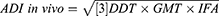Back to Journals » Journal of Experimental Pharmacology » Volume 13
Investigation of Antibacterial and Anti-Diarrhoeal Activities of 80% Methanol Leaf and Fruit Extract of Leonotis ocymifolia (Burm. F) Iwarsson (Lamiaceae)
Authors Mengie Ayele T , Chekol Abebe E , Bogale Kassie A
Received 18 May 2021
Accepted for publication 13 June 2021
Published 29 June 2021 Volume 2021:13 Pages 613—626
DOI https://doi.org/10.2147/JEP.S319981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Paola Rogliani
This paper has been retracted.
Teklie Mengie Ayele,1 Endeshaw Chekol Abebe,2 Achenef Bogale Kassie1
1Department of Pharmacy, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia; 2Departments of Biochemistry, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Teklie Mengie Ayele Tel +251 910111531
Email [email protected]
Background: Leonotis ocymifolia (Burm.F) Iwarsson (Lamiaceae) is among the medicinal plants that are claimed to have various pharmacologic activities. However the leaves and fruits of L. ocymifolia have not yet been explored scientifically for antibacterial and anti-diarrhoeal activities. This study was aimed at investigating the anti-diarrhoeal and antibacterial activities of 80% methanol leaf and fruit extract of Leonotis ocymifolia in mice and selected diarrhea causing bacterial species.
Methods: The leaves and fruits of Leonotis ocymifolia were extracted using 80% methanol through maceration technique. The anti-diarrheal activity was evaluated using a castor oil induced diarrheal model, prostaglandin induced anti-enteropooling, and castor oil induced charcoal meal test in mice of either sex. Data were analyzed using one-way analysis of variance followed by Tukey post-hoc test. The antibacterial activity was evaluated on using an agar well diffusion assay. Bacterial species used were Salmonella typhi, Salmonella paratyphi, Salmonella typhimurium, Shigella species, Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. For anti-diarrhoeal activity, the extract was tested at 100, 200, and 400 mg/kg. Positive and negative control groups were treated with loperamide (3 mg/kg) and 2% tween 80 (10 mL/kg), respectively.
Results: A significant (p< 0.05) reduction in frequency of wet stools and watery content of diarrhea as well as in delaying onset of diarrhea as compared to controls was observed in mice at the stated doses. The extract showed a dose-dependent inhibition in all used models. L. ocymifolia leaf and fruit extract also showed antimicrobial activity against all tested organisms.
Conclusion: Results from this study collectively indicated that 80% methanol leaf and fruit extracts of L. ocymifolia possessed significant anti-diarrhoeal activity and antibacterial activities, hence provides the scientific base for its traditional use as a diarrhea treatment.
Keywords: anti-diarrhoeal, antibacterial, castor oil induced diarrhea, gastrointestinal transit, anti-enteropooling, L. ocymifolia
Introduction
Definition and Classification
Diarrheal diseases are a leading cause of childhood morbidity and mortality in developing nations and an important cause of malnutrition.1 It is a common symptom of gastrointestinal infections which can be caused by a wide range of pathogens. There are numerous agents which cause diarrhea. Among these, bacteria covers the majority of the causes.2 Fungal infections have also been recognized to cause diarrhea in humans.3
Even though the availability of variety of approaches for diarrhea management, the majority of people in developing countries depends on herbal drugs for the management of diarrhea. The World Health Organization has encouraged studies for treatment and prevention of diarrheal diseases depending on traditional medical practices.4 The recognition of traditional medicine as an alternative and complementary medicine and the emerging of microbial resistance to the existing antibiotics have led scientists to explore the antimicrobial activity of medicinal plants. Herbal extracts containing secondary metabolites have been investigated to have antimicrobial activity. The effort towards evaluation and use of herbal medicine for a diarrheal disease continued to be an important preventive strategy, particularly in developing countries.5
In developing nations, the majority of people entirely use traditional medicines in treating a variety of diseases, including diarrhea. Searching plants having antidiarrheal claims that could be used against any type of diarrheal disease is therefore interesting. A variety of herbal medicines with anti-diarrheal and antimicrobial properties have been extensively utilized by herbalists. However, their healing properties have not been scientifically investigated.6 In Ethiopia, there is Leonotis ocymifolia (Burm. F) Iwarsson (Lamiaceae), commonly known as Ras-kimir or Yeferes Zeng. Traditionally, it is used for the treatment of headache and ulcers of the neck and swelling.7 Furthermore, L.ocymifolia also has various pharmacologic activities that have been explored scientifically. For instance, the various L.ocymifolia parts investigated include; anthelmintic (aerial part),8 antimicrobial (aerial part, flower, leaf),9–11 analgesic and anti-inflammatory activities (leaf).7
Moreover, the various solvent fractions of leaf extract of L.ocymifolia of showed the presence of a variety of secondary metabolites,12 such as labdane diterpenoids13 and essential oils.11
There are numerous plants used for the treatment of diarrhea, and L.ocymifolia is one of them. By tradition, L.ocymifolia dried leaf and fruit mixed with honey is given orally for the treatment of diarrhea. However this plant has not yet been explored scientifically for antibacterial and anti-diarrheal activities. Thus, it is necessary to establish the scientific basis for the antibacterial and anti-diarrheal action of L.ocymifolia as this may serve as the source for the advance of more effective drugs.
The objective of this study was to investigate the possible anti-diarrheal and antimicrobial properties of the leaves and fruits extract of L.ocymifolia in order to establish its claimed biological activity.
Materials and Methods
Drugs and Chemicals
Castor oil (Amman Pharmaceutical Industries, Jordan), activated charcoal (Acuro Organics Ltd, New Delhi), loperamide hydrochloride (Medochemie Ltd, Cyprus (EU)), misoprostol (Mylan Laboratories Ltd., India), distilled water (department of Chemistry department of Debre Tabor University), methanol (Blulux, India), petroleum ether (Carlo Erba Regents S.A.S, Italy), McFarland standard (Remel, Lenexa Kansas 66215, USA), Brain Heart Infusion (BHI) (Difco Laboratories, Detroit Michigan, USA), ciprofloxacin disk 5mcg (Ecton DickinsonPty Ltd, Australia), Mueller Hinton agar (Himedia laboratories Pvt Ltd, India), Muller Hinton broth (Himedia laboratories Pvt Ltd, India) were used in this study. All reagents were of analytical grade.
Plant Materials
The leaves and fruits of L.ocymifolia (Burm.F) (Lamiaceae) (Figure 1) were collected from Libo kemkem woreda, Addis Zemen, South Gondar, Amhara regional state. After collecting the plants, identification and authentication of the plants specimens was done by taxonomists at the Department of Biology, College of Natural Sciences and Computation, Debre Tabor University and the voucher number TM001/2021 with specimens was deposited for future reference.
 |
Figure 1 Pictures of Leonotis ocymifolia from the site of collection (A) before collection; (B) after collected. |
Experimental Animals
Mice of either sex (20–30 g) were obtained from the Animal House Unit of Ethiopian Public Health Institute, Addis Ababa. The animals were housed in polypropylene cages under standard environmental conditions on a 12 hour light–dark cycle with free access to pellet food and water ad libitum. The animals were acclimatized for a week before beginning the actual experiment. All experiments were conducted during the light period. All procedures and techniques used in this experiment were carried out in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.14
Test Strains
Bacterial species used for this study were S. typhi, S. paratyphi, S. typhimurium, Shigella, P. aeruginosa, S. aureus, and E. coli., and were obtained from the Microbiology Department, Addis Ababa University.
Preparation of 80% Methanol Extract
The dried leaves and fruits of L.ocymifolia were initially washed using distilled water to remove dust materials. It was then ground by using a grinder and powdered coarsely using a mortar and pestle prior to extraction. The powdered L.ocymifolia leaf and fruit was extracted using 80% methanol through cold maceration. One hundred grams of coarse powder L.ocymifolia was macerated with 1 Liter of 80% methanol at room temperature for 3 days. For proper mixing, the plant material was shaken with the solvent continuously on a horizontal orbit shaker. The mixture was then filtered by using Whatman No. 1 filter paper and the residue was then re-macerated 2-times with fresh solvent to thoroughly extract the L.ocymifolia. The organic solvent was then removed from the extract via a Rota-vapor. The extract was then lyophilized to remove the water residual. The percentage yield of dried 80% methanol and fruit extract of L.ocymifolia was found to be 15.5%. Lastly, the dried extract was stored at −20°C and was reconstituted with 2% tween 80.
Acute Toxicity Test
Initially, the test was done based on the limit test recommendations of the Organization for Economic Cooperation and Development (OECD) 425 Guideline (OECD, 2008). First, a sighting study was performed to determine the starting dose, in which a single female mouse for each fraction was given 2,000 mg/kg of the respective fraction as a single dose using oral gavage. Since no death was observed within 24 hours, an additional four female mice were used for each of the fractions, and administered the same dose of fractions. The mice were observed continuously for 4 hours at 30 minute gaps and after that for 14 successive days at an interval of 24 hours for the general signs and symptoms of physical and behavioral toxicities. After an acute toxicity test, three dose levels were selected. These were a middle dose, which is one-tenth of the maximum dose obtained during an acute toxicity study; a low dose, which is half of the middle dose; and a high dose, which is twice of the middle dose.
Grouping and Dosing
Mice of either sex (weighing 20–30 g) were arbitrarily grouped into five groups (six mice per group) and were fasted for 18 hours before the commencement of the experiment with free access to water. Group I was assigned as the negative control and provided an intervention of 10 mL/kg 2% tween 80. Group II was assigned as a standard (positive control) and treated with Loperamide (3 mg/kg). Groups III, IV, and V were given 100, 200, and 400 mg/kg of methanol leaf and fruit extract of L.ocymifolia orally. Misoprostol, a PGE2 analog, was considered in an entropooling model to induce diarrhea, while castor oil is replaced in the remaining models for the same function. All doses were administered orally.
Phytochemical Screening of the Extract
Preliminary phytochemical screening of secondary metabolites of 80% methanol leaf and fruit extract of L.ocymifolia was carried out using standard tests.15,16
Test for Saponins
To 0.25 g of 80% methanol leaf and fruit extract of L.ocymifolia, 5 mL of distilled water was added. Then, the solution was shaken vigorously and observed for a stable persistent froth. Formation of a stable froth that persists for about half an hour indicated the presence of saponins.
Test for Terpenoids
To 0.25 g of 80% methanol leaf and fruit extract of L.ocymifolia, 2 mL of chloroform was added. Then, 3 mL of concentrated sulfuric acid was carefully added to form a layer. A reddish brown coloration of the interface indicated the presence of terpenoids.
Test for Tannins
About 0.25 g of 80% methanol leaf and fruit extract of L.ocymifolia was boiled in 10 mL of water in a test tube and then filtered with filter paper (Whatman No. 1). A few drops of 0.1% ferric chloride were added to the filtrate. A brownish green or a blue-black precipitate indicated the presence of tannins.
Test for Flavonoids
About 10 mL of ethyl acetate was added to 0.2 g of 80% methanol leaf and fruit extract of L.ocymifolia, and heated in a water bath for 3 minutes. The mixture was cooled and filtered. Then, about 4 mL of the filtrate was taken and shaken with 1 mL of dilute ammonia solution. The layers were allowed to separate and the yellow color in the ammonia layer indicated the presence of flavonoids.
Test for Cardiac Glycosides
To 0.25 g of 80% methanol leaf and fruit extract of L.ocymifolia, diluted with 5 mL of water, 2 mL of glacial acetic acid containing one drop of ferric chloride solution was added. This was underlayed with 1 mL of concentrated sulfuric acid. A brown ring at the interface indicated the presence of a deoxysugar characteristic of cardenolides.
Test for Steroids
Two milliliters of acetic anhydride was added to 0.25 g of 80% methanol leaf and fruit extract of L.ocymifolia with 2 mL sulfuric acid. The color change from violet to blue or green in some samples indicated the presence of steroids.
Test for Alkaloids
Then 0.5 g each of 80% methanol leaf and fruit extract of L.ocymifolia was taken and a few drops of freshly prepared Mayer’s reagent were added. The formation of cream was taken as positive for the presence of alkaloids.
Determination of Anti-Diarrheal Activity
Castor Oil-Induced Diarrhea
Anti-diarrheal activity of 80% methanol leaf and fruit extract of L.ocymifolia was investigated with the castor oil-induced diarrheal model in mice mentioned by Umer et al.17 Thirty mice of both sexes were randomly grouped into five groups (six mice/group) and fasted overnight.18 Mice were dosed as described in the Grouping and Dosing section. One hour after dosing, 0.5 mL of castor oil was administered to each mouse orally. The mice were then kept separately in the cage, the base of which was wrinkled with white paper for examination of the number and consistency of fecal droppings. The papers were changed every l hour to make the fecal droppings able to be seen for counting and to make sure of stool consistency. Diarrhea was graded as follows: Normal pelleted feces (0), discrete soft-formed feces (1), soft-formed feces (2), soft watery stool (3), and watery stool with little solid matter (4).18
The mice were followed for the duration of 4 hours, in which there was the onset of diarrhea, the quantity of both dry and wet fecal matter excreted by the mice, was counted and compared with the negative and positive controls for investigating the antidiarrheal activity of 80% methanol and fruit extract of L.ocymifolia. The onset was considered as the time gap in minutes between the running of castor oil and the emergence of the initial fecal matter. The total amount of fecal matter for the negative control was taken as 100% and the percentage of diarrheal inhibition for wet and watery content of feces was determined via the following formula:
where AWFC=average weight of the fecal matter of controls and AWFT=average weight of fecal matter of test groups.18
Prostaglandin (PGE2)-Induced Enteropooling
In this technique, prostaglandin served as a diarrhea producing agent. Thirty mice of both sexes were randomly assigned into five groups (six mice per group) and used after overnight fasting.18
Mice were dosed as mentioned in the Grouping and Dosing section. Misoprostol was administered 1 hour after dosing. Then, 1 hour after administration of 100 µg/kg of PGE2, all mice were euthanized by means of cervical dislocation, and the small intestine with collected fluid was ligated both at the pyloric sphincter and at the ileocecal junctions and dissected out. The tied intestine was weighed (m1); the content was emptied and measured using a graduated cylinder. Then, emptied intestine was weighed (m0) and a difference between the empty and intact intestine was used to calculate the percentage inhibition of intestinal secretion compared with the control group using the following formula:
where A=average volume or weight of intestine in the control group and B=average volume or weight of intestine in the test groups.18
The volume of the intestinal content was read from the graduated measuring cylinder, whereas the weight was recorded as (m1-m0) g.
Charcoal Meal Test in Normal Mice
The experimental procedure described by Bahekar and Kale19 was used for the present study with slight modification. Thirty mice of both sexes at random were split into five groups (six mice per group) and fasted for 18 hours prior to commencement of the study. However, the mice had free access to water. One hour after dosing, as described in the Grouping and Dosing section, 1 mL of freshly prepared 10% activated charcoal suspension in 2% tween 80 was administered for each mouse orally. The mice were euthanized 1 hour after charcoal administration. The abdomen was opened up and the section from the pylorus to caecum was removed. The distance covered by the charcoal, from the section of pylorus to caecum was considered and described as the percentage of distance covered by the following formula:
where T0=total length of intestine and
T1=distance traveled by charcoal in the intestine.
Charcoal Meal Test Following Induction of Diarrhea
The effect of the L.ocymifolia leaf and fruit extract on gastrointestinal motility was evaluated as mentioned by Umer et al17 with little change. Thirty mice of both sexes at random were assigned into five groups (six mice per group) and used following overnight fasting.18 One hour after a dosing, 0.5 mL of castor oil was given to each animal orally. After an hour of castor oil administration, all mice were given 1 mL of 10% charcoal suspension orally and euthanized after 30 minutes. The small intestine was dissected out and the distance covered by charcoal from a section of the pylorus to caecum was considered and expressed as a percentage of the total distance of the small intestine. The intestine of each mouse was kept in formalin to hold peristalsis and then washed in distilled water before measuring the distance covered by the charcoal. Charcoal movement was expressed as a peristaltic index (PI) as follows:
where A=distance covered by charcoal and B=length of the full intestine. Percentage inhibition was determined as follow:
where APIC=average PI of control and APIT=average PI of the test group.18
In vivo Anti-Diarrheal Index (ADI)
The ADI of treatment groups was calculated from the data obtained via all anti-diarrheal models used in the present study by using the formula described below.20
where
DDT is the delay in defecation time (as % of control),
GMT is the gastrointestinal motility by decrease in charcoal travel (as a % of control), and
IFA is the decrease in the intestinal fluid accumulation (as % of control).
Antimicrobial Activity
Inoculums Preparation and Standardization
The bacteria were selected based on availability and considering the likely bacterial strains that can cause diarrhea for which L.ocymifolia is indicated traditionally. Nutrient agar was set by using the manufacturer’s procedure. After cooling of the culture medium at 45°C, it was poured into a prelabeled sterile petri dish and given time for congealing of the agar. The test bacteria were then inoculated and spread on the prepared agar with an inoculating wire loop following aseptic condition and incubated for 24 hours at 37°C.
The bacterial turbidity of every bacterium was set and standardized as described by Chikezie.21 The bacterial suspension in a broth was set by the growth method as follows. After preparing nutrient broth in distilled water, 5 mL of the broth was added to test tubes and sterilized. Isolated colonies of similar morphology of every bacterium from three-to-five wells were picked up by wire loop from fresh agar plates of bacterial culture and aseptically transferred into pre-labeled test tubes containing the sterile nutrient broth and incubated for about 6 hours. The inoculum tube was adjusted visually by either adding bacterial colonies or by adding sterile normal saline solution to that of the already prepared 0.5 McFarland standard which is assumed to contain a bacterial concentration of 1×108 colony forming unit (CFU)/mL. The adjustment and comparison of turbidity of inoculum tube and that of 0.5 McFarland standard was performed by visually observing them with the naked eye against a 0.5 McFarland turbidity equivalence standard card with white background and contrasting black lines in the presence of adequate light.
Determination of Minimum Inhibitory Concentration (MIC)
The extract of L.ocymifolia that showed antibacterial activity by agar well diffusion method were subjected to serial micro broth dilution technique to determine MIC as described by previous study reports.22,23 Successive dilutions were set from 1,000 mg/mL of the L.ocymifolia extract using distilled water to make 1,000, 500, 250, 125, 62.5, 31.25, and 15.625 mg/mL. The wells were inoculated with 0.1 mL aliquot of test bacteria (108 colony forming unit (CFU/mL) having serial dilutions of the L.ocymifolia extract 50 μL, each). The plate was incubated at 37±1°C for 24 hours. Dilution of the L.ocymifolia extract equivalent to respective test bacteria showing no visible growth was considered as MIC.
Determination of Minimum Bactericidal Concentration (MBC)
The lowest concentration by which bacterial growth was not observed is called MBC. This was determined by aseptically subculturing the contents of wells from the MIC results for each bacteria to antimicrobial free agar, as described in different study reports.23–25 In this technique, the contents of all wells containing a concentration of test material above the MIC value from each triplicate, in the MIC determination test, was streaked using a sterile wire loop on Mueller Hinton Agar aseptically and incubated at 37°C for 24 hours. The lowest concentrations of L.ocymifolia extract which showed no bacterial growth after incubation was observed for each triplicate and noted as the MBC. The average value was considered for the MBC of test material against every bacterium.
Statistical Analysis
Results are expressed as mean±standard error of the mean (SEM). The experimental results of the present study were analyzed using the software Statistical Package for Social Sciences (SPSS), version 20 and statistical significance was determined by one way analysis of variance (ANOVA) followed by Tukey Kramer post Hoc test. A P-value of less than 0.05 was considered as statistically significant. The analyzed data was presented using tables and figures.
Results
Phytochemical Screening
Phytochemical screening of the 80% methanol leaf and fruit extract of L.ocymifolia revealed the presence of alkaloids, tannins, flavonoids and saponins (Table 8).
 |
Table 1 The Effect of 80% Methanol Leaf and Fruit Extract of L.ocymifolia on Castor Oil Induced Diarrhea Model in Mice |
 |
Table 2 The Effect of 80% Methanol Leaf and Fruit Extract of L.ocymifolia on Prostaglandin Induced Entropooling in Mice |
 |
Table 3 The Effect of 80% Methanol Leaf and Fruit Extract of L.ocymifolia on Castor Oil Induced Gastrointestinal Transit in Mice |
 |
Table 4 The Effect of 80% Methanol Leaf and Fruit Extract of L.ocymifolia on Normal Gastrointestinal Transit in Mice |
 |
Table 5 In-vivo ADI of 80% Methanol Leaf and Fruit Extract of L.ocymifolia |
 |
Table 6 Antimicrobial Effect of 80% Methanol Leaf and Fruit Extract of L.ocymifolia and Ciprofloxacin Using Disk Diffusion Techniques |
 |
Table 7 Antimicrobial Effect of 80% Methanol Leaf and Fruit Extract of L.ocymifolia Using Micro-Dilution Techniques |
 |
Table 8 Preliminary Phytochemical Screening of 80% Methanol Leaf and Fruit Extract of L.ocymifolia |
Acute Toxicity Test
The acute toxicity study of 80% methanol leaf and fruit extract of L.ocymifolia indicates that physical and behavioral changes or mortality were not observed within 24 hours and for the next 14 days. Along with the “Limit Test” of OECD guideline 425,26 the oral LD50 of L.ocymifolia extract was greater than 2,000 mg/kg in mice. Subsequently, the 100, 200, and 400 mg/kg doses of L.ocymifolia extract were determined and used for the experiment.
The Effect of L.ocymifolia Extract on Castor Oil- Induced Diarrhea
During the 4-hour observation period, all animals in the control group had either wet stool or watery diarrhea. The 80% leaf and fruit extract of L.ocymifolia produced a significant effect (p<0.001) on the onset only at 400 mg/kg. The L.ocymifolia extract, at all dose levels, was able to significantly reduce the frequency of diarrhea (p<0.001) (Table 1). Moreover, L.ocymifolia extract delayed the onset of diarrhea (R2=1.00) and reduced the number of occurrences of defecation (R2=0.893) dose dependently as compared to the negative control. The percentage of inhibition for the total weight of wet stool as well as watery content of stool relative to negative controls was determined. The data showed that, all other doses of L.ocymifolia extract produced a significant decrease both in the total weight of wet and watery content of the stool compared to negative control (p<0.05). Otherwise, there was no detectable difference between standard and extracts as well as among various doses of L.ocymifolia extract (Table 1).
The Effect of L.ocymifolia Extract on Prostaglandin Induce Enteropooling
The percentage inhibition fluid accumulation by 80% methanol leaf and fruit extract of L.ocymifolia was 39.62%, 52.83%, and 62.26%, for 100, 200, and 400 mg/kg doses, respectively (Table 2). The anti-secretory effect of the extract increased with dose (R2=0.92). The extract also showed a significant reduction for both average weight and volume of small intestine content at all doses (p<0.05). However, there was no a significant difference in terms of volume of intestinal fluid and weight of intestinal contents when all doses of the extract were compared with the standard drug.
The Effect of L.ocymifolia Extract on Castor Oil Induced Gastrointestinal Propulsion
The 80% methanol leaf and fruit extract of L.ocymifolia exhibited a significant anti-motility effect against castor oil induced diarrhea compared to negative control (p<0.001) (Table 3). The intestinal transit of charcoal was inhibited at all doses of L.ocymifolia extract, with the maximum effect observed at a higher dose (61.1%). The effect was dose dependent, (R2=0.861). The higher effect in standard drug exhibited significantly compared to negative control as well as in the lower dose of the extract (p<0.001).
The Effect of L.ocymifolia Extract on Normal Gastrointestinal Transit in Mice
The 80% methanol leaf and fruit extract of L.ocymifolia tended to decrease the intestinal transit of the charcoal through the GI compared to negative control group (Table 4). The percentage of inhibition by L.ocymifolia extract as well as the standard was under 30%, indicating the transit time for charcoal was shorter in normal than castor oil treated animals. However, the effect was dose dependent (R2=0.81). The inhibition obtained with the standard drug, however, was significantly greater than the control group (29.6%, p<0.05).
The In-Vivo Anti-Diarrheal Index
The ADI for the different doses of the extract is presented in Table 5.
Antimicrobial Activity
The 80% methanol leaf and fruit extract of L.ocymifolia inhibited the growth of all the bacterial species used at different extents. However, ciprofloxacin at a concentration of 1 mg/mL entirely inhibited the growth of all the bacterial species except Shigella spps (Table 6). The L.ocymifolia extract showed highest activity against S. typhi and E. coli among the tested microorganisms (Table 7).
Discussion
The present study was conducted to investigate the anti-diarrheal and antibacterial activity of 80% methanol leaf and fruit extract of L.ocymifolia in mice and selected bacterial strains, respectively, and the probable underlying mechanism. The results showed that the plant possesses anti-diarrheal and antibacterial activity in the models used.
Numerous mechanisms are used to elucidate the diarrheal effect of castor oil. These include stimulating the release of inflammatory mediators through preventing reabsorption of NaCl and water,27 and inhibiting Na+/K+-ATPase activity via decreasing normal fluid absorption.28 This model embraces both secretory and abnormal motility diarrhea.29 Therefore, the use of such as agent as a diarrhea inducer is plausible as it mimics the abnormal processes and allows for the examination of quantifiable changes in the number of fecal matter, intestinal transit, and enteropooling.
Administering a synthetic PG analog (misoprostol) directly is also another option. Among the physiological compounds that are known to disturb the motility of the GI tract, PGs are the major ones. PGE2 induces diarrhea by inhibiting absorption of glucose, thus resulting in accumulation of fluid in the intestinal lumen. PGE2 agonists act on PG receptors coupled to G-protein that makes use of inositol triphosphate (IP3), diacylglycerol (DAG), or cyclic adenosine monophosphate (cAMP) transducer mechanism. Activation of E-type prostanoid receptor-1 (EP1) causes contraction of smooth muscles via IP3, DAG, or cAMP, resulting in secretion of water and electrolytes. In this regard, agents that have the potential to inhibit the activity of PGs could be useful in preventing the enteropooling effect of PGE2.18
A study conducted by Riviere et al30 testing diarrhea inducing abilities of PGE2 confirmed its dose- and time-dependent effect. The study also demonstrated that PGE2 at a dose of 200 µg/kg also produces fluid accumulation in the small intestine, which results in a condition known as enteropooling. However, based on the study, PGE2 treatment did not altergastric emptying and GI propulsion. Due to this evidence PG was used only for the testing of enteropooling effect. Additionally, this smooth muscle stimulating action of PGs has been shown to be blocked by loperamide in several laboratory animals.31
Loperamide hydrochloride (the standard drug) not only regulates the GI tract, but also slows down the peristalsis across the small intestine. Nowadays, loperamide is widely used in a different diarrheal model to investigate the anti-diarrheal activities of various experimental plants. This is because of its documented antisecretory and antimotility properties.32
In the present study, 80% methanol leaf and fruit extracts of L.ocymifolia exhibited anti-diarrheal activity via significant reduction in both castor oil and PG induced diarrhea in the entire models used. The most likely reason could be the presence of phytochemicals in the L.ocymifolia extract (Table 8). Both flavonoids and phenolic compounds having antioxidant properties33 appear to be responsible for the antidiarrheal effect.34 These phytochemicals might act through enzymatic inhibition, possibly via blocking the arachidonic acid metabolism, thus reducing PG induced fluid secretion.35 In addition to these, phytochemical constituents like tannins and saponins are also endowed with anti-diarrheal activity.36
In the present study, significant reduction (p<0.05) in the number and weight of both wet and watery content of fecal matter as well as delayed onset of diarrhea was observed. The effect was increased dose-dependently. This is similar to other studies of various plants wherein extracts of these plants are revealed to exert an antidiarrheal effect dose dependently.37
The significant decrease in frequency of fecal out (number of wet stools), weight of wet and watery content of stools signifies the efficacy of 80% methanol leaf and fruit extract of L.ocymifolia as an antidiarrheal agent. This finding is supported by previous claims about anti-diarrheal plants. Antidiarrheal plants are identified in reducing the number of wet fecal matter, as reported for Eremomastax speciosa and Xylocarpus granatum.38,39 Castor oil produces diarrhea by inhibiting fluid and electrolyte absorption, thus resulting in intestinal peristalsis.40 One of the possible mechanisms of anti-diarrheal activity of the test leaf and fruit extract of L.ocymifolia might be due to the capability of facilitate fluid and electrolyte absorption through the GI tract.
Moreover, significantly (p<0.05) delayed induction of diarrhea, reduced frequency of fecal matter (number of wet feces) following the administration of the L.ocymifolia extract imply its antidiarrheal activity at all stated doses. This finding was further supported with the increased inhibition of fecal output. The comparable percentage of inhibition of fecal output at 400 mg/kg dose of the L.ocymifolia extract with the standard drug suggests that the L.ocymifolia has a promising effect and may serve as an alternative agent in the future. The L.ocymifolia extract might have exerted its anti-diarrheal activity via an antisecretory mechanism as evident from reduction in the total number of fecal matter, both wet and watery content. Furthermore, this anti-diarrheal activity might be due to the inhibitory activity of the L.ocymifolia extract on PGs synthesis, nitric oxide (NO), and platelet activating factors production, as these modes of action are known to delay diarrhea induced by castor oil.41–43 Furthermore, studies reported that an increase in the sodium-potassium ATPase (Na+K+ATPase) activity and decreased nitric oxide (NO) content in the small intestine was observed and proposed this could be the possible mechanism of anti-diarrheal action of medicinal plants.44,45
Percentage inhibition of diarrhea calculated as a function of weight of watery content of diarrhea is higher than that of weight of wet stool diarrhea in 80% methanol leaf and fruit extract treated mice. This indicates that the most probable mechanisms of the plant extracts are increasing absorption or decreasing secretion or both, of fluid and electrolytes. This is a salient point since this nature of the plant may fill the less proabsorptive and antisecretory property of the standard drug, loperamide.
For further evaluation of the mode of anti-diarrheal action, the study was extended to determine its anti-enteropooling effect. In PG induced enteropooling, the 80% methanol leaf and fruit extract of L.ocymifolia significantly blocked the intestinal fluid collection and weight of intestinal content at all levels of the tested doses as compared to the negative control. The effect of the extract against PG induced fluid accumulation is comparable. It may be due to liable active metabolites which inhibit fluid accumulation in L.ocymifolia extract. This finding further strengthens that the plant extract has a dose-dependent anti-enteropooling effect. This effect of L.ocymifolia might be credited to the existence of secondary metabolites such as terpenoids, steroids, flavonoids, and tannins. Terpenoids,46 flavonoids,47 and steroids48 have been shown to inhibit production of PGE2, which had a critical role in the activation of intestinal secretions through causing secretion of water and electrolytes.49 Tannins reduce fluid discharge through inhibition of CFTR and CaCC, via generating a protein-precipitating reaction to the GI mucosa,50 which make the mucosa more resistant to chemical alteration.35,43
Both parasympathetic and sympathetic systems extrinsically innervate the small intestine.51 Para-sympathetic systems activate intestinal homeostasis by making use of neurotransmitters such as acetylcholine and vasoactive intestinal peptides (VIP), while a sympathetic one stimulates intestinal absorption through α2 adrenergic agents such as enkephalins and somatostatins. Phytochemicals such as flavonoids from herbal origin might activate α2 adrenoreceptors in the absorptive cells of the GI tract.52 Besides regulating electrolyte movement, fluid transport across the epithelium of the GI tract is also controlled by managing aquaporin (AQP) type water channels. Tannins were found to inhibit specific AQPs expressions via down-regulating the various kinases. Particularly, AQP down-regulates the protein kinase signal pathway, which partially accounts for the anti-secretory and hence anti-diarrheal effects.53 Therefore, anti-secretory activity of L.ocymifolia could probably be due to the presence and synergistic effects of phytochemicals. Keeping this in mind, in this study, the L.ocymifolia extract more likely decreases diarrhea by either stimulating reabsorption of fluid and electrolytes through sympathetic activation or by blocking the fluid secretion into the intestine by altering parasympathetic activity.
Increasing intestinal motility is one way of increasing formation of diarrhea. To investigate the antimotility activity of the L.ocymifolia extract, the study was performed by using charcoal meal as a marker. GI motility is primarily modulated by the sympathetic and parasympathetic nerves, with the latter considered as the major factor. Escalating activation of the parasympathetic nerves enhances intestinal transit, while increasing stimulation of the sympathetic nerves inhibits it.51 Loperamide, which was used as a standard drug, was known to suppress movement of the charcoal meal due to its anticholinergic, antihistamine and PG blocking effects.54 Beside cholinergic receptors, stimulation of α2 adrenoreceptors in the GI tract is capable of inhibiting peristalsis, decreasing GI smooth muscle contraction, improving gastric emptying, and encouraging protection of stomach mucosa.55,56 The reduction in distance traveled might be used as a tool to explain the intestinal smooth muscle relaxation. From previous knowledge, contractions of all smooth muscles absolutely depend on the presence of Ca2+ which activates the contractile elements and their relaxation, a mechanism drawn in the antidiarrheal effect of different drugs. Therefore, the L.ocymifolia could have caused the reduction in distance covered by the charcoal through increasing the intracellular Ca2+ release.
In the present study, the charcoal meal test showed that the graded doses 80% methanol leaf and fruit extract significantly reduced intestinal propulsive movement in castor oil induced intestinal transit as compared to the negative control. The inhibitory effect of the L.ocymifolia extract in castor oil induced intestinal transit was greater as compared to that of the normal intestinal transit. According to the literature, drugs with anti-diarrheal effects are renowned for stimulating GI relaxation and thereby slowing the emptying time,57 allowing more time for better absorption fluids.35,58 The observed effect is therefore possibly due to the extracts’ ability to inhibit the intestinal movement, which in turn accounts for the anti-diarrheal effect of the extract of L.ocymifolia. In other words, the more the intestinal motility the greater would be the inhibitory effect of the extracts. The importance of this finding should not be underestimated since the related development of constipation, a major problem of most conventional drugs, including loperamide, as a side-effect would be lower. The inhibitory effect on the intestinal transit seems comparable for L.ocymifolia extract could be attributed to the presence of phytochemicals that are responsible for anti-motility effect of the L.ocymifolia.
According to reports tannins reduce the intracellular Ca2+ inward current or by stimulation of the calcium channel, which induces the muscle relaxation, ascribed for their spasmolytic and calcium channel blocking activities resulting inhibition of intestinal peristalsis.35,59 Flavonoids are also known to have the same antimotility mechanism,60 through relaxing intestinal smooth muscles35,61 while terpenoids, on the other hand, were reported to inhibit intestinal motility through inhibition of the release of autacoids.35 Furthermore, anticholinergic drugs are known to slow down GI hyper-motility, as indicated earlier. Therefore, it is probable that the observed antimotility effect of the L.ocymifolia extract might be due to an interaction with acetylcholine activity.
The ADI is a means to quantify the pooled effects of different parameters of diarrhea such as reduction in GI motility, onset of diarrheal stools, and fluid accumulation.35 As indicated in the literature, the larger the ADI value, the better the efficacy of the extract in curing diarrhea.50 The ADI value further corroborated that 80% methanol leaf and fruit extract of L.ocymifolia comparable to antidiarrheal activity with the standard drug.
The 80% methanol leaf and fruit extract of L.ocymifolia showed a broad spectrum of antibacterial activity. Results from the present study showed that L.ocymifolia extract inhibited the growth of all pathogenic bacteria species tested moderately (Table 6). Increased inhibition was found against E. coli and Shigella spp. MICs of L.ocymifolia extract is described in Table 7. The phytochemical screening of L.ocymifolia showed the existence of a number of phytochemicals which is summarized in Table 8. The anti-microbial effect of L.ocymifolia extract might be as a result of the existence of these phytochemicals. In addition to the above-mentioned antidiarrheal mechanisms, tannins and flavonoids are generally reported to have anti-diarrheal activity through antimicrobial action.17 Several reports support the present study. For instance, a study conducted in Tanzania reported that from essential oils isolated from leaves of Leonotis ocymifolia (Burm. F.) lwarsson var. raineriana showed significant antimicrobial activity.11 Another study conducted in Eastern Cape, South Africa also reported that essential oils of the leaf and flower of L. leonurus and L. ocymifolia exhibited broad spectrum antibacterial activity against gram-positive and Gram-negative bacteria.9
Moreover, a study conducted in Ethiopia has reported that essential oils extracted from L.ocymifolia showed trypanocidal activity.62 Furthermore, the hydro alcoholic extract of the aerial part of L.ocymifolia exhibited antibacterial activity against the tested organism.10
In conclusion, the results from the present study suggest that the 80% methanol leaf and fruit extract of L.ocymifolia has significant anti-diarrheal activity, probably related to its pro-absorptive, antisecretory, and anti-motility effects. Moreover, L.ocymifolia has an appreciable antimicrobial effect, ruling out this activity as a possible mechanism. This may be associated with the presence of secondary metabolites in the hydro alcoholic extract of L.ocymifolia.
Abbreviations
ADI, antidiarrheal index; cAMP, cyclic adenosine mono phosphate; AQPs, aqua porins; CFU, colony forming unit; DAG, diacyl glycerol; EP-1, E-type prostanoid receptor-1; GI, gastrointestinal; IP3, inositol triphosphate; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; PGE-2, prostaglandin E-2; PG, prostaglandins; OECD, organization for economic cooperation and development; VIP, Vasoactive intestinal peptide.
Ethics Approval
Ethical clearance and permission was obtained from Debre Tabor University Research and Ethical Review Committee. National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Acknowledgments
We are very grateful to Debre Tabor University for funding the study.
Author Contributions
All authors made a significant contribution to the work reported, that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
Debre Tabor University provided all the required resources for conducting this research project.
Disclosure
The authors have no conflicts of interest to disclose.
References
1. World Health Organization. Diarrheal disease. [Online]; 2013. Available from: http://www.who.int/mediacentre/factsheets/fs330/en/.
2. Toyin Y, Khadijat O, Saoban S, et al. Antidiarrheal activity of aqueous leaf extract of Ceratotheca sesamoides in rats. Bangladesh J Pharmacol. 2012;7:14–20.
3. Robert K, Egon S, Daniela B, et al. Role of Candida in antibiotic-associated diarrhoea. J Infect Dis. 2001;184(8):1065–1069. doi:10.1086/323550
4. Atta AHMS, Mouneir SM. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. J Ethnopharmacol. 2004;92(2–3):303–309. doi:10.1016/j.jep.2004.03.017
5. Latha LSRP. Antimicrobial, antidiarrhoeal and analysis of phytochemical constituents of of Sphaeranthus amaranthoides. Indian J Sci Technol. 2009;2(3):45–48. doi:10.17485/ijst/2009/v2i3.3
6. Chitme HR, Pradesh U, Chandra R, Kaushik S. Studies on anti-diarrheal activity of. Group. J Pharm Pharmaceu Sci. 2004;7(1):70–75.
7. Alemu A Evaluation of the in vivo analgesic and anti-inflammatory activities of 80 % methanol extract of Leonotis ocymifolia (Burm. F.) Iwarsson leaves Asnakech Alemu A Thesis submitted to The department of pharmacology and clinical pharmacy, school of pharma; 2017.
8. Eguale T, Tadesse D, Giday M. In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus. J Ethnopharmacol. 2011;137(1):108–113. doi:10.1016/j.jep.2011.04.063
9. Oyedeji OA, Afolayan AJ, Eloff JN. Comparative study of the essential oil composition and antimicrobial activity of Leonotis leonurus and L. ocymifolia in the Eastern Cape, South Africa. South Afr J Bot. 2005;71(1):114–116. doi:10.1016/S0254-6299(15)30160-5
10. Habtamu Y, Eguale T, Wubete A, Sori T. In vitro antimicrobial activity of selected Ethiopian medicinal plants against some bacteria of veterinary importance. Afr J Microbiol Res. 2010;4(12):1230–1234.
11. Vagionas K, Graikou K, Chinou IB, Runyoro D, Ngassapa O. Chemical Analysis and antimicrobial activity of essential oils from the aromatic plants Artemisia afra Jacq. and Leonotis ocymifolia (Burm. F.) Iwarsson var.raineriana (Vision1) Iwarsson growing In Tanzania. J Essent Oil Res. 2007;19(4):396–400. doi:10.1080/10412905.2007.9699314
12. Yeshitila A. Phytochemical investigation on the leaves of leonotis ocymifolia, AAU; 2006.
13. Hussein AA, Meyer MJJ, Rodríguez B. Complete 1H and 13C NMR assignments of three labdane diterpenoids isolated from Leonotis ocymifolia and six other related compounds. Magn Reson Chem. 2003;41(2):147–151. doi:10.1002/mrc.1136
14. Hock FJ. Drug discovery and evaluation: pharmacological assays, fourth edition. In: Drug Discovery and Evaluation: Pharmacological Assay.
15. Sasidharan S, Chen Y, Saravanan D, et al. Extraction, isolation and characterization of bioactive compounds from plants’ extracts Institute for Research in Molecular Medicine (INFORM), Universiti Sains Malaysia, Minden 11800. African J Tradit Complement Altern Med. 2011;8:1–10.
16. Ayoola GA, Coker HAB, Adesegun SA, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res. 2008;7(September):1019–24.
17. Umer S, Tekewe A, Kebede N. Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complement Altern Med. 2013;13:13. doi:10.1186/1472-6882-13-21
18. Erhirhie E, Emudainohwo T, Igboeme S, et al. Preclinical screening techniques for antidiarrheal drugs: a comprehensive review. Am J Physiol Biochem Pharmacol. 2018;7(2):61. doi:10.5455/ajpbp.20180329014330
19. Bahekar SE, Kale RS. Antidiarrheal activity of ethanolic extract of Manihot esculenta Crantz leaves in Wistar rats. J Ayurveda Integr Med. 2015;6(1):35–40. doi:10.4103/0975-9476.146542
20. Mokomane M, Kasvosve I, de ME, Pernica JM, Goldfarb DM. The global problem of childhood diarrhoeal diseases: emerging strategies in prevention and management. Ther Adv Infect Dis. 2018;5(1):29–43. doi:10.1177/2049936117744429
21. Chikezie IO. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afr J Microbiol Res. 2017;11(23):977–980. doi:10.5897/AJMR2017.8545
22. Gahlaut A, Chhillar AK. Evaluation of antibacterial potential of plant extracts using Resazurin based microtiter dilution assay. Int J Pharm Pharm Sci. 2013;5(2):372–376.
23. Rouis Z, Abid N, Koudja S, et al. Evaluation of the cytotoxic effect and antibacterial, antifungal, and antiviral activities of Hypericum triquetrifolium Turra essential oils from Tunisia. BMC Complement Altern Med. 2013;13:13. doi:10.1186/1472-6882-13-24
24. Nikolic M, Vasic S, Djurdjevic J, Stefanovic O, Comic L. Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic extract. Kragujev J Sci. 2014;36(36):129–136. doi:10.5937/KgJSci1436129N
25. Powthong P, Jantrapanukorn B, Thongmee A, Suntornthiticharoen P. Screening of antimicrobial activities of the endophytic fungi isolated from Sesbania grandiflora (L.). Pers J Agric Sci Technol. 2013;15(SUPPL):1513–1522.
26. Chemicals O guidelines for the testing of. Oecd guidelines for the testing of chemicals; 2008.
27. Sarin RV, Narwal S, Bafna PA. Anti-diarrhoeal activity of aqueous extract of Ocimum kilimandscharicum. J Ethnopharmacol. 2013;148(1):223–228. doi:10.1016/j.jep.2013.03.083
28. Imam MZ, Sultana S, Akter S. Antinociceptive, antidiarrheal, and neuropharmacological activities of Barringtonia acutangula. Pharm Biol. 2012;50(9):1078–1084. doi:10.3109/13880209.2012.656850
29. Achenef B. A Thesis paper submitted to the department of pharmacology and presented in partial fulfillment of the requirements for the degree of. 2019.;
30. Riviere PJM, Farmer SC, Burks TF, et al. Prostaglandin E2- induced diarrhea in mice: importance of colonic secretion. J Pharmacol Exper Ther. 1991;256(2):547–552.
31. Dodge JA, Hamdi I, Walker S. Arch Dis Child. Prostaglandin-induced diarrhoea. 1977;52(10):800–802. doi:10.1136/adc.52.10.800
32. Salgado HRN, Roncari AFF, Moreira R. Antidiarrhoeal effects of Mikania glomerata Spreng. (Asteraceae) leaf extract in mice. Bras J Farmacogn. 2005;15(3):205–208. doi:10.1590/S0102-695X2005000300007
33. Mostafa KE, Kharrassi YE, Badreddine A, et al. Nopal Cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health andDisease. Molecules. 2014;19(9):14879–14901. doi:10.3390/molecules190914879
34. Shahed-Al-Mahmud M, Jahan T, Towhidul Islam M. Antidiarrheal activities of hydroalcoholic extract of Sida cordifolia roots in Wister albino rats. Orient Pharm Exp Med. 2018;18(1):51–58. doi:10.1007/s13596-017-0295-5
35. Yaecob T, Shibeshi W, Nedi T. Antidiarrheal activity of 80 % methanol extract of the aerial part of Ajuga remota Benth (Lamiaceae) in mice. BMC Complement Altern Med. 2016;16:1. doi:10.1186/s12906-016-1277-8
36. Muthukumaran P, Saraswathy N, Aswitha V, et al. Assessment of total phenolic, flavonoid, tannin content and phytochemical screening of leaf and flower extracts from Peltophorum pterocarpum (DC.) Backer ex K.Heyne: a comparative study. Pharmacogn J. 2016;8(2):5530. doi:10.5530/pj.2016.2.7
37. Trease GE, Evans WC. A Textbook of Pharmacognosy. Vol. (1989. London: Bailliere Tindall Ltd.; 1989:1989.
38. Oben JE, Assi SE, Agbor GA, Musoro D. Effect of Eremomastax speciosa on experimental diarrhoea. Afr J Tradit Complement Altern Med. 2006;3(1):95–100.
39. Rouf R, Uddin SJ, Shilpi JA, Alamgir M. Assessment of antidiarrhoeal activity of thmethanol extract of Xylocarpus granatum bark in mice model. J Ethnopharmacol. 2007;109(3):539–542. doi:10.1016/j.jep.2006.08.01
40. Rehman NU, Khan A, Fatima U, et al. Presence of laxative and antidiarrheal activities in Periploca aphylla: a Saudi medicinal plant. Int J Pharmacol. 2013;9(3):190–196. doi:10.3923/ijp.2013.190.196
41. Tangpu V, Yadav AK. Antidiarrhoeal activity of Rhus javanica extract in albino mice. Fitoterapia. 2004;75(1):39–44. doi:10.1016/j.fitote.2003.08.015
42. Adzu B, Amos S, Amizan MB, Gmaniel K. Evaluation of the antidiarrhoeal effects of Zizyphus spinachristi (Combretaceae) stem bark in rats. Acta Trop. 2003;87(2):245–250. doi:10.1016/S0001-706X(03)00114-1
43. Balaji G, Chalamaiah M, Ramesh B, Reddy AY. Antidiarrhoeal activity of ethanol and aqueous extracts of Carum copticum seeds in experimental rats. Asian Pac J Trop Biomed. 2012;2(2):1151–1155. doi:10.1016/S2221-1691(12)60376-1
44. Shahed-Al-Mahmud M, Shawon MJA, Islam T, Rahman MM, Rahman MR. In vivo anti-diarrheal activity of methanolic extract of streblus asper leaves stimulating the Na+/K+-ATPase in Swiss Albino Rats. Indian J Clin Biochem. 2020;35(1):72–79. doi:10.1007/s12291-018-0781-7
45. Sahoo HB, Sagar R, Kumar A, Bhaiji A, Bhattamishra SK. Antidiarrhoeal investigation of Apium leptophyllum (Pers.) by modulation of Na+K+ATPase, nitrous oxide and intestinal transit in rats. Biomed J. 2016;39(6):376–381. doi:10.1016/j.bj.2016.11.003
46. Prakash V. Terpenoids as source of anti-inflammatory compounds. Asian J Pharmaceut Clin Res. 2017;10(3). doi:10.22159/ajpcr.2017.v10i3.16435
47. Hamalainen M, Nieminen R, Asmawi MZ, Vuorela P, Vapaatalo H, Moilanen E. Effects of flavonoids on prostaglandin E2 production and on COX-2 and mPGES- 1 expressions in activated macrophages. Planta Med. 2011;77(13):1504–1511. doi:10.1055/s-0030-1270762
48. Awad AB, Toczek J, Fink CS. Phytosterols decrease prostaglandin release in cultured P388D1/MAB macrophages. Prostaglandins Leukot Essent Fatty Acids. 2004;70(6):511–520. doi:10.1016/j.plefa.2003.11.005
49. Pierce NF, Carpenter CC, Elliot HL, Greenough WB. Effects of prostaglandins, theophylline and cholera exotoxin upon transmucosal water and electrolyte movement in canine jejunum. Gastroenterology. 1971;60(1):22–32. doi:10.1016/S0016-5085(71)80003-3
50. Tadesse E, Engidawork E, Nedi T, Mengistu G. Evaluation of the anti- diarrheal activity of the aqueous stem extract of Lantana camara Linn (Verbenaceae) in mice. BMC Complement Altern Med. 2017;17(1):190. doi:10.1186/s12906-017-1696-1
51. Tortora GJ, Derrickson B. Principles of Anatomy and Physiology. In: Menard M, Fagelson E, Gaines K, editors. The Digestive System; Neural Innervation of the Gastrointestinal System. Springer Nature. 2009:925–926.
52. Degu A, Engidawork E, Shibeshi W. Evaluation of the antidiarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del. (Euphorbiaceae) in mice model. BMC Complement Altern Med. 2016;16(1):379. doi:10.1186/s12906-016-1357-9
53. Liu C, Zheng Y, Xu W, Wang H, Lin H. Rhubarb tannins extract inhibits the expression of Aquaporins 2 and 3 in magnesium sulphate-induced diarrhea model. Bio-Medical Res Int. 2014;2014:465–619. doi:10.1155/2014/619465
54. Kartm SMM, Adaikan PG. The effect of loperamide on prostaglandin induced diarrhoea in rat and man. Prostaglandins.. 1977;8(13):2.
55. Beserra FP, Santos RC, Périco LL, et al. Cissus sicyoides: pharmacological mechanisms involved in the anti- inflammatory and antidiarrheal activities. Int J Mol Sci. 2016;17(2):
56. Suleyman H. The role of alpha-2 adrenergic receptors in anti-ulcer activity. Eurasian J Med. 2012;44(1):43–45. doi:10.5152/eajm.2012.09
57. Kumar PM, Suba V, Ramireddy BBSP. Evaluation of antidiarrhoeal activity of ethanolic extract of celtis timorensis leaves in experimental rats. Asian J Pharmaceut Clin Res. 2014;7(2):185–188.
58. Ihekwereme PC, Erhirhie EO, Mbagwu IS, Ilodigwe EE, Ajaghaku DL, Okoye OFB. Antidiarrheal property of Napoleona imperialis may be due to procyanidins and ellagic acid derivatives. Astrophys J Suppl. 2016;6(03):101–106. doi:10.7324/JAPS.2016.60317
59. Wansi LS, Deumeni RM, Kamani PL, Sama FL, Tchoumi TM, Kuiate R. Antidiarrhoeal activity of aqueous and methanolic Alchornea laxiflora (Euphorbiaceae) leaves extracts in rats. J Med Plants Stud. 2017;5(1):205–211.
60. AL-Maamori JAI. Evaluation of the anti-motility-related diarrhoeal activity of the sage tea salvia officinalis L. in laboratory mice. Int J Biol. 2011;3(4):1. doi:10.5539/ijb.v3n4p36
61. Damabi NM, Moazedi AA, Seyyednejad SM. The role of alpha and Beta adrenergic receptors in the spasmolytic effects on rat ileum of Petroselinum crispum Latifolum (parsley). Asian Pac J Trop Med. 2010;3(11):866–870. doi:10.1016/S1995-7645(10)60208-8
62. Nibret E, Phytomedicine WM. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Eur J Integr Med. 2010;17(12):911–920.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.