Back to Journals » Medical Devices: Evidence and Research » Volume 14
Invention and Pilot Study of the Efficacy and Safety of the SUPRAtube Device in Continuous Supraglottic Aspiration for Intubated and Mechanically Ventilated Patients
Authors Ramírez-Sarmiento A , Aya O, Cáceres-Rivera D, Reyes CF, Espitia A , Pizarro C, Gea J, Castillo VR, Orozco-Levi M
Received 9 July 2021
Accepted for publication 26 August 2021
Published 5 October 2021 Volume 2021:14 Pages 287—297
DOI https://doi.org/10.2147/MDER.S328485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Alba Ramírez-Sarmiento,1– 3 Orlando Aya,3,4 Diana Cáceres-Rivera,1,2 Carlos F Reyes,2– 4 Angela Espitia,1,2 Camilo Pizarro,3,4 Joaquim Gea,5,6 Victor R Castillo,7,8 Mauricio Orozco-Levi1– 3,6
1Respiratory Department, Instituto Cardiovascular y Hospital Internacional de Colombia, Fundación Cardiovascular de Colombia, Floridablanca, Santander, Colombia; 2EMICON Research Group MINCIENCIAS, Bogotá, Cundinamarca, Colombia; 3Universidad de Santander (UDES), Bucaramanga, Santander, Colombia; 4Department of Critical Care, Fundación Cardiovascular de Colombia, Floridablanca, Santander, Colombia; 5Respiratory Department, Parc de Salut Mar, Barcelona, Catalonia, Spain; 6CEXS-Universidad Pompeu Fabra, Barcelona, Catalonia, Spain; 7Department of Cardiovascular Surgery, Fundación Cardiovascular de Colombia, Floridablanca, Santander, Colombia; 8Biomedical and Translational Research Group, Fundación Cardiovascular de Colombia, Floridablanca, Santander, Colombia
Correspondence: Mauricio Orozco-Levi
Respiratory Department, ICV-HIC, Fundación Cardiovascular de Colombia, Calle 155a no. 23-58, El Bosque, Floridablanca, Santander, Colombia
Tel +57 3175741421
Email [email protected]
Background: Bronchoaspiration of content that accumulates in the supraglottic area (eg, saliva, gastroesophageal reflux) is a risk factor for ventilator-associated pneumonia. A continuous supraglottic suction system may decrease the risk of bronchoaspiration in these patients.
Objective: (1) Constructing a conceptual model and functional prototype of a continuous supraglottic suction device for use in humans; (2) defining functional characteristics in ex vivo swine head models; and (3) evaluating its efficacy and safety in mechanically ventilated patients.
Methods: Study conducted in three phases. First phase: definition of distances and diameters of the triangle determined by dental arch, posterior oropharynx and vallecula, and diameter of the oropharynx in axial projection; and identification of the declining area of supraglottic suction. Second phase: design engineering and functional prototype evaluated in ex vivo models. Third phase: evaluation of device use in terms of safety and efficacy in ventilated patients.
Results: We obtained a final functional model of the SUPRAtube device injected into PVC for medical use. Device effectiveness in in vitro simulation showed a high and fast suction capacity of liquid and thick volumes. Study of swine heads allowed to validate the shape, size and functional fenestration of the device. Study in intubated and mechanically ventilated patients showed a high supraglottic suction capacity and the absence of local adverse events during 72 (7– 240) hours of continuous operation.
Conclusion: Our study describes the process of conceptualization, design and production of a practical, safe, low-cost continuous supraglottic suction device without representing antibiotic pressure, which appears to be a new complementary preventive strategy for the standard management of intubated and mechanically ventilated patients.
Keywords: mechanical ventilation, bronchoaspiration, supraglottic suction, SUPRAtube, endotracheal tube, gastroesophageal reflux
Introduction
The present study describes the conceptual design, functional prototyping and final deliverable of a technological innovation device designed for continuous supraglottic suction in intubated and mechanically ventilated patients, named with the acronym SUPRAtube. This device has received a patent as a utility model (ref. NC2016/0002059 Resolution 466 of the Superintendence of Industry and Commerce, Colombia) due to its novel nature, in the absence of similar devices on market, as a complementary solution for the prevention of complications such as nosocomial pneumonia in these patients.
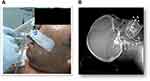
Patients under invasive mechanical ventilation (MV) suffer a high incidence of gastroesophageal reflux (GER) as well as an accumulation of saliva and other secretions in the oropharyngeal area.1,2 Macroaspiration in critically ill patients witnessed by health personnel range from 1% to 12% of frequency. Microaspiration (small volume and clinically silent) is much more common, which can have a frequency of up to 94% of patients who are in MV.3 The consequences of bronchoaspiration depend on chemical composition and volume of the aspirated material, as well as of the presence and virulence of infectious agents and morbid conditions of the patient Figure 1A. Composition of the aspirated material, not only acid or liquid but also non-acid or gaseous, and in addition the presence of pepsin and bile salts, are aggressions for the delicate respiratory epithelium. Bronchoaspiration of such oropharyngeal contents is the main risk factor for pneumonia in intubated and mechanically ventilated patients.4
Clinical experience shows that current preventive strategies are insufficient to abolish GER as a risk of ventilator-associated pneumonia (VAP). It is pertinent, reasonable and mandatory, to search for additional strategies that allow complementing the current conventional measures to reduce the risk of bronchoaspiration in these patients.5 In this sense, we have considered that there is both high clinical interest and scientific plausibility regarding an additional practical utility of SUPRAtube, understanding that it is directly related to nosocomial pneumonia risk management. Suction of the oropharyngeal and gastroesophageal reflux (GER) contents that accumulate in the supraglottic sphere represents one of the main risk factors for nosocomial pneumonia, especially in patients under mechanical ventilation. Our impression is that there is a conformism considering that we already do everything necessary to prevent bronchoaspiration and GER in these patients. It is evident that the usual treatment simplified to a prokinetic, oropharyngeal decontamination, semirecumbent body position, gastric acidification, suction of bronchoaspirated secretions, saliva ejector, esophageal balloon probes, among others, are insufficient to prevent nosocomial pneumonia, for which multiple other additional prevention strategies are needed. Antibiotics seem to be few or inadequate in this line because of the risk of multi-resistance selection. In the real-life clinical setting, identifying the causes of GER involves indisputable diagnostic complexity that varies as the patient is under critical care. GER is modifiable in these patients, but therapeutic interventions require the consideration of a comprehensive diagnosis of its causes in order to intervene in a multimodal way and mitigate risk. We consider that applied research should continue evaluating additional innovative strategies such as continuous or intermittent suction devices, artificial intelligence, augmented reality, application of information and communication technologies (ICTs), among others, to identify new forms of preventive and therapeutic intervention against the risk and consequences of NN in patients hospitalized in the ICU for various causes.
The objectives of the present study were to determine the ideal shape, size and positioning of a device that allows continuous oropharyngeal suction in mechanically ventilated humans; to evaluate performance of the SUPRAtube supraglottic suction device functional model, in terms of suction capacity (velocity–volume ratio), adequate design, and ideal material(s) to achieve suction, thus avoiding device collapse and adhesion to the oropharynx soft tissues; and to build a definitive prototype of the SUPRAtube, and evaluate its performance in mechanically ventilated patients.
Methods
Methodological aspects are further described in the repository addendum available on the website (Supplementary Material).
Ethical Considerations
The study protocol was reviewed and approved by both Institutional Review Board and Ethics committee (Comité de Ética de la Fundación Cardiovascular de Colombia, CEI-FCV); certificate ref. 361, December 2014 for phases 1 and 2; certificate ref. 424, May 2017 for Phase 3. The patient's next of kin have provided informed consent for the images to be published.
Aspects Related to the Study on Swine Heads
The studies were conducted on swine heads obtained from the meat industry. The swine heads did not imply a biological risk, as they were obtained from meat production of certified animals for human consumption. The heads were cremated following the sanitary protocol of the institution.
Aspects Related to the Study on Humans
CT scans from intubated and non-intubated patient groups were retrospectively identified from the inventory of brain and neck images obtained from adult individuals for any medical indication and that were available in the institution’s radiology department. To be evaluated, the images were always anonymized. Images of cases with craniocervical trauma, apparent deformities or craniocervical surgery were excluded. In addition, 10 intubated and mechanically ventilated patients were included (Table 1). The regulations of the World Medical Association for Research on Humans (Helsinki Declaration) were complied with. Instrumentation was always in accordance with the rules of good clinical practice. Participation in the study included informed and written consent from the close responsible family members of each intubated individual (next of kin). The Written Informed Consent Sheet was signed by the next of kin after reading and knowing the objectives, techniques to be used and any inconvenience that participation could cause. Patient confidentiality was always respected. The study was registered in clinicaltrials.gov (identifier NCT03573609). The present study includes the interim analysis conducted before data collection has been completed for the clinical trial. The authors intend to share with other individuals deidentified.
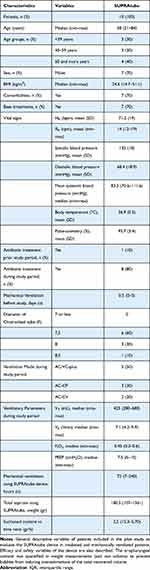 |
Table 1 General Characteristics of the Study Population |
Project Operational Organization
For the Development and Scope of the Main Objective and each of the Specific Objectives of the SUPRAtube Project, components and activities were operatively organized into three complementary “Work Phases”.
The first phase was focused on “ANTHROPOMETRIC STUDY” in head and neck tomography (50 of non-intubated patients, 50 of intubated patients) (Figure 1B).
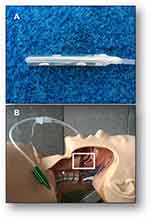
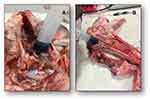
The second phase resolved the “DESIGN OF THE SUPRAtube DEVICE AND VALIDATION IN SWINE HEADS”. We designed the conceptual (theoretical) model (Figure 2) and evaluated the functional prototype of the device on porcine heads (Figure 3). We verified the absence of adhesion to the soft tissue in the anatomical portion. We conducted analysis of suction speeds and volumes. We designed plans, renders and technical reports that demonstrate functionality of the prototype. In a group of functional models, we built and verified operation of the SUPRAtube device, including design of the eventual fixation system to the orotracheal tube; we documented the construction process and the tests carried out, as well as their results. We designed a detailed engineering model of the selected SUPRAtube model with modelling, construction plans, renders, simulation of the designed system, and technical support.
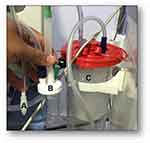
The third phase presented the “EXPLORATORY EVALUATION OF THE SUPRAtube IN PATIENTS”. We prospectively identified patients hospitalized in the Intensive Care Unit, with orotracheal intubation, and requiring mechanical ventilation. The effect of SUPRAtube was studied in ten (n = 10) patients, who received standard treatment throughout the study without their participation in the study implying an omission or a therapeutic addition different from those conventional and required by their medical condition that led to intubation and mechanical ventilation in the Intensive Care Unit. Participation in the study did not change the basic treatment or commodities of any patient. All of them received the usual and necessary medical strategies for the management of their underlying disease that justified the hospitalization in the ICU as well as for eventual complications. Patients were studied in one condition and only once, with the SUPRAtube being inserted by members of the research group. The sample size was defined by consensus and convenience. All patients had a nasogastric tube. During the study, the standard treatment was maintained, without the patients’ participation in the study implying an omission or a therapeutic addition different from those conventional and required by their medical condition that led to intubation and mechanical ventilation. The device was inserted buccally, and its positioning was verified using a video bronchoscope for supraglottis evaluations. It was connected to continuous wall suction through conventional tubing with a collecting trap flask inserted in circuit. Suction was maintained continuously during the mechanical ventilation period (Figures 4 and 5).
Statistical Analysis of the Results
Volume of oropharyngeal secretions aspirated per unit of time. We recorded demographic and anthropometric characteristics, patient morbidity, orotracheal tube used, ventilatory modality used, airway pressure, tidal volume, minute volume, respiratory rate, mean pressure, SUPRAtube suction cannula peaks and valleys during continuous oropharyngeal aspiration, volume of oropharyngeal secretions aspirated per unit of time, and complications during device usage (erosions, lacerations, bleeding, displacement, migration, need for removal). As it is the functional design and definitive prototype of a biomedical device, the data obtained relevant to the objectives were those corresponding to the volumes and weights of aspirated secretions, which were analyzed as a dependent variable and in absolute values.
Results
Phase 1
The anthropometric study allowed obtaining average measurements of the triangle distances, determined by dental arch, posterior oropharynx and vallecula. We measured the oropharynx diameter in axial projection (Table 1). We identified the nadir decline zone (distance and diameter) as the “target” zone for ideal supraglottic suction. We designed the SUPRAtube device through the Bioengineering Design and Development process (Figure 6A). Several primary conceptual designs were converted into functional models. We tested as many new designs as needed. Evaluation of the various functional models allowed the identification of several fundamental safety challenges, such as: preventing adherence-suction of oropharyngeal tissues; preventing a bloody tip; preventing negative pressure collapse and preventing obstruction of the suction end by secretions and thick fluids. The solutions to challenges made it possible to reach a sufficient and consensual design as an ideal end device, the SUPRAtube. Specifically, multiple foramina sequentially aligned in four quadrants were included in conjunction with a distal apical foramen to prevent adhesion-suction of oropharyngeal tissues (Figure 6B). We developed a suction indirectly distributed on the suction thimble by means of a coaxial tube of smaller diameter that reduces the risk of adhesion. We decided that the distal end of the suction device should be blunt, soft and malleable to make it less bloody. And, to prevent dynamic collapse problems, we designed the distal O-ring, which provides stability against transverse axial forces, maintaining malleability of the end against longitudinal forces. We built device prototypes with all the expected features of safety, reliability, ergonomics and function. The device was based on current quality standards for the manufacture of biomedical equipment.
Phase 2
Evaluations in porcine models confirmed its correct functioning even in small-diameter spaces, as a representation of the nasal passages and sinuses.
Phase 3
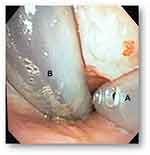
Sequentially, we proceeded to evaluate functional characteristics in terms of suction capacity in ICU patients, with endotracheal intubation and requiring mechanical ventilation (video available). We performed the second endoscopic study upon study completion, also by video bronchoscopy, emphasizing potential complications due to device pressure, suction, vacuum or contusion. We evaluated complications and the presence of secretions in vallecula, pyriform sinuses, vocal cords or some other areas of the oropharynx, through endoscopic evaluation (Figure 7). Demographic and anthropometric characteristics, and patient morbidity did not demonstrate any connection with device insertion or operation difficulties. The SUPRAtube device did not affect the patients’ orotracheal tube at all, nor was it related to the ventilatory modality used, airway pressure, tidal volume, minute volume, or respiratory rate. Mean pressure, peak and valley, in the SUPRAtube suction cannula always remained stable between values of −20 to −30 mmHg. Measurement of the aspirated volume was made difficult by the presence of foam, for which weight was assumed as the objective variable less susceptible to measurement bias. One of the most striking points is that the initial oropharyngeal suction volume in some cases reached up to 415 mL in only approximately 6 hours, despite the fact that patients were receiving standardized conventional respiratory therapy strategies (Figure 8). The weight of oropharyngeal secretions aspirated per unit of time varied widely among volunteers. Higher values were quantified within the first hour (65±110 gr) and decreased in the following hours. No erosions, lacerations, bleeding, displacement, migration, or need for removal were observed in any case due to adverse events related to the device (Table 2).
 |
Table 2 Fiberendoscopic Assessment of Supraglottic Adverse Events in Patients Submitted to Supratube Treatment: Baseline vs Final Evaluations |
Discussion
The present study describes an original technological development motivated by a real need perceived within our clinical experience: the potential accumulation of naso-oropharyngeal secretions and gastroesophageal reflux products in the oropharynx area of patients who are admitted to the ICU with mechanical ventilation. These secretions represent a widely recognized risk factor for the development of health care-associated pneumonia and other respiratory complications.6 It is reasonable to try that the content that accumulates in the oropharynx does not pass through the glottis, as an attempt to reduce colonization and infection of the lower respiratory tract.7 This oropharyngeal content is the result of an eventual combination of saliva, sinus secretions, nasal secretions, bleeding and gastroesophageal reflux. We have hypothesized that a pre-bronchoaspiration suction system could have a beneficial impact in addition to the multiple usual strategies for the prevention of bronchoaspiration of oropharyngeal content of any origin and, consequently, could reduce the risk of tracheobronchitis, pneumonia and other complications associated with health care. Several aspects must be highlighted:
The first aspect is the original nature of the study. As far as we have been able to find, there is no specific device for continuous suction of oropharyngeal (supraglottic) content in intubated and mechanically ventilated patients. Our device had to be conceptualized, designed, prototyped and validated without being able to compare it with another device with similar characteristics. This becomes a strength of the study itself and determines that the SUPRAtube device has been patented as a utility model.
The second aspect is relevance of the SUPRAtube device. Our results suggest that the fluid accumulated in the supraglottis is frequent and abundant, and often underestimated. In this sense, it is suggested that saliva production can reach up to 800–1000 mL/24 hours in healthy individuals. If they are intubated, the accumulation of liquid content in supraglottis is even greater due to presence of the orotracheal (or nasotracheal) tube, supine body position, decreased abdominal compliance, gastric distension, compression of the esophagus by the pneumotracheal balloon, alteration of swallowing dynamics or absence of swallowing reflex, muscle relaxant drugs, sedation, and presence of nasogastric or orogastric tube, among others.
The third aspect is clinical relevance of the aspiration of contents accumulated in supraglottis. It has been suggested that 45% of healthy individuals habitually aspirate during sleep. Commonly, hospitalized patients are colonized by microorganisms acquired from hospital environment, and up to 75% of seriously ill patients get colonized within 48h.8 The first route of lung infection is precisely through microaspiration of microorganisms colonizing the oropharyngeal secretions. The main complication of bronchoaspiration is infection of the respiratory system, with bacterial tracheobronchitis and hospital-acquired pneumonia (HAP) being the most frequent.9 HAP leads to the causes of hospital-acquired infection deaths with a mortality of between 20–50%10–12 and, far from being an eradicated problem, it affects a high percentage of patients admitted to any hospital both in Colombia and outside the country. Ventilator-associated pneumonia (VAP) is the most frequent nosocomial infection in critically ill patients and is one of the main causes of prolonged hospitalization and ICU mortality. Severity depends on the number and virulence of pathogens entering the lower respiratory tract.13 Bronchoaspiration-associated lung injury is characterized by lung inflammation, capillary leakage, and oxidative damage. Possible outcomes range from mild pneumonitis to acute respiratory distress and death. The incidence of VAP ranges between 10% and 20% of patients with more than 48 hours on mechanical ventilation, and the attributable mortality doubles that of patients not suffering from it.14–16
The fourth aspect to highlight has to do with technical features of the SUPRAtube device. The materials of the different parts are biocompatible. Device usage is intuitive, and its placement technique is simple. The fixation system is universal regardless of the type of endotracheal tube used. The suction system connections have been designed so that they have connectivity with conventional tubes. The possibility of interspersing a collecting vessel allows continuous quantification of the volume of oropharyngeal content aspirated, a variable that to date has not been controlled or identified in the conventional clinical settings in which we have considered use of the device. The tube is translucent, which makes it possible to evaluate the characteristics of aspirated material. The novel design appears to guarantee that the device aspirates without the possibility of creating a local vacuum in front of the oropharyngeal epithelium. The aspiration capacity (a few seconds even for volumes greater than 200 mL) allows us to assume that the device will be useful and especially indicated in cases of emesis.
The fifth aspect to highlight is related to device production with polyvinyl chloride (PVC). Life cycle analyses show that the environmental impact of PVC is equivalent to or even more favorable than other materials. The application of existing technology makes PVC one of the most respectful materials with human beings and the environment, being very difficult to replace in some medical devices.
Another relevant aspect is the potential clinical impact of SUPRAtube in preventive terms of tracheobronchitis and pneumonia, associated both with mechanical ventilation and other pathologies or interventions that lead to increased oropharyngeal content. Given that low production price and functional concept of the device provide a possibility for its globalization, future clinical trials may be representing real-life studies in the prevention of morbidity and mortality associated with accumulation of biological fluids from gastroesophageal reflux and the inevitable oropharyngeal colonization in instrumentalized patients.
The present study has limitations that deserve to be mentioned. A potential limitation, intrinsic to future clinical trial in humans, is the impossibility of predefining which patients will present vomiting, macro-regurgitations, sinus secretions or oropharyngeal secretions of greater magnitude. We have not evaluated the possibility of using the SUPRAtube in pediatric patients. There are no data regarding safety of the device operation for several consecutive days. Although we promote its insertion by buccal route, we must keep in mind the possible alternative of nasal introduction. Finally, we did not evaluate the complementary benefits of intermittently instilling some antiseptic solutions through the SUPRAtube itself to reduce risks related to bacterial biofilm and reduce oropharyngeal inoculum. We are convinced that the SUPRAtube device should be evaluated in clinical trials to determine its impact in terms of safety and clinical efficacy in preventing VAP.
The present study allows concluding that the SUPRAtube device is effective and safe to be used in continuous supraglottic suction for intubated and mechanically ventilated patients. SUPRAtube is practical and shows a low production cost. The device appears to be beneficial as a preventive non-redundant strategy that does not exert antibiotic pressure and is complementary to the standard management of intubated and mechanically ventilated patients. These results justify the design of studies to evaluate the efficacy and safety of device operation during all days of mechanical ventilation.
Data Sharing Statement
The authors intend to share with other individuals deidentified participant data including demographics, clinical characteristics, and specific data regarding safety and efficacy of the device. Data are available on request from the corresponding author ([email protected]).
Acknowledgments
We thank to Nidya López for her assistance in acquisition of data. This study was financed in part by MINCIENCIAS (COLCIENCIAS) Ref. 656677758334 contrato No. 833-2017, and Fundación Cardiovascular de Colombia.
Disclosure
The authors ARS and MOL declare to be inventors with intellectual property of the device. The patrimonial property of the same belongs to the FCV institution. ARS reports grants from MINCIENCIAS, during the conduct of the study, and has a patent Patente Modelo de Utilidad licensed to ref NC2016/0002059. MOL reports grants from Ministerio de Ciencia y Tecnologia de Colombia MINCIENCIAS, during the conduct of the study, and has a patent Patente de modelo de utilidad licensed to ref NC2016/0002059. The authors report no other potential conflicts of interest for this work.
References
1. Orozco-Levi M, Félez M, Martínez J, et al. Gastroesophageal reflux in mechanically ventilated patients: effects of an oesophageal balloon. Eur Respir J. 2003;22:348–353. doi:10.1183/09031936.03.00048902
2. Schallom M, Orr J, Metheny M, Pierce J. Gastroesophageal reflux in critically ill patients. Dimens Crit Care Nurs. 2013;32:69–77. doi:10.1097/DCC.0b013e318280836b
3. Nseir S, Zerimech F, Jaillette E, Artru F, Balduyck M. Microaspiration in intubated critically ill patients: diagnosis and prevention. Infect Disord Drug Targets. 2011;11:413–423. doi:10.2174/187152611796504827
4. Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 2017;36:1999–2006. doi:10.1007/s10096-016-2703-z
5. Ferrer M, Bassi GL, Torres A. Medidas prácticas para la prevención de la neumonía nosocomial [Practical measures for the prevention of nosocomial pneumonia]. Medicina respiratoria. 2013;6:33–44. Spanish.
6. Messika J, La Combe B, Ricard J. Oropharyngeal colonization: epidemiology, treatment and ventilator-associated pneumonia prevention. Ann Transl Med. 2018;6(21):426–427. doi:10.21037/atm.2018.10.17
7. Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med. 2005;118:11–18. doi:10.1016/j.amjmed.2004.07.051
8. Venditti M, Falcone M, Corrao S, Licata G, Serra P. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med. 2009;150:19. doi:10.7326/0003-4819-150-1-200901060-00005
9. Yang CS, Qiu HB, Zhu YP, Huang YZ, Xu XT. Effect of continuous aspiration of subglottic secretions on the prevention of ventilator associated pneumonia in mechanically ventilated patients: prospective, randomized, controlled clinical trial. Zhonghua Nei Ke Za Zhi. 2008;47:625–629.
10. Craven DE, Palladino R, McQuillen DP. Healthcare-associated pneumonia in adults: management principles to improve outcomes. Infect Dis Clin North Am. 2004;18:939. doi:10.1016/j.idc.2004.08.001
11. Blanquer J, Aspa J, Anzueto A, et al. Sociedad Española de Neumología y Cirugía Torácica. SEPAR Guidelines for Nosocomial Pneumonia. Arch Bronconeumol. 2011;47:510–520. doi:10.1016/j.arbres.2011.05.013
12. Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J. 2017;50:1700582. doi:10.1183/13993003.00582-2017
13. Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med. 2005;118:11–18. doi:10.1016/j.amjmed.2004.07.051
14. Pace F, Bianchi Porro G. Gastroesophageal reflux disease: a typical spectrum disease a new conceptual framework is not needed. Am J Gastroenterol. 2004;99(5):946–949. doi:10.1111/j.1572-0241.2004.04164.x
15. Nind G, Chen WH, Protheroe R, et al. Mechanisms of gastroesophageal reflux in critically ill mechanically ventilated patients. Gastroenterology. 2005;128(3):600–606. doi:10.1053/j.gastro.2004.12.034
16. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest. 2006;129(5):1210. doi:10.1378/chest.129.5.1210
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.