4E6.
8-OH-dG was measured using the Cloud-Clone Crop 8-OH-dG Competitive Inhibition Enzyme Immunoassay Technique Kit (Cloud-Clone Crop., USCN Life Science Inc., Houston, TX, USA). Used for the quantitative determination of 8-OH-dG in human serum (CV < 10%). A competitive inhibition reaction between biotinylated 8-OH-dG and unlabeled 8-OH-dG in standards or samples was then initiated with a pre-coated antibody specific for 8-OH-dG. The concentration of 8-OH-dG in the sample was then calculated since the amount of bound HRP conjugate is inversely proportional to the concentration of 8-OH-dG. The amount of 8-OH-dG in serum was calculated in pg/mL from a standard curve. Data were summarized using descriptive statistics, and the method of averages and independent-sample t-tests were used to compare various measured parameters between groups. Pearson’s correlation coefficient was used to assess the association between various lipid parameters and oxidized LDL and 8-OH-dG.
Statistical Analysis
Statistical analysis of our data was performed using the Statistical Package for the Social Sciences (SPSS) for Windows version 20. Results were expressed as mean ± SD. Descriptive statistics was used in tabulation of data, comparison between the means of different measured parameters of lipids profile and oxidized LDL was done using the mean procedures and independent-sample t-test. The Pearson correlation coefficient was used to evaluate the association between the different lipid parameters and oxidized LDL and 8-OH-dG. P<0.05 was considered as statistically significant.
Results
One hundred and fourteen qualified participants were enrolled in the present study, the ages of the study group range between 20 and 54 years old. There was a positive correlation between cholesterol levels mg/dL and oxidized LDL levels in the studied subjects (r = 0.42; p<0.001) as shown in Figure 1A. In addition, the same positive correlation was also seen between triglyceride levels and oxidized LDL levels in the participants (r = 0.31; p<0.001) (Figure 1B). Interestingly, high levels of LDL were also correlated positively with LDL oxidation (r = 0.45; p<0.001) (Figure 1C).
 |
Figure 1 Correlation between levels of oxidized LDL and lipid profile parameters, (A) Ox-LDL and cholesterol, (B) Ox-LDL and triglycerides, (C) Ox-LDL and LDL-C. |
After assessing the correlation between oxidized LDL and different lipid profile parameters, the correlation between 8-OH-dG and different lipid profile parameters was then assessed. There was a positive correlation between cholesterol levels mg/dL and 8-OH-dG levels in the studied subjects (r = 0.53; p<0.001) as shown in Figure 2A. In addition, the same positive correlation was also seen between triglycerides and 8-OH-dG levels in the study group (r = 0.41; p<0.001) (Figure 2B). Moreover, high levels of LDL were also correlated positively with 8-OH-dG (r = 0.60; p<0.001) (Figure 2C).
 |
Figure 2 Correlation between levels of 8-OH-dG and lipid profile parameters, (A) 8-OH-dG and cholesterol, (B) 8-OH-dG and triglycerides, (C) 8-OH dG and LDL-C. |
After that oxidized LDL status was investigated among different lipid profile categories. Oxidized LDL was significantly high in the hypercholesterolemia group comparing to the normal cholesterol group (p<0.001). Moreover, the hypertriglyceridemia group showed a significant increase in oxidized LDL comparing to the normal triglyceride group (p<0.001). When the high LDL group was compared with the normal LDL group, also a significant high level of oxidized LDL was seen in the high LDL group comparing to the control (p<0.001) as shown in Figure 3.
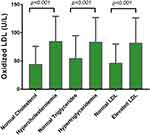 |
Figure 3 Levels of oxidized LDL in normal and elevated lipid profile parameter groups. |
After exploring the status of oxidized LDL among different groups of abnormal lipid profile, the 8-OH-dG was then measured in the different abnormal lipid profile groups. 8-OH-dG was significantly high in the hypercholesterolemia group comparing to the normal cholesterol group (p<0.001). Moreover, the hypercholesterolemia group showed a significant increase in 8-OH-dG comparing to the normal cholesterol group (p<0.01) as shown in Table 1. When the high LDL group was compared with the normal LDL group, also a significant high level of 8-OH-dG was seen in the high LDL group comparing to the control (p<0.001) as shown in Figure 4.
 |
Table 1 Age, BMI, Lipid Profile, and 8-OH-dG in Subjects of Normal Cholesterol and Cases of Hypercholesterolemia |
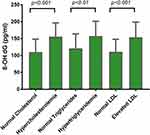 |
Figure 4 Levels of 8-OH-dG in normal and elevated lipid profile parameter groups. |
Most of the subjects in the studied group (79.8%) show normal triglyceride concentrations as shown in Table 2. The remaining group of hypertriglyceridemia also shows increased levels in oxidized LDL (P<0.001) and 8-OH-dG (P<0.01) compared to the normal triglyceride group.
 |
Table 2 Age, BMI, Lipid Profile, and 8-OH-dG in Subjects of Normal Triglycerides and Cases of Hypertriglyceridemia |
Regarding the levels of LDL, we found that (40.3%) of the study group have elevated LDL levels as shown in Table 3. This group shows significant increased levels in total cholesterol (P<0.001), triglycerides (P<0.01), as well as increased oxidized LDL and 8-OH-dG (P<0.001) compared to the normal LDL group.
 |
Table 3 Age, BMI, Lipid Profile, and 8-OH-dG in Subjects of Normal LDL-C and Cases of Elevated LDL-C |
Discussion
Hyperlipidemia is a major risk factor of metabolic syndrome and cardiovascular diseases,1 and affects millions of people worldwide. People with hyperlipidemia have increased levels of serum cholesterol, triglycerides, and LDL and are at increased risk of thrombosis.18 Previous studies have shown that oxidized lipoproteins, such as oxidized low-density lipoproteins, contribute to the development of a prothrombotic state.19,20 Previous studies considered oxidized LDL as a biomarker of cardiovascular diseases; it has been established as a pro-inflammatory pathogenic particle leading to endothelial dysfunction.21 Falih et al described the role of oxidized low-density lipoproteins in many pathologic scenarios.16
In this study, we found a significant positive correlation between the levels of oxidized LDL and lipid profile parameters. This may indicate that the oxidation of the native LDL particles occurs in hyperlipidemic subjects as shown in Figure 1; hypercholesterolemia and increased levels of LDL are particularly found to be associated with higher levels of oxidized LDL in previous studies.8 The mean oxidized LDL was found to be significantly higher in subjects with hyperlipidemia as shown in Figure 2. Many studies suggest that plasma oxidized LDL levels may represent a useful biomarker for predicting cardiovascular diseases; although substantial differences were observed between the methods used for evaluation and measurement of oxidized LDL.13 Validation of methods and more focus on the role of oxidized LDL in different pathologies may be important in the management of these pathological conditions.
8-Hydroxy-2-deoxyguanosine (8-OH-dG) is generated following the repair of DNA damage which is caused by reactive oxygen species (ROS); thus, it is considered as one of the most widely known markers of DNA oxidative damage.22 Previous works have shown that DNA damage and oxidative stress caused by different factors23 are significantly associated with the atherosclerosis and heart failure,24 and the role of this oxidative damage in the onset and contribution to progression of this pathologic condition need to be more clarified. On the other hand, previous studies demonstrate an association between increased levels of 8-OH-dG and hyperlipidemia.25 In consistence with these findings, the present study shows higher levels of 8-OH-dG in groups of hypercholesterolemia, hypertriglyceridemia, and elevated LDL cholesterol as shown in Figure 3. There is a significant positive correlation in the present study between these lipid parameters and levels of 8-OH-dG as shown in Figure 4.
Shen et al evaluate 8-OH-dG as a potential survival biomarker in patients with non-small-cell lung cancer; they suggest an inverse relationship with survival, where patients with low levels of 8-OH-dG show longer survival times compared with those with high levels of 8-OH-dG.26 The same study shows that over 3-fold increased hazard of death was observed in patients with increased levels of 8-OH-dG. The levels of 8-OH-dG need to be well addressed in clinical research and practice as an oxidative DNA damage marker.
The situation of oxidative stress, leading to oxidative damage of biomolecules, plays an important role in the development and progression of many diseases including diabetes mellitus, atherosclerosis, or cancer. The hypothesis that oxidative stress may be responsible and/or plays a significant role in the etiology or development of metabolic disorders is supported by several studies.27 Moreover, food and vegetable intake as antioxidants was found to be independently associated with reduced ox-LDL and 8-OH-dG as biomarkers of oxidative stress.28 Burgos Alves et al previously suggest reference values of oxidative stress biomarkers in a young healthy Spanish population and demonstrate different correlations between oxidized LDL and isoprostanes by gender with lipid profile parameters;29 however, validation of such findings is important in larger studies. The limitation of the present study is the relatively small sample size, and studies recruiting larger sample sizes are recommended for validation and to evaluate the impact of dyslipidemia on oxidized LDL and 8-OH-dG; the specified gender and region are also limitations of this study, and different regions and ethnic groups may show different findings. As some levels of oxidative damage are present in every individual, the normal versus pathological elevation of oxidative stress biomarkers such as oxidized LDL and 8-OH-dG should be defined, and the levels associated with disturbance in lipid profile and pathologies should be established in larger studies.
Conclusion
In conclusion, the present study suggests that hypercholesterolemia, hypertriglyceridemia, and elevated LDL may be associated with increased levels of oxidized low-density lipoproteins and 8-hydroxy-2´-deoxyguanosine. More studies are recommended to validate this relationship and possible strategies for prevention of oxidative damage.
Abbreviations
LDL, low-density lipoproteins; 8-OH-dG, 8-hydroxy-2´-deoxyguanosine; Oxidized LDL, oxidized low-density lipoproteins.
Data Sharing Statement
For data requests, please contact the corresponding author.
Ethical Approval and Consent to Participate
A written consent was obtained from all participants. The study methodology and protocols were approved by the Biomedical Ethics Committee, College of Medicine, Umm Al-Qura University, Makkah Al-Mukarramah, Kingdom of Saudi Arabia.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4300045DSR03).
Disclosure
The authors declare that there is no conflicts of interest regarding the publication of this paper.
References
1. Fischer S, Schatz U, Julius U. Practical recommendations for the management of hyperlipidemia. Atheroscler Suppl. 2015;18:194–198. doi:10.1016/j.atherosclerosissup.2015.02.029
2. Rai S, Bhatnagar S. Hyperlipidemia, disease associations, and top 10 potential drug targets: a network view. OMICS. 2016;20(3):152–168. doi:10.1089/omi.2015.0172
3. Hussein HK, Aubead M, Kzar HH, et al. Association of cord blood asprosin concentration with atherogenic lipid profile and anthropometric indices. Diabetol Metab Syndr. 2022;14:1. doi:10.1186/s13098-022-00844-7
4. Ferrieres J, Amber V, Crisan O, Chazelle F, Junger C, Wood D. Total lipid management and cardiovascular disease in the dyslipidemia international study. Cardiology. 2013;125(3):154–163. doi:10.1159/000348859
5. Cifkova R, Krajcoviechova A. Dyslipidemia and cardiovascular disease in women. Curr Cardiol Rep. 2015;17(7):609. doi:10.1007/s11886-015-0609-5
6. Maiolino G, Rossitto G, Caielli P, Bisogni V, Rossi GP, Calo LA. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediators Inflamm. 2013;2013:714653. doi:10.1155/2013/714653
7. Wan F, Qin X, Zhang G, et al. Oxidized low-density lipoprotein is associated with advanced-stage prostate cancer. Tumour Biol. 2015;36(5):3573–3582. doi:10.1007/s13277-014-2994-6
8. Nour Eldin EEM, Almarzouki A, Assiri AM, Elsheikh OM, Mohamed BEA, Babakr AT. Oxidized low density lipoprotein and total antioxidant capacity in type-2 diabetic and impaired glucose tolerance Saudi men. Diabetol Metab Syndr. 2014;6(1). doi:10.1186/1758-5996-6-94
9. Smith JA, Park S, Krause JS, Banik NL. Oxidative stress, DNA damage, and the telomeric complex as therapeutic targets in acute neurodegeneration. Neurochem Int. 2013;62(5):764–775. doi:10.1016/j.neuint.2013.02.013
10. Nijhawan P, Arora S, Behl T. Intricate role of oxidative stress in the progression of obesity. Obes Med. 2019;15:100125. doi:10.1016/J.OBMED.2019.100125
11. Abuarrah M, Setianto BY, Faisal A, Sadewa AH. 8-hydroxy-2-deoxyguanosine as oxidative DNA damage biomarker of medical ionizing radiation: a scoping review. J Biomed Phys Eng. 2021;11(3):389–402. doi:10.31661/JBPE.V0I0.2101-1258
12. Chen HI, Liou SH, Ho SF, et al. Oxidative DNA damage estimated by plasma 8-hydroxydeoxyguanosine (8-OHdG): influence of 4, 4′-methylenebis (2-chloroaniline) exposure and smoking. J Occup Health. 2007;49(5):389–398. doi:10.1539/joh.49.389
13. Itabe H, Ueda M. Measurement of plasma oxidized low-density lipoprotein and its clinical implications. J Atheroscler Thromb. 2007;14(1):1–11. doi:10.5551/jat.14.1
14. Gao Y, Wang P, Wang Z, et al. Serum 8-hydroxy-2′-deoxyguanosine level as a potential biomarker of oxidative DNA damage induced by ionizing radiation in human peripheral blood. Dose-Response. 2019;17(1):155932581882064. doi:10.1177/1559325818820649
15. Varghese DS, Ali BR. Pathological crosstalk between oxidized LDL and ER stress in human diseases: a comprehensive review. Front Cell Dev Biol. 2021;9. doi:10.3389/fcell.2021.674103
16. Falih IQ, Alobeady MAH, Banoon SR, Saleh MY. Role of oxidized low-density lipoprotein in human diseases: a review. J Chem Health Risks. 2021;11(Special Issue):71–83. doi:10.22034/jchr.2021.684227
17. Holvoet P, Macy E, Landeloos M, et al. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin Chem. 2006;52(4):760–764. doi:10.1373/clinchem.2005.064337
18. Owens AP, Byrnes JR, Mackman N. Hyperlipidemia, tissue factor, coagulation, and simvastatin. Trends Cardiovasc Med. 2014;24(3):95–98. doi:10.1016/j.tcm.2013.07.003
19. Trpkovic A, Resanovic I, Stanimirovic J, et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52(2):70–85. doi:10.3109/10408363.2014.992063
20. Ghazizadeh H, Saberi-Karimian M, Aghasizadeh M, et al. Pro-oxidant–antioxidant balance (PAB) as a prognostic index in assessing the cardiovascular risk factors: a narrative review. Obes Med. 2020;19:100272. doi:10.1016/J.OBMED.2020.100272
21. Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J. 2001;15(12):2073–2084. doi:10.1096/fj.01-0273rev
22. Di Minno A, Turnu L, Porro B, et al. 8-hydroxy-2-deoxyguanosine levels and cardiovascular disease: a systematic review and meta-analysis of the literature. Antioxid Redox Signal. 2016;24(10):548–555. doi:10.1089/ars.2015.6508
23. Adly HM, Saleh SA. The association of increased oxidative stress and tumor biomarkers related to polyaromatic hydrocarbons exposure for different occupational workers in Makkah, Saudi Arabia. Cureus. 2022. doi:10.7759/cureus.32981
24. Di Minno A, Turnu L, Porro B, et al. 8-hydroxy-2-deoxyguanosine levels and heart failure: a systematic review and meta-analysis of the literature. Nutr Metab Cardiovasc Dis. 2017;27(3):201–208. doi:10.1016/j.numecd.2016.10.009
25. Fentoglu O, Kirzioglu FY, Bulut MT, et al. Evaluation of lipid peroxidation and oxidative DNA damage in patients with periodontitis and hyperlipidemia. J Periodontol. 2015;86(5):682–688. doi:10.1902/jop.2015.140561
26. Shen J, Deininger P, Hunt JD, Zhao H. 8-hydroxy-2′-deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer. 2007;109(3):574–580. doi:10.1002/cncr.22417
27. Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev. 2014;2014:908539. doi:10.1155/2014/908539
28. Cocate PG, Natali AJ, Oliveira A, et al. Fruit and vegetable intake and related nutrients are associated with oxidative stress markers in middle-aged men. Nutrition. 2014;30(6):660–665. doi:10.1016/j.nut.2013.10.015
29. Burgos Alves MI, Avilés Plaza F, Martínez-Tomás R, et al. Oxidized LDL and its correlation with lipid profile and oxidative stress biomarkers in young healthy Spanish subjects. J Physiol Biochem. 2010;66:3. doi:10.1007/s13105-010-0028-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
