Back to Journals » Journal of Inflammation Research » Volume 11
Investigation of chronic efficacy and safety profile of two potential anti-inflammatory bipyrazole-based compounds in experimental animals
Authors Domiati S , Mehanna M, Ragab H , Nakkash Chmaisse H , El Mallah A
Received 24 November 2017
Accepted for publication 10 January 2018
Published 3 April 2018 Volume 2018:11 Pages 143—153
DOI https://doi.org/10.2147/JIR.S157955
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Video abstract presented by Domiati et al.
Views: 276
Souraya Domiati,1 Mohammed Mehanna,2,3 Hanan Ragab,4 Hania Nakkash Chmaisse,1 Ahmed El Mallah5
1Department of Pharmacology and Therapeutics, Faculty of Pharmacy, Beirut Arab University, Beirut, Lebanon; 2Department of Pharmaceutical Technology, Faculty of Pharmacy, Beirut Arab University, Beirut, Lebanon; 3Department of Industrial Pharmacy, Faculty of Pharmacy, Alexandria University, Alexandria, Egypt; 4Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Alexandria University, Alexandria, Egypt; 5Department of Pharmacology, Faculty of Pharmacy, Alexandria University, Alexandria, Egypt
Purpose: Although nonsteroidal anti-inflammatory drugs are widely used to treat a variety of disorders, their administration is associated with gastrointestinal side effects, acute kidney injury and liver enzymes’ elevation. Accordingly, researchers are encouraged to create novel agents with better safety profile. The aim of the current study was to evaluate the chronic efficacy and safety profile of two compounds previously proven to have acceptable acute anti-inflammatory and analgesic activities.
Materials and methods: Doses were determined through formalin-induced mice paw edema-based dose–response curves. Granuloma weight was used to assess the chronic effect of the investigated compounds as compared to the vehicle and diclofenac representing the positive and the negative controls, respectively. Mice kidneys, livers and stomachs were histologically examined. Moreover, troponin I, alanine aminotransferase, aspartate aminotransferase, serum creatinine and blood urea nitrogen levels were measured.
Results: The results highlight that the granulomas and exudates developed in mice after 7 days of treatment, with compound I and compound II were significantly lower than that of the negative control group. Moreover, compound I showed significantly better anti-inflammatory effect than diclofenac. Troponin level was undetected in all groups. Histopathological examination of the stomach revealed normal mucosa for both tested compounds and controls. Likewise, kidneys showed neither significant histologic alteration nor biomarkers increase as compared to the control over both 7- and 30-day treatment periods. Mice that received the tested compounds or diclofenac exhibited transient liver damage specifically; congestion, vacuolization, necrosis and inflammation after 7 days of treatment which decreased significantly after 30 days of treatment as emphasized by the Suzuki score and biomarker levels.
Conclusion: Since the tested compounds, specifically compound I, presented a satisfactory chronic safety profile as well as anti-inflammatory effect, it is worth conducting further molecular pharmacological, toxicological and bioavailability studies to elucidate the efficacy of these potential anti-inflammatory bipyrazole compounds.
Keywords: peptic ulcer, hepatic injury, kidney injury, nonsteroidal anti-inflammatory drugs, pyrazole, histology
Introduction
Osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, gouty arthritis, dysmenorrhea, dental pain and headache are varieties of disorders treated by nonsteroidal anti-inflammatory drugs (NSAIDs).1 The main NSAIDs mechanism of action is the inhibition of prostaglandin (PG) production via the cyclooxygenase (COX) pathway. The COX enzymes catalyze the rate limiting step of PGs, thromboxane and eicosanoids biosynthesis. Initially, COX converts arachidonic acid, a PG precursor, to prostaglandin G2, which is reduced subsequently to prostaglandin H2 through peroxidase action.2,3 Two isoforms of COX enzymes exist, namely, COX-1 which is constitutively expressed in the gastrointestinal tract and many other tissues and COX-2 which is completely absent in most cells but induced mainly at inflammation and pain sites. NSAIDs are classified according to their COX inhibition selectivity into traditional agents and COX-2 selective ones.
Since traditional NSAIDs inhibit both COX-1 and COX-2 enzymes, they are associated with increased gastrointestinal side effects mainly due to inhibition of the production of cytoprotective PGs, explicitly PGE2 and PGI2 found in the stomach, which in turn lead to reduced intestinal mucus formation, disturbed micro-circulation causing increased intestinal motility, and increased mucosal permeability to many inflammatory mediators including neutrophils and cytokines.4–6 These gastrointestinal side effects can range from dyspepsia to severe peptic ulcer and bleeding, endoscopically ranging from subepithelial hemorrhages and erosions to total destruction of epithelial membrane and full thickness ulcer.7,8
Other NSAIDs side effects include renal, hepatic and cardiovascular adverse reactions which are related to the agent as well as the duration and frequency of therapy.4 Specifically, NSAIDs can cause acute kidney injury; electrolyte and acid-base disorders; acute interstitial nephritis, which may be accompanied by the nephrotic syndrome; and papillary necrosis.9 These side effects are mainly mediated through inhibition of PG which is involved in the regulation of sodium reabsorption in the renal tubules and acts as a counter-regulatory factor under conditions of increased sodium reabsorption. It also increases potassium secretion mainly through activation of the renin-angiotensin system, resulting in increased secretion of aldosterone. Moreover, PGs increase renal blood flow and glomerular filtration rate under conditions associated with decreased circulating volume, causing a greater tubular flow and secretion of potassium. In healthy, hydrated individuals, renal PGs do not play a major role in sodium and water homeostasis while under conditions of decreased renal perfusion, PGs serve as a vital compensatory mechanism. Thus, renal side effects are relatively mild and rare in healthy individuals.9,10
Prolonged administration of NSAIDs is commonly associated with elevation of liver enzymes, even though this is true, liver failure rarely occurs. The serum aminotransferase enzymes return to normal levels upon discontinuation of the drug. In a retrospective study, out of 625,000 patients who received NSAIDs, only 23 cases developed acute liver injury over 4 years. Among NSAIDs, sulindac showed the greatest hepatic disorders induction.11–13
Both nonselective and COX-2 selective NSAIDs increase risk of cardiovascular events specifically ischemic cardiovascular disease, heart failure, increased blood pressure, and possible atrial fibrillation which are agent, dose and COX-2 selectivity-dependent. Selective COX-2 inhibition is associated with reduced prostacyclin production by vascular endothelium with little or no inhibition of thromboxane A2 production.14–15 Consequently, some selective NSAIDs were withdrawn from the market, but not celecoxib.16–17
The indispensability of NSAIDs medical use accompanied by their side effects encourages continuous research to create novel agents with better safety profile. As an example, Huang et al developed three novel modified NSAIDs, phospho-aspirin, phospho-ibuprofen and phospho-sulindac. Their results are suggestive but not definitive that the three compounds have good efficacy with less gastrointestinal toxicity.18 Moreover, Pan et al synthesized novel benzimidazole derivatives and identified, in vitro, out of 34 compounds, several with an anti-inflammatory effect.19 Faour et al also evaluated four series of newly synthesized bipyrazoles for their anti-inflammatory and analgesic activities. Two compounds showed superior anti-inflammatory activity compared to diclofenac and similar analgesic activity with minimal ulcerogenic side effect.20 In fact these compounds reduced COX-2 protein expression as well as inducible nitric oxide synthase (iNos) to levels comparable to control unstimulated cells.20
In the light of the aforementioned by Faour et al,20 the purpose of the current study is to evaluate the chronic efficacy and safety profile of two bipyrazole compounds previously demonstrated to have a good acute anti-inflammatory effect. Based on the probit method, doses were determined to assess the chronic anti-inflammatory effect of the investigated compounds. Troponin I was used to assess drug induced cardiac toxicity. Histological and biochemical markers of the kidney and liver function were evaluated after chronic use of the tested compounds. Moreover, histological features of the stomach were studied.
Materials and methods
Experimental animals
Male BALB/c mice weighing 25–30 g were used. Animals were housed under standard laboratory conditions, kept under diurnal light and had free access to water and a standard chow diet throughout the experiments.
Ethical consideration
All experiments were performed at Beirut Arab University laboratories after obtaining approval from the Investigation Review Board, number 2016A-0043-P-P-0165. Animal care and handling for the research were performed in accordance with the regulations and guidelines stipulated by the Institutional Animal Care and Use Guidelines (IACUG) at Beirut Arab University, Lebanon, authenticated by the Ministry of Public Health (1/141).
Drugs and drugs preparation
Compounds I and II were synthesized in the pharmaceutical chemistry laboratory, faculty of pharmacy, Alexandria University, Egypt. The chemical entities (Figure 1) were dissolved in equivalent volumes of dimethyl-sulfoxide and polyethylene glycol 400 for intraperitoneal administration. Diclofenac sodium, supplied by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA), was dissolved in normal saline to achieve appropriate concentration. Benzathine benzylpenicillin (Retarpen; Sandoz GmbH, Kundl, Austria) was dissolved in normal saline and administered intramuscularly. Ether supplied by POCH SA (Gliwice, Poland) was used to anesthetize the animals when needed. Formalin supplied by Sigma-Aldrich Co. (St Louis, MO, USA) was used for the determination of the doses of compounds I and II.
  | Figure 1 Chemical structure and molecular data of the investigated compounds. Abbreviation: MW, molecular weight. |
Procedures
Assessment of chronic anti-inflammatory activity
Formalin-induced mice paw edema method
Formalin-induced mice paw edema was used to assess the acute anti-inflammatory activity of different doses of the tested compounds as to determine the dose–response curve and the equivalent doses to 10 mg/kg diclofenac sodium. After 1 h of injecting each compound in five different doses as well as the control, the mice were challenged by a 200 µL subcutaneous injection of 5% v/v (volume/volume) formalin solution into the plantar side of the right hind paw. The paw volume was measured plethysmographically immediately after the injection and 4 h later.21,22 The doses of compounds I and II equivalent to 10 mg/kg diclofenac sodium were calculated using probit analysis with 95% CI.
Cotton pellet induced granuloma method
Chronic anti-inflammatory effects of the different compounds were studied using the cotton pellet granuloma test. Male mice were anesthetized with ether. The back skin was shaved and disinfected with 70% v/v ethanol. An incision was made in the lumbar region. With sterile forceps, a subcutaneous tunnel was formed, and a sterilized cotton pellet was placed in the scapular region. After the surgery, each mouse was given 60,000 IU of benzathine penicillin intramuscularly. Each group of mice was treated with either vehicle or tested compounds, or diclofenac sodium for 7 consecutive days. On the 8th day, the animals were sacrificed, and the cotton pellets surrounded by the granulomatous tissue were removed surgically. Extraneous tissues were removed, and then, the moist pellets were weighed (wet weight). The granuloma weight was considered as the wet weight minus the weight of the original cotton which was 10 mg. The pellets were then dried at 90°C for around 3 h until reaching a constant weight which was considered the dry weight.22,23
Chronic safety profile of compound I and compound II
Cardiac biomarker
Before sacrificing the mice to remove the cotton pellet, blood was collected by cardiac puncture from the anesthetized mice to determine the cardiac troponin I which is a sensitive biomarker of drug-induced cardiac toxicity.24 A chromatographic immunoassay test device for qualitative detection of cardiac troponin I in serum supplied by ACON Laboratories Inc. (San Diego, CA, USA) was used for this purpose.
Renal and hepatic functions biomarkers
Blood collected was also used to determine the sub-chronic toxicity of compounds I and II on both the liver and kidneys. Another group of mice also kept in single cages during the whole procedure with free water and food either received the vehicle, compound I, compound II or diclofenac sodium intraperitoneally for 30 consecutive days to determine the chronic toxicity. Creatinine, blood urea nitrogen (BUN), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using a ready-made chemical kit supplied by SPINREACT Co., Girona, Spain.25
Histological features of the stomach, kidney and liver
After sacrificing the animals for biomarkers’ study, their stomachs, kidneys and livers were removed for histological analysis.
The isolated stomachs were opened along the greater curvature, washed with normal saline and examined macroscopically for any apparent damage. The isolated stomachs were then fixed in a buffered neutral formalin solution, trimmed after, dehydrated and embedded in paraffin, cut and stained with hematoxylin and eosin appropriately then subjected to microscopic evaluation for any histological changes.26
Kidneys were also trimmed and fixed in neutral buffered formalin solution. Paraffin blocks were made and cut into sections and adhered to glass slides using egg albumin. Tubular, endothelial, glomerular and tubulointerstitial tissues were scored according to the endothelial, glomerular, tubular and interstitial (EGTI) histology scoring system.27
Livers were immediately fixed with Carnoy’s solution. Samples were then embedded in paraffin wax, cut into 6-μm sections, and stained with hematoxylin and eosin prior to microscopic examination. Liver damage was graded according to Suzuki score.28
Three expert pathologists who were blinded to the experimental conditions examined the specimens using a standard light microscope.
Statistical analysis
Results were analyzed using Statistical Package for the Social Science version 20 (IBM Corporation, Armonk, NY, USA). Continuous data was expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by post hoc analysis was used when appropriate. Difference was considered significant at P-value less or equal to 0.05. The effective concentration was calculated using the regression analysis, probit.
Results
Assessment of chronic anti-inflammatory activity
Formalin-induced mice paw edema test
Through the construction of the dose–response curves for compounds I, II and diclofenac sodium, the equieffective doses of the two compounds to 10 mg/kg diclofenac sodium calculated using probit analysis method were 25.66 mg/kg and 17.01 mg/kg, respectively (Figure 2) which were used in all other tests.
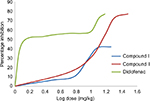  | Figure 2 Dose–response relationship of compounds I, II and diclofenac sodium based on formalin-induced paw edema method. |
Cotton pellet granuloma test
After 7 days of treatment, the tested compounds as well as diclofenac sodium showed an anti-inflammatory activity illustrated by the significant lower weight of the granuloma and exudate developed in mice receiving these compounds compared to the control group. Moreover, compound I showed a significantly lower granuloma development as compared to diclofenac sodium as shown in Table 1.
Chronic safety profile of compound I and compound II
Cardiac biomarker
The cardiac biomarker, troponin I, was not detected in mice groups treated with either compound 1, compound 2, or diclofenac comparable to the control.
Renal and hepatic functions biomarkers
The biomarkers of the renal function, BUN and serum creatinine (Scr), of the three treated groups did not show any significant difference compared to the control group over both periods, 7 and 30 days. Liver enzymes ALT and AST showed significant increase compared to the control after 7 days but not after 30 days. Nevertheless, all levels were within normal limits (<45 IU/L) (Table 2).
Histological features of the stomach, kidney and liver
Histological examination of the gastric structure revealed an intact mucosa for all tested compounds as well as the control group after 7 days treatment, while for 30 days, minimal petechia was noticed in mice treated with compounds I and II, as well as diclofenac sodium.
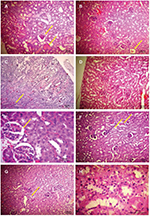
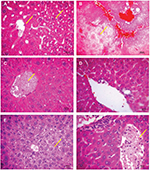
Concerning the kidney histological structures, EGTI scores of mice on compounds I, II and diclofenac sodium for 7 days treatment period, were significantly higher than the score of the control group while after 30 days of treatment only compound II and diclofenac sodium showed statistical difference compared to the control. In fact, EGTI score for compound I was comparable to the control and statistically lower than that of diclofenac sodium group (Table 3). Even though there was an increase in some scores, different treatment modalities induced no actual damage. In fact, in less than 25% of the tubular cells, a minor loss of the bundle brush was detected. Retraction of the glomerular tuft was noticed in most of the studied samples. Moreover, non-effective endothelial swelling and inflammation were noticed as shown in Figure 3. These changes did not indicate any alteration in the kidney functions other than a transient and mild histologic change.
Regarding the hepatic side effects of compound I, II and diclofenac sodium administered for 7 days to mice, it was obvious that they cause liver changes manifested as congestion, vacuolization, necrosis as well as inflammation. In the group treated with compound I, the mice liver showed mild lobular and portal inflammation while in those treated with compound II, the liver showed only lobular inflammation. Additionally, diclofenac sodium group showed discrete portal inflammation (Figure 4). The control group did not show any significant changes. Suzuki score was used to evaluate liver damage extend depending on; the congestion, vacuolization and necrosis. There was statistical difference between the different groups and control animals in Suzuki scores. Alternatively, hepatocytes showed binucleation and nuclear activation in compounds I, and II, and diclofenac sodium groups. After 30 days of treatment, compound I, and II, and diclofenac sodium also showed an elevated Suzuki score as compared to the control, but those scores were significantly lower than the corresponding ones after 7 days of treatment (Table 4).
Discussion
NSAIDs are the most widely used drugs worldwide for a diverse range of conditions. Nevertheless, several side effects are consequences of their administration especially with chronic use. For this purpose, researchers are still investigating new molecules to discover ideal anti-inflammatory drugs with better safety profile than the former ones. Compound I and compound II showed not only acute anti-inflammatory effect as suggested by Faour et al,20 but also chronic efficacy as demonstrated by the results which were obtained in our current study with the cotton granuloma test. In fact, on chronic administration of the investigated compounds and diclofenac for 7 days, both granulomas and exudates formation were significantly lower than those of the group receiving only the vehicle.
As a second step, drug safety is one of the crucial aspects which is evaluated during preclinical, early clinical, late stage clinical and post-marketing phases of the pharmaceutical discovery process. For this purpose, monitoring of the possible side effects of the molecule in question is a necessity.29 Most important side effects of NSAIDs include cardiovascular problems, alterations in gastrointestinal mucosal integrity, renal function disorders and hepatic injuries.1
The motive to introduce more efficient anti-inflammatory and analgesic agents with reduced adverse effects led to the evolvement of selective COX-2 inhibitors, nevertheless, these agents showed higher cardiovascular side effects.3 Since compound I and compound II were not considered as selective NSAIDs having a COX-1IC50/COX-2IC50 ratio of 1.75 and 1.59, respectively, as suggested by Faour et al,20 cardiovascular side effects are less probable. Moreover, since troponin I is a useful tool to assess drug induced cardiac toxicity in laboratory animals,27 it was measured in in all treated groups to reveal a negative value implicating a safe cardiovascular outcome.
However, traditional NSAIDs are associated with a significant risk of serious gastrointestinal side effects mediated by inhibition of COX-1 which plays an important protective role in the gut by stimulating the synthesis and secretion of mucus and bicarbonate, increasing mucosal blood flow and promoting epithelial proliferation. Thus, when NSAIDs inhibit this enzyme, they create a gastric environment prone to ulceration.7,30 Even though this is true, the obtained results revealed that compound I and compound II caused no alteration in the gastric mucosa when administered for 7 days and minimal petechia occurred after continuous administration for 30 days comparable to diclofenac sodium. In fact, the risk of gastric bleeding induced by traditional NSAIDs may vary from one product to the other. As mentioned by Van Walsem et al, for example, major gastrointestinal bleeding events with diclofenac were lower compared to naproxen and ibuprofen, comparable to celecoxib and higher than etoricoxib.31
NSAIDs can cause acute kidney injury mainly mediated by PG inhibition.9 In the current study, compounds I and II as well as diclofenac showed some histologic changes but no real damage to the kidneys with no elevations in serum creatinine and BUN in both 7 and 30-day time periods. This is explained by the fact that NSAIDs induced acute kidney injury is relatively mild and rare in healthy hydrated individuals since renal PGs serve mainly for compensatory mechanism in case of decreased renal perfusion.9,10
Drug-induced liver injury (DILI) is the most challenging disorder upon continuous administration of NSAIDs. Two types of DILI are defined namely; intrinsic and idiosyncratic. The intrinsic type refers to liver injury caused by drugs when given at high doses exemplified by acetaminophen, while the idiosyncratic type is less common as it affects only susceptible individuals, has less consistent relationship to dose, and is more varied in its presentation.32 NSAIDs are an example of DILI the idiosyncratic type.33 In fact, NSAIDs along with anti-infectious agents are listed as the most common cause of DILI. Several NSAIDs have been abandoned after being developed or introduced into practice, due to serious liver injury such as bromfenac, ibufenac, oxicams and sudoxicam.32 In fact, these hepatic injuries may be present in a variety of forms as it may be asymptomatic, transient, hypertransaminasemia or fulminant.34 The exact mechanism of NSAID-induced liver injury is still fully unexplained but mitochondrial toxicity, endoplasmic reticulum stress and inflammation may play a role. The liver toxicity of NSAIDs appears to be compound specific rather than class or family effect. In the same chemical family, some compounds carry this side effect while others do not. The most common agents that have been reported to have relatively high risk of liver injury are: diclofenac, ibuprofen, naproxen, nimesulide, piroxicam and sulindac.33 In the current study, the liver injury detected after compound I, compound II and diclofenac sodium administration for 7 consecutive days was most probably transient since ALT and AST levels increased significantly as compared to the control but upon continuation of administration over 30 days these levels decreased. Nonetheless, all levels were within normal limits (<45 IU/L). Similarly, even though the histologic results showed significantly elevated Suzuki scores for compounds I, II and diclofenac as compared to the control after 30 days of treatment, those scores significant dropped as compared to the 7 days treatment samples. Hepatocytes after 7 days of treatment with compound I, II and diclofenac sodium also showed bi-nucleation and nuclear activation which is a sign of liver regeneration. These results match with the fact that elevations in AST and ALT values occur in 5 to 15% of patients taking NSAIDs as a class, remain less than three times the upper normal limits, and in some cases may resolve despite continuation of the NSAID.33
Conclusion
The investigated compounds showed a marked anti-inflammatory activity in the chronic experimental animal model which was approved via a statistically significant lower formation of granuloma and exudate. Moreover, these compounds showed a satisfactory safety profile, specially demonstrated by neither gastric nor kidney side effects. In fact, compound I had an even better anti-inflammatory and kidney safety profile as compared to the standard US Food and Drug Administration approved NSAID, diclofenac sodium. The chronic administration of the tested compounds resulted in transient liver changes comparable to those of diclofenac sodium. As a consequence of the adequate anti-inflammatory efficacy and favorable safety profile of the investigated bipyrazole compounds, particularly compound I, advanced investigation is recommended.
Acknowledgments
The authors are grateful to Mrs Amina Fayed, senior specialist at the histology laboratory, Beirut Arab University; Dr Mona Majed, Beirut Arab University; Leyla Akil, Chief of Pathology Department at Bahman Hospital; and Hassan Sidani, chief of Pathology Department at Makassed General Hospital.
Author contributions
Souraya Domiati undertook all laboratory procedures and wrote the article. Hanan Ragab synthesized the molecules. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ong CKS, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res. 2007;5(1):19–34. | ||
Grosser T, Smyth E, FitzGerald GA. Anti-inflammatory, antipyretic and analgesic agents; pharmacotherapy of gout. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. California: McGraw-Hill Education; 2011:959–1004. | ||
Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11:81–110s. | ||
Frölich JC. A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. Trends Pharmacol Sci. 1997;18:30–34. | ||
Shah AA, Fitzgerald DJ, Murray FE. Non-steroidal anti-inflammatory drugs (NSAIDs) and gastro-intestinal toxicity: current issues. Ir J Med Sci. 1999;168(4):242–245. | ||
Higuchi K, Umegaki E, Watanabe T, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44(9):879–888. | ||
Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24(2):121–132. | ||
Laine L. The gastrointestinal effects of nonselective NSAIDs and COX-2-selective inhibitors. Semin Arthritis Rheum. 2002;32(3):25–32. | ||
Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106(5):13S–24S. | ||
Whelton A. Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDs and non-NSAID analgesics. Am J Ther. 2000;7(2):63–74. | ||
Carson JL, Strom BL, Duff A, Das K. Safety of nonsteroidal anti-inflammatory drugs with respect to acute liver disease. Arch Intern Med. 1993;153(11):1331–1336. | ||
Rubenstein JH, Laine L. The hepatotoxicity of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2004;20(4):373–380. | ||
Garcia-Rodriguez LA, Williams R, Derby LE, Dean AD, Jick H. Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch Int Med. 1994;154(3):311–316. | ||
Antman EM, Bennett JS, Daugherty A, et al; American Heart Association. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634–1642. | ||
Caughey GE, Cleland LG, Penglis PS, Gamble JR, James MJ. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J Immunol. 2001;167(5):2831–2838. | ||
Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. | ||
Johnsen SP, Larsson H, Tarone RE, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Int Med. 2005;165(9):978–984. | ||
Huang L, Mackenzie G, Ouyang N, et al. The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;162(7):1521–1533. | ||
Pan L, Hang N, Zhang C, et al. Synthesis and biological evaluation of novel benzimidazole derivatives and analogs targeting the NLRP3 inflammasome. Molecules. 2017;22(2):213. | ||
Faour WH, Mroueh M, Daher CF, et al. Synthesis of some new amide-linked bipyrazoles and their evaluation as anti-inflammatory and analgesic agents. J Enzyme Inhib Med Chem. 2016;31(6):1079–1094. | ||
Morris CJ. Carrageenan-induced edema in the rat and mouse. In: Winyard PG, Willoughby DA, editors. Methods in Molecular Biology: Inflammation Protocols. Vol 225. New Jersey: Humana Press; 2003:115. | ||
Vogel GH, editor. Analgesic, anti-inflammatory, and anti-pyretic activity. In: Drug Discovery and Evaluation: Pharmacologic Assays. 3rd ed. Berlin, Germany: Springer. 2008; 983–1116 | ||
Bailey PJ, Sturm A, Lopez-Ramos, B. A biochemical and morphological study of the cotton pellet granuloma in the rat: effects of dexamethasone and indomethacin. Inflammation: Mechanisms and Treatment. Volume 4. Dordrecht: Springer. 1981;345–346. | ||
Gaze DC, Collinson PO. Cardiac troponins as biomarkers of drug- and toxin-induced cardiac toxicity and cardioprotection. Expert Opin Drug Metab Toxicol. 2005;1(4):715–725. | ||
Lerma EV. Blood urea nitrogen (BUN). 2012. Available from: http://emedicine.medscape.com/article/2073979-overview. Accessed May 1, 2016. | ||
Seo PJ, Kim N, Kim J-H, et al. Comparison of indomethacin, diclofenac and aspirin-induced gastric damage according to age in rats. Gut Liver. 2012;6(2):210–217. | ||
Khalid U, Pino-Chavez G, Nesargikar P, et al. Kidney ischaemia reperfusion injury in the rat: the EGTI scoring system as a valid and reliable tool for histological assessment. Journal of Histology and Histopathology. 2016;(3):1. | ||
Behrends M, Martinez-Palli G, Niemann CU, Cohen S, Ramachandran R, Hirose R. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg. 2010;14(3):528–535. | ||
Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul Toxicol Pharmacol. 1998;27(2):119–130. | ||
Gandhi MN, Challa SR, Prasanth P, Gandhi TR. Role of leukotrienes in NSAID induced gastric ulceration and inflammation in wistar rats. Asian Pac J Trop Dis. 2012;2(3):215–219. | ||
Van Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A, Moore RA. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis Res Ther. 2015;17(1):66. | ||
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950–966. | ||
Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In: Kaplowitz N, DeLeve LD, editors. Drug-Induced Liver Disease. 3rd ed. Amsterdam: Elsevier. 2013:370–402. | ||
Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol. 2010;16(45):5651–5661. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.