Back to Journals » Infection and Drug Resistance » Volume 13
Investigating the Effect of Nano-Curcumin on the Expression of Biofilm Regulatory Genes of Pseudomonas aeruginosa
Authors Sharifian P, Yaslianifard S , Fallah P, Aynesazi S, Bakhtiyari M, Mohammadzadeh M
Received 2 June 2020
Accepted for publication 9 July 2020
Published 21 July 2020 Volume 2020:13 Pages 2477—2484
DOI https://doi.org/10.2147/IDR.S263387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Parastoo Sharifian,1 Somayeh Yaslianifard,2 Parviz Fallah,3 Siavash Aynesazi,4 Mahmood Bakhtiyari,5 Mohammad Mohammadzadeh2
1Department of Microbiology, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran; 2Department of Microbiology, School of Medicine, Dietary Supplements and Probiotic Research Center, Alborz University of Medical Sciences, Karaj, Iran; 3Department of Laboratory Science, Faculty of Allied Medicine, Alborz University of Medical Sciences, Karaj, Iran; 4Department of Microbiology, Faculty of Science, North Branch, Islamic Azad, Tehran, Iran; 5Department of Community Medicine and Epidemiology, School of Medicine, Non-Communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran
Correspondence: Mohammad Mohammadzadeh Tel +989126767995
Email [email protected]
Background: Pseudomonas aeruginosa is an opportunistic pathogen that causes serious nosocomial infections, especially in immunodeficient patients and cystic fibrosis, cancer, and burned individuals. The biofilm that plays an important role in the virulence of P. aeruginosa is under the regulation of quorum sensing and two-component regulatory systems of bacteria. Curcumin, an active phenolic extract of turmeric has shown an inhibitory effect on the biofilm formation of some pathogenic bacteria. Thus, the present study aims to evaluate the effect of Nano-Curcumin on the expression of major regulatory genes involved in biofilm formation of P. aeruginosa.
Materials and Methods: The biofilm formation of P. aeruginosa ATCC 10145 was assessed in the presence of 15, 20, and 25 μg/mL concentrations of Nano-Curcumin using the microplate titer method. The effect of Nano-Curcumin on the expression level of regulatory genes were determined by relative reverse transcriptase-realtime PCR.
Results: In the absence of Nano-Curcumin, P. aeruginosa strain ATCC 10145 strongly produced biofilm (3+) and in the presence of 15 and 20 μg/mL, biofilm formation was reduced to moderate (2+) and weak biofilm producer (1+), respectively. Nano-Curcumin at a concentration of 25μg/mL inhibited biofilm formation in P. aeruginosa. The expression of regulatory genes was not affected by biofilm inhibitory concentrations of Nano-Curcumin.
Conclusion: The antibiofilm mechanism of Curcumin is not related to the downregulation of regulatory systems of P. aeruginosa and probably it prevents the formation of a complete biofilm structure.
Keywords: Pseudomonas aeruginosa, biofilm formation, Nano-Curcumin
Introduction
Pseudomonas aeruginosa is a gram-negative opportunistic pathogen found everywhere and able to infect immunocompromised patients including those with cystic fibrosis and extensive burn wounds.1 Because of more antibiotic resistance of bacterial cells living as biofilms, the relevant infections with biofilm producer strains are very hard to eradicate.2
The biofilm matrix of Pseudomonas aeruginosa is formed from extracellular polymeric materials, including polysaccharides, extracellular DNA, and proteins.3 The complex regulation of biofilm formation involves multiple bacterial types of machinery, including QS systems and two-component regulatory systems that both interact mainly with extracellular polysaccharide (EPS) production.4 Two-component regulatory systems such as GacS/GacA and RetS/LadS play the main role in controlling factors associated with biofilm formation. The GacS/GacA system acts as a super-regulator of the QS system and is involved in the production of multiple virulence factors as well as in biofilm formation.5 The Gac system consists of a transmembrane sensor kinase (GacS) and a transcriptional regulator (GacA) which has a positive effect on the upregulation of the QS systems involved in biofilm formation. It has also been reported that RetS interacts with the GacS/GacA system and inhibits the phosphorylation of GacA.6 Furthermore, P. aeruginosa possesses two main QS systems (Las and Rhl) which drive the production of autoinducer signaling acyl-homoserine lactone molecules.7 The QS regulator LasR and RhlR can bind to the promoter region of the psl and pel operons and lead to enhancement of biofilm formation.8
Because of the emergence of multidrug-resistant (MDR) P. aeruginosa in developing countries,9 complementary therapy for control of infections associated with these bacteria can be very beneficial. Recently, dietary phytochemicals with a long history of medical use in humans have been investigated to control biofilms as they are non-toxic.1,10 It has been shown that the phenolic compound Curcuma longa (Curcumin) can inhibit biofilm formation in several bacteria such as P. aeruginosa.11–13 Different mechanisms have been suggested for the anti-biofilm activity of Curcumin by some studies, but the molecular mechanism of Curcumin for inhibition of biofilm is until unclear. Curcumin may affect biofilm formation by changing the expression of regulatory genes associated with P. aeruginosa signaling pathways. Curcumin has low solubility in aqueous solutions that lead to lower tissue diffusion. The Curcumin nanoparticle formulation (Nano-Curcumin) is more soluble than Curcumin and more resistant to enzymatic hydrolysis. Moreover, Nano-Curcumin has increased tissue uptake and also has a longer half-life than Curcumin that makes it more appropriate to investigate its effects on the biofilm formation.14 According to the evidences, this study aimed to evaluate the effect of Nano-Curcumin on the expression of major genes involved in the regulation of biofilm in P. aeruginosa.
Materials and Methods
Bacterial Strains and Culture Conditions
Pseudomonas aeruginosa ATCC 10145 was used as a positive biofilm producer strain and P. aeruginosa ATCC 27853 was used as a biofilm negative control. Bacteria were cultured in Trypticase soy broth (TSB) medium (Merck, Germany). The cultures were incubated at 37 °C overnight, then were preserved at −70 °C for long term storage after adding 20% glycerol.
Biofilm Formation Assay
Biofilm Assay Optimization
The microtiter plate assay was performed for optimizing biofilm formation conditions.15 Initial 1:100 dilution was prepared from fresh 18 hours culture of bacterial strains in Brain heart infusion (BHI) (Merck, Germany). Then, 100 μL was added in 96-well plate and incubated for 48 hours at 37°C. After 48 hours, planktonic bacteria were removed and the wells were washed two times with tap water with the plate left at room temperature for 3 hours. The walls were stained with 125 μL of 0.1% (w/v) crystal violet for 10 min at room temperature, then washed with tap water. After drying, 200 μL of 30% (v/v) acetic acid was added for 10 min, and finally, OD of positive and negative controls was measured at 570nm using Microplate Readers (Awareness, USA). Biofilm formation results were obtained regarding OD of controls as follows: ODco+ ≤ ODco-= no biofilm producer; ODco+ > ODco-: ≤2×ODco-= weak biofilm producer; ODco+ > ODco-: 2–4×ODco-= moderate biofilm producer; ODco+ > ODco-: >4×ODco-=strong biofilm producer. OD values were measured based on the mean OD of four assessments.
Biofilm Inhibition Assay
For increasing the solubility of Curcumin, the polymeric nanoparticles of Curcumin (Nano-Curcumin) from source Curcumin (Merck, Germany) were used in this study. The Planetary Ball Mill technology was performed to synthesize Nano-Curcumin by the reference laboratory of Amirkabir University of Technology. Biofilm formation of P. aeruginosa ATCC 10145 strain was assessed at the final 15, 20, and 25µg/mL concentrations of Nano-Curcumin. Nano-Curcumin was dissolved into Brain-Heart Infusion Broth (Merck, Germany) containing 10% of Dimethyl Sulfoxide (DMSO). Bacterial culture in BHI with 10% DMSO without Nano-Curcumin was used as a control.
Bacterial Growth Inhibition Assay
The effect of Nano-Curcumin on bacterial growth was assessed through measuring OD at 600nm. P. aeruginosa ATCC 10145 cultures at the initial concentration of 0.5 McFarland was incubated for 48 hours in the absence and presence of different concentrations of Nano-Curcumin (15, 20, 25μg/mL) in BHI with 10% DMSO. Then, OD values of different samples with Nano-Curcumin were compared with the control sample.
RNA Extraction
RNA was extracted from three samples; Pseudomonas aeruginosa ATCC 10145 strain in the presence and the absence of Nano-Curcumin after 48 hours of incubation under optimal biofilm formation conditions and one 16-hour fresh planktonic bacteria. All samples were centrifuged and the supernatant was discarded. All samples were diluted with sterile normal saline to 3.0 McFarland. The total RNA was extracted using the High Pure RNA Isolation Kit (Roche, Switzerland) according to the kit protocol. The quantity of RNA samples was analyzed through OD measurement by NanoDrop (Boeco, Germany). RNA was adjusted to the same concentration for all samples. The purity of RNA was assessed by the negative results of a house-keeping tufB gene using traditional PCR.
cDNA Synthesis
Reverse transcriptase reaction was accomplished for cDNA synthesis using Transcriptor First Strand cDNA Synthesis Kit (Roche, Switzerland) regarding kit protocol. The quantity and quality of cDNA samples were assessed by OD measurement and positive results of tufB gene in PCR, respectively. cDNA was adjusted as the same concentration for all samples. The primer sequences of tufB are listed in Table 1.
 |
Table 1 Sequences of Primers and PCR Conditions of Evaluating Genes |
Polymerase Chain Reaction (PCR)
PCR was carried out for controlling the specific primers and optimizing annealing temperatures for amplification of gacA, retS, lasR, and rhlR genes. Amplifications were performed in reactions containing 12 µL of 2X ready to use Master Mix (Fermentase, Germany), 1µL of each 20 pmol Forward and reverse primers, 10µL of sterile distilled water, and 1 µL of 1µg adjusted cDNA. Electrophoresis on 1.5% agarose gel was done for evaluating the PCR amplicons. The primer sequences and PCR conditions are mentioned in Table 1.
Real-Time PCR
Relative Real-time PCR was used to investigate the effect of Nano-Curcumin on the expression of genes gacA, retS, lasR, and rhlR. The comparison of gene expression was performed in five cDNAs prepared from samples with different culture conditions: a fresh culture (bacterial planktonic form), a sample of bacteria isolated from the optimal biofilm formation condition, and three samples of bacteria isolated from bacterial biofilm conditions in the presence of 15, 20, and 25µg/mL of Nano-Curcumin. Amplifications were performed in double reaction in final volume 20µL including 10 µL of 2X ready to use SYBR Green Master Mix (TaKaRa, Japan), 1µL of each 20 pmol Forward and Reverse primers, 7µL double-distilled water, and 1µL of adjusted cDNA (1µg/µL). Reactions were carried out in 35 cycles via Rotor-Gene Q 5plex (Qiagen, USA) according to thermal conditions as mentioned above. For normalization of the expression, the house-keeping tufB gene was used as an internal control. No template controls were used for each reaction for the screening of unwanted amplifications. The differences in gene expression were calculated through the 2–∆∆Ct method.
Statistical Analysis
The experiments were performed in five times. The means and standard deviations of ΔCt values were analyzed using GraphPad Prism 8.0 Software (San Diego, Canada). The ANOVA test was carried out to calculate the significance of the results. A P value of less than 0.05 was considered significant.
Results
Determination of Optimum Condition of Biofilm Formation
Based on the microtiter plate assay, the control positive P. aeruginosa ATCC 10145 strain was confirmed as a strong biofilm producer strain. The OD ratios for biofilm quantification have been mentioned in Table 2.
 |
Table 2 OD Values and Biofilm Formation Level of P. aeruginosa ATCC 10145 Strain in 570 nm |
Nano-Curcumin Blocks Biofilm Formation in P. aeruginosa in a Concentration-Dependent Way, but Does Not Affect Bacterial Growth
Nano-Curcumin was dissolved at a maximum concentration of 25μg/mL in BHI containing 10% DMSO. Bacterial growth was not inhibited by Nano-Curcumin, but biofilm formation was inhibited completely. In the presence of 15 and 20µg/mL, biofilm formation levels were reduced to moderate (2+) and weak (1+), respectively (Figures 1 and 2). The OD values of bacterial growth in the presence of different concentrations of Nano-Curcumin are listed in Table 3.
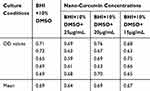 |
Table 3 Bacterial Growth in the Absence and the Presence of Different Concentrations of Nano-Curcumin According to OD Values of 600 nm After 48 Hours |
 |
Figure 1 Nano-Curcumin inhibits the Biofilm in a concentration-dependent manner. The biofilm formation was reduced to 2+, 1+ and negative in 15, 20 and 25 µg/mL concentrations of Nano-Curcumin. |
Quality Control of RNA and cDNA
The extracted RNA samples were adjusted to 250 ng/µL, and cDNA samples were provided as 1µg/µL for PCR. The purity of RNA as well as the quality of cDNA and primers was confirmed by negative results for RNA and positive PCR results for cDNA samples. The results of the PCR of tufB gene in RNA samples were negative, suggesting the purity of RNA. Further, positive PCR results of tufB, gacA, retS, lasR, and rhlR genes showed the reliability of primers and cDNA of samples.
Nano-Curcumin Does Not Affect Gene Expression of Biofilm Regulatory Factors
The relative expression of genes gacA, retS, lasR, and rhlR was determined with Ct values. The mean amounts of expression of target genes were normalized to the mean amount of the tufB reference gene in the same sample. The level of expression of gacA, lasR, and rhlR regulators showed a significant increase in samples from biofilm conditions in the absence and presence of different concentrations of Nano-Curcumin in comparison to the sample of the fresh planktonic form of bacteria. However, there were no significant differences between the expression level of retS gene in all samples. The results showed that while biofilm inhibited with Nano-Curcumin, it did not affect on the expression of regulator genes involved in biofilm formation (Figure 3).
Discussion
P. aeruginosa is an important human opportunistic pathogen causing acute and chronic infections, especially in immunocompromised patients. In recent decades, the excessive use of antibiotics has led to the emergence of resistant strains in these bacteria.16
Biofilm formation is one of the mechanisms of these bacteria that reduce the effect of antibiotic therapy. P. aeruginosa biofilm is a structure composed of different biomolecules that evolve as these components come together. Bacterial attachment factors, extracellular polysaccharides (EPS), and extracellular DNA (eDNA) are involved in the formation of this structure.6 Regulation of the expression of essential genes in the process of secretion and formation of biofilm components is under the direct and indirect control of two-component regulatory systems and bacterial quorum sensing. Failure in these systems results in halting biofilm production or it's defective structure.17
The natural compounds with antimicrobial effects as an alternative or complementary therapy has been recently considered. The antimicrobial activity of essential oils of some plants on multi-drug-resistant P. aeruginosa isolates in vitro has been shown by some previous studies.18,19 Curcumin is an active phenolic component of Curcuma longa that has been investigated due to its pharmaceutical attributes.6,20
Some studies confirming the effect of Curcumin on biofilm production in P. aeruginosa suggest that this effect is likely due to the QS of the bacterium and the downregulation of the regulatory genes involved in this process. In a study, Roudashti et al21 using RT-PCR showed that C12-HSL and C4-HSL signals of P. aeruginosa PAO1 strain significantly diminished in the presence of Curcumin. Rudrappa et al1 hypothesized that Curcumin may target QS responses in P. aeruginosa PAO1 through delays in the construction of the HSL or by disabling the receiving QS signals. Nevertheless, other studies showed it affects the expression of the genes directly involved in the biofilm production.22
In the present study, the inhibitory effect of Curcumin was surveyed on the biofilm formation and QS of P. aeruginosa.
The results of this study indicated that although Nano-Curcumin has an inhibitory effect on biofilm formation in P. aeruginosa in a concentration-dependent manner, it has no significant effect on the expression of major regulatory genes that play an important role in biofilm formation.
This may suggest that Nano-Curcumin has a different role other than changing the expression of regulatory genes that are involved in bacterial biofilm synthesis.
A research indicated that Curcumin at concentrations ≥25 μM had an inhibitory effect on bacterial growth, causing leakage on the bacterial membranes, that lead to the death of some bacterial species such as Staphylococcus aureus, Escherichia coli, and P. aeruginosa.23 The blockade of the proteolytic activity of autotransporter proteins of Enteroaggregative and Enteropathogenic Escherichia coli by Curcumin was mentioned in a more recent study. It was also observed that Curcumin does not affect bacterial growth and gene expression of those auto-transporters, but it prevents their release through binding to their cleavage site.24
Degradation of bacterial biofilms by Curcumin through its interaction with biofilm matrix proteins of some bacterial species was reported by a study.20 Furthermore, a novel protein CdrA was shown in the biofilm structure. This protein is an auto-transporter which mediates bacterial aggregation and static biofilm formation with interaction with Psl; a major EPS of P. aeruginosa biofilm.25 Curcumin may destabilize the biofilm structure and reduce bacterial aggregation by inhibiting the release of this protein to the extracellular matrix or biofilm degradation resulting in interaction with this type of biofilm component proteins.
The results of some other studies indicated the inhibitory effect of Curcumin on bacterial cell division26,27 as well as its synergistic effect on some antibiotics in the treatment of antibiotic-resistant bacteria.28,29 Despite the remarkable effects of Curcumin on many bacteria, there are some limitations to using it as an antibacterial agent. Curcumin is poorly water-soluble while water is the most important constituent of the human body. Further, it has been shown that Curcumin may be toxic to eukaryotic cells at the concentration above 10 µg/mL.30 In the present study, we used Nano-Curcumin that has more solubility than exact Curcumin in water. However, Nano-Curcumin was dissolved in a maximum at a concentration of 25 µg/mL in the presence of 10% DMSO. This concentration inhibited biofilm formation in the strong biofilm producer strain of P. aeruginosa, while it did not affect bacterial growth.
The results of this study revealed that non-toxic concentrations of Curcumin in eukaryotic cells were not able to inhibit bacterial growth. For this reason, the systemically use of Curcumin to treat P. aeruginosa infections seem unexpected.
The cytotoxic effect of plant oils in high-concentrations also has been reported in some studies, which lead to the limited application of these compounds as a therapeutic agent in Invivo conditions.31,32
According to the results of this study and similar studies, it seems that the anti-biofilm effect of Curcumin in P. aeruginosa is more related to inhibition of secretion of different components of the biofilm to extracellular space of bacteria or direct disruption of biofilm stability due to reaction with its structural protein component rather than affecting the expression of regulatory genes related to QS and two-component regulatory systems that control biofilm formation in this bacterium. Further studies seem to be necessary to determine the exact mechanism of biofilm inhibition. Further, due to the low solubility of Curcumin and its minor inhibitory effect on bacterial growth at it's soluble concentrations, it's topical use can be considered as a complementary therapy in superficial infection with P. aeruginosa.
Acknowledgments
We wish to thank the Deputy of Research and Technology and Department of Microbiology, Alborz University of Medical Sciences for supporting this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rudrappa T, Bais HP. Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J Agric Food Chem. 2008;56(6):1955–1962. doi:10.1021/jf072591j
2. Skindersoe ME, Alhede M, Phipps R, et al. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52(10):3648–3663. doi:10.1128/AAC.01230-07
3. Taghadosi R, Shakibaie MR, Ghanbarpour R, Hosseini-Nave H. Role of antigen-43 on biofilm formation and horizontal antibiotic resistance gene transfer in non-O157 Shiga toxin producing Escherichia coli strains. Iran J Microbiol. 2017;9(2):89.
4. De Kievit T. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2009;11(2):279–288. doi:10.1111/j.1462-2920.2008.01792.x
5. Parkins MD, Ceri H, Storey DG. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol Microbiol. 2001;40(5):1215–1226. doi:10.1046/j.1365-2958.2001.02469.x
6. Rasamiravaka T, Labtani Q, Duez P, El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int. 2015;2015:1–17. doi:10.1155/2015/759348
7. Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76(1):46–65. doi:10.1128/MMBR.05007-11
8. Sakuragi Y, Kolter R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J Bacteriol. 2007;189(14):5383–5386. doi:10.1128/JB.00137-07
9. Tuon FF, Gortz LW, Rocha JL. Risk factors for pan-resistant Pseudomonas aeruginosa bacteremia and the adequacy of antibiotic therapy. Braz J Infect Dis. 2012;16(4):351–356. doi:10.1016/j.bjid.2012.06.009
10. Brackman G, Defoirdt T, Miyamoto C, et al. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008;8(1):149. doi:10.1186/1471-2180-8-149
11. Li B, Li X, Lin H, Zhou Y. Curcumin as a promising antibacterial agent: effects on metabolism and biofilm formation in S. mutans. Biomed Res Int. 2018;2018.
12. Raorane CJ, Lee J-H, Kim Y-G, Rajasekharan SK, García-Contreras R, Lee J. Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front Microbiol. 2019;10:990. doi:10.3389/fmicb.2019.00990
13. Vaughn AR, Haas KN, Burney W, et al. Potential role of curcumin against biofilm‐producing organisms on the skin: a review. Phytother Res. 2017;31(12):1807–1816. doi:10.1002/ptr.5912
14. Kurita T, Makino Y. Novel curcumin oral delivery systems. Anticancer Res. 2013;33(7):2807–2821.
15. O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47:e2437.
16. Tielen P, Kuhn H, Rosenau F, Jaeger K-E, Flemming H-C, Wingender J. Interaction between extracellular lipase LipA and the polysaccharide alginate of Pseudomonas aeruginosa. BMC Microbiol. 2013;13(1):159. doi:10.1186/1471-2180-13-159
17. Ganesh PS, Rai VR. Attenuation of quorum-sensing-dependent virulence factors and biofilm formation by medicinal plants against antibiotic resistant Pseudomonas aeruginosa. J Tradit Complement Med. 2018;8(1):170–177. doi:10.1016/j.jtcme.2017.05.008
18. Donadu M, Usai D, Pinna A, et al. In vitro activity of hybrid lavender essential oils against multidrug resistant strains of Pseudomonas aeruginosa. J Infect Dev Ctries. 2018;12(01):009–014. doi:10.3855/jidc.9920
19. Trong Le N, Viet Ho D, Quoc Doan T, et al. In vitro antimicrobial activity of essential oil extracted from leaves of Leoheo domatiophorus Chaowasku, DT Ngo and HT Le in Vietnam. Plants. 2020;9(4):453. doi:10.3390/plants9040453
20. Singh AK, Prakash P, Singh R, et al. Curcumin quantum dots mediated degradation of bacterial biofilms. Front Microbiol. 2017;8:1517. doi:10.3389/fmicb.2017.01517
21. Roudashti S, Zeighami H, Mirshahabi H, Bahari S, Soltani A, Haghi F. Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World J Microbiol Biotechnol. 2017;33(3):50. doi:10.1007/s11274-016-2195-0
22. Shariati A, Asadian E, Fallah F, et al. Evaluation of Nano-curcumin effects on expression levels of virulence genes and biofilm production of multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infection in Tehran, Iran. Infect Drug Resist. 2019;12:2223. doi:10.2147/IDR.S213200
23. Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One. 2015;10(3):e0121313. doi:10.1371/journal.pone.0121313
24. Sanchez-Villamil JI, Navarro-Garcia F, Castillo-Romero A, Gutierrez-Gutierrez F, Tapia D, Tapia-Pastrana G. Curcumin blocks cytotoxicity of enteroaggregative and enteropathogenic Escherichia coli by blocking Pet and EspC proteolytic release from bacterial outer membrane. Front Cell Infect Microbiol. 2019;9:334. doi:10.3389/fcimb.2019.00334
25. Reichhardt C, Wong C, da Silva DP, Wozniak DJ, Parsek MR. CdrA interactions within the Pseudomonas aeruginosa biofilm matrix safeguard it from proteolysis and promote cellular packing. MBio. 2018;9(5):e01376–01318. doi:10.1128/mBio.01376-18
26. Kaur S, Modi NH, Panda D, Roy N. Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ–a structural insight to unveil antibacterial activity of curcumin. Eur J Med Chem. 2010;45(9):4209–4214. doi:10.1016/j.ejmech.2010.06.015
27. Rai D, Singh JK, Roy N, Panda D. Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem J. 2008;410(1):147–155. doi:10.1042/BJ20070891
28. Kali A, Devaraj Bhuvaneshwar P, Charles M, Seetha KS. Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J Basic Clin Pharmacol. 2016;7(3):93. doi:10.4103/0976-0105.183265
29. Sasidharan NK, Sreekala SR, Jacob J, Nambisan B. In vitro synergistic effect of curcumin in combination with third generation cephalosporins against bacteria associated with infectious diarrhea. Biomed Res Int. 2014;2014:1–8. doi:10.1155/2014/561456
30. Damarla SR, Komma R, Bhatnagar U, Rajesh N, Mulla SMA. An evaluation of the genotoxicity and subchronic oral toxicity of synthetic curcumin. J Toxicol. 2018;2018:1–27. doi:10.1155/2018/6872753
31. Amorese V, Donadu M, Usai D, et al. In vitro activity of essential oils against Pseudomonas aeruginosa isolated from infected hip implants. J Infect Dev Ctries. 2018;12(11):996–1001. doi:10.3855/jidc.10988
32. Bua A, Usai D, Donadu M, et al. Antimicrobial activity of Austroeupatorium inulaefolium (HBK) against intracellular and extracellular organisms. Nat Prod Res. 2018;32(23):2869–2871. doi:10.1080/14786419.2017.1385014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


