Back to Journals » OncoTargets and Therapy » Volume 13
Invasive Micropapillary Carcinoma with CEP17 Monosomy of the Bilateral Breast: A Rare Case Report and Review of the Literature
Authors Zhang L , Wang Y, Zhang L, Xing H, Niu C, Yu Q, Tang L
Received 29 February 2020
Accepted for publication 19 June 2020
Published 2 July 2020 Volume 2020:13 Pages 6425—6432
DOI https://doi.org/10.2147/OTT.S251934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Le Zhang,1,* Yuechen Wang,2,* Leichao Zhang,3 Hua Xing,1 Chunbo Niu,3 Qiong Yu,4 Lu Tang1
1Department of Breast Surgery, The Third Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China; 2Department of Breast Surgery, Tohoku University Hospital, Sendai, Miyagi 980-0000, Japan; 3Department of Pathology, The Third Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China; 4Department of Radiology, The Third Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lu Tang Phone/ Fax +86 431-84995495
Email [email protected]
Abstract: Invasive micropapillary carcinoma (IMPC) is a novel type of breast cancer which is potentially very aggressive and may show early lymphatic infiltration. Monosomy of chromosome 17 (m17) is rare in breast cancer, and according to the 2018 guidelines of the American Society of Clinical Oncology/College of American Pathologists, the decision to administer trastuzumab treatment should be made based on positive human epidermal growth factor receptor 2 results by immunohistochemistry. Here, we report a rare case of bilateral local advanced IMPC involving m17. A 33-year-old woman found a mass measuring 30 mm on the left breast that increased to 100 mm over 3 months. A diagnosis of IMPC was made based on the findings of core needle biopsies of bilateral breast masses and left axillary lymph node, and m17 was detected by fluorescence in situ hybridization (FISH). The patient underwent 6 cycles of neoadjuvant chemotherapy (docetaxel, epirubicin, and cyclophosphamide) and left-side modified radical mastectomy, left axillary lymph node dissection, right breast-conserving surgery, and right sentinel lymph node biopsy. Postoperative pathologic analysis of both breasts revealed IMPC, and m17 was confirmed by FISH. The patient received radiotherapy and endocrine therapy but rejected trastuzumab treatment. The patient was still alive at the 30-month follow-up, without recurrence or metastasis. Our findings suggest that loss of chromosome 17 may influence prognosis or therapeutic response, which needs to be further confirmed.
Keywords: human epidermal growth factor receptor 2, breast cancer, fluorescence in situ hybridization, chromosome enumeration probe 17, CEP17
Introduction
Invasive micropapillary carcinoma (IMPC), described as an invasive papillary cancer with an exfoliative appearance,1 was first described by Siriaunkgul and Tavassoli in 1993 as a rare subtype of epithelial breast tumor. These authors proposed the recognition of “invasive micropapillary carcinoma of the breast” as a novel entity with a potentially high degree of aggressiveness; it was listed in the 2003 World Health Organization histologic classification of tumors of the breast as an invasive carcinoma.2,4
The gene encoding human epidermal growth factor receptor 2 (HER2) is located on the long arm of chromosome 17 (17q11.2–q12.0); its amplification is generally associated with overexpression of the oncoprotein.5,6 Monosomy of chromosome 17 (m17) is observed in 1.4% of breast carcinoma cases.7 HER2-amplified breast cancer combined with m17 may exhibit unique prognostic or therapeutic response profiles compared to other HER2-positive tumors.8 In this report, we describe the clinical features of a case of bilateral breast cancer of the IMPC type with chromosome enumeration probe 17 (CEP17) monosomy in accordance with CARE guidelines,9 with the aim of facilitating the diagnosis and treatment of this rare malignancy.
Case Report
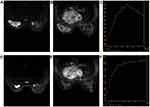
A 33-year-old woman without pain or fever detected a firm mass measuring 30 mm on the central part of the left breast, which quickly grew to a size of 100×70 mm in 3 months. During this period she did not receive any treatment. The patient had a 6-year-old son and no family history or history of medication or psychosocial issues. Physical examination revealed a 150×120 mm mass in the central quadrant of the left breast that caused the nipple to retract (Figure 1A). Superficial lymph nodes in both axillae were not significantly enlarged, and no masses were detected in the right breast. An ultrasound (US) examination revealed a low-echoic lesion in the left breast whose size was too large to estimate (Figure 2A), while no hypoechoic mass was observed in the right breast. There were 6 enlarged lymph nodes in the left axilla with an indistinct medullary boundary (Figure 2B). Mammography revealed dense glands in both breasts that were asymmetrically distributed. Diffuse, microscopic calcifications and spiculated masses were present throughout the left breast (Breast Imaging–Reporting and Data System 4), and spiculated masses were also observed in the right breast. Breast magnetic resonance imaging (MRI) imaging revealed heterogeneous enhancement of the left breast with nipple inversion. Strikingly, there was an ovoid, poorly defined mass in the upper quadrant of the right breast; although visible enhancement was observed by MRI (Figure 3A–C), the color Doppler US examination result was negative. Staging workups including chest and brain computed tomography, liver US, and whole-body bone scintigraphy were negative for metastatic disease.
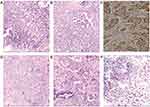
Core needle biopsy of bilateral breast masses suggested IMPC, while cytologic detection of metastatic breast carcinoma in the left axillary lymph node yielded positive findings (Figure 4A–C). Immunohistochemistry (IHC) of the left breast showed that cancer cells were positive for estrogen receptor (ER), progesterone receptor (PR), and HER2 (2+) (Figure 5A); fluorescence in situ hybridization (FISH) revealed m17 (Figure 5C); and the Ki-67 proliferation index was 20%, with similar IHC and FISH results obtained for the right breast mass (Figure 5B, D). The pathologic diagnosis was locally advanced, stage IIIA cancer (cT3N1M0) in the left breast and stage IA cancer (cT1N0M0) in the right breast. The patient received neoadjuvant chemotherapy consisting of docetaxel (120 mg), epirubicin (140 mg), and cyclophosphamide (800 mg) every 21 days for 6 cycles.
During the treatment course, the size of the tumor decreased significantly as a result of neoadjuvant chemotherapy as determined by physical examination, US, and breast MRI (Figures 1–3, 4D–F), and there were no severe adverse effects. We recommended mastectomy on the left side with immediate breast reconstruction but this was rejected by the patient, who instead elected to undergo left-side modified radical mastectomy, right breast-conserving surgery, and right sentinel lymph node biopsy. The results of pathologic analysis of the left breast mass were consistent with chemotherapy-associated changes in IMPC, which occupied a large area and could not be evaluated in terms of size. Dissected left axillary lymph nodes showed 12 of 16 signs of metastasis. The left breast mass was positive for ER, PR, HER2 (2+), Ki-67 (20%), and epithelial membrane antigen (EMA) by IHC. The right-side lesion was determined as multifocal IMPC (0.5–2 mm in diameter). There was no metastasis in the sentinel lymph nodes. The right breast mass was positive for ER, PR, HER2 (2+), and EMA by IHC, and the Ki-67 proliferation index was 10%. FISH confirmed m17 on both sides. Postoperatively, the patient received radiation therapy and ovarian function suppression plus aromatase inhibitor therapy but refused trastuzumab treatment. At the 30-month follow-up, the patient was still alive and there was no evidence of metastasis (Figure 6).
Discussion
IMPC is characterized by early lymph node metastasis and progression, which is the reason for the late-stage diagnosis in our patient. Based on the pathologic characteristics, we speculated that the right breast cancer was caused by metastasis from the left side. HER2 protein localization in IMPC deviates from the circumferential pattern that is often seen in nonspecific invasive breast cancer. Among 21 cases of IMPC that were identified as HER2-amplified by FISH, 9 were IHC 1+10,11 This may be due to the distinct IHC staining pattern in IMPC, especially at cell-cell contacts or the basolateral membrane in micropapillary structures, in contrast to the typical staining of the cytoplasmic membrane facing the stroma in invasive ductal carcinoma not otherwise specified (IDC-NOS).12 Therefore, even when IMPC is HER2-positive by IHC, FISH should be performed to determine whether there is HER2 gene amplification.
A retrospective multicenter study of 534 patients with IMPC or IDC-NOS found that while 5- and 10-year overall survival rates were similar between the cohorts (both >90%, p=0.67), recurrence-free rate at 2 years was lower for IMPC than for IDC-NOS (p=0.007).13 An analysis of 327 IMPC and 4979 IDC patients who underwent primary resection between 2008 and 2012 reported no differences in overall survival (p=0.752) or disease-free survival (p=0.578) between cohorts.14 Another study found that IMPC is more aggressive and has worse prognosis than IDC irrespective of the molecular subtype, which may be attributable to the higher incidence and greater aggressiveness of tumor–node–metastasis stage III tumors.15
According to the 2013 guidelines American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP), cases with a HER2/CEP17 ratio ≥2.0 and mean HER2 signals/cell <4.0 should be reported as HER2-positive based primarily on the results of the Herceptin Adjuvant clinical trial.16 This subset of patients showed no obvious trend of nonresponsiveness to HER2-targeted therapy.17 Many studies have since investigated whether these patients should be considered as having HER2 amplification and would thus benefit from trastuzumab therapy. A recent study in which patients with HER2-amplified m17 breast cancer were treated with trastuzumab yielded results that were similar to those of a large Phase III adjuvant trastuzumab trial of HER2-positive patients, supporting the key role of HER2-targeted therapy in this patient population,18 although it did not specifically evaluate cases with HER2 copy number <4.0. According to the 2018 ASCO/CAP guidelines, cases that are IHC 2+ with HER2 signals/cell <4.0 and HER2/CEP17 ratio ≥2.0 should be diagnosed as HER2-negative.11
HER2 signals/cell <4.0 and HER2/CEP17 ratio ≥2.0 are frequently observed in conjunction with chromosome 17 copy number variation (ie, true monosomy or loss of a portion of chromosome 17).18 When 10 samples with HER2 signals/cell <4.0 and HER2/CEP17 ratio ≥2.0 were retested with an alternative chromosome 17 probe (D17S122), 10% had HER2/D17S122 ratio ≥2.0 and HER2 signals/cell ≥4.0; 40% had HER2/D17S122 ratio <2.0 and HER2 signals/cell ≥4.0; and 50% were unchanged.19 These results were confirmed in another report.20 The incidence of m17 was found to be 0.8% using FISH to detect tumor protein P53 (TP53), retinoic acid receptor alpha (RARA), and CEP1721 or Smith-Magenis syndrome (SMS), RARA, and TP53 genes.20,22,23
Chromosome 17 contains additional genes that play a critical role in breast cancer pathogenesis and DNA repair including topoisomerase II α (TOP2A), breast cancer 1 (BRCA1), TP53, and Erb-B2 receptor tyrosine kinase 2 (ERBB2, encoding HER2). As such, loss of chromosome 17 may influence prognosis or therapeutic response.24,25 For example, TOP2A expression has been linked to sensitivity to anthracycline; therefore, loss of TOP2A may result in chemotherapy resistance.26 The mechanism of doxorubicin resistance in IMPC has been investigated using MCF-7 breast cancer cells cultured into 3-dimensional spheroids.27 However, it remains to be determined whether IMPC is sensitive to paclitaxel and anthracycline chemotherapy.
IMPC has a unique growth pattern in which tumor cells adhere to cell clusters. Therefore, IHC detection of HER2-positive tumor cells may be insufficient for diagnosis, and FISH should be performed to determine whether HER2 is amplified. Our case report also demonstrates that if the tumor is on the inner side of the breast, the contralateral breast should be examined because of the presence of lymphatic branches in both breasts. A limitation of our study is that the patient was treated with chemotherapy and surgery from 2016 to 2017; anti-HER2 treatment should have been administered in accordance with 2013 ASCO/CAP guidelines but was not carried out because the patient could not afford the drug as it was not covered by insurance. Another limitation is that we examined only 1 case, and were therefore unable to evaluate the clinical significance of chromosome 17 deletion in IMPC.
Conclusion
The results of this study show that IMPC is a novel, distinct type of breast cancer with a potentially high degree of aggressiveness and early lymphovascular invasion. If the cancer occurs on the inner side of the breast, the contralateral breast should be examined because of the presence of lymphatic branches in both breasts. m17 is rare in breast cancer, and whether anti-HER2 treatment is appropriate should be determined based on positive HER2 IHC results. Finally, chromosome 17 deletion may have biological implications in terms of therapeutic response and prognosis.
Patient’s Perspective
At first, I agreed to undergo neoadjuvant chemotherapy because I did not want to lose my breasts, but at the end of the 6 cycles of chemotherapy, the doctor told me that I could not keep my breasts in any case. I felt very sad. Although the doctor strongly recommended anti-HER2 treatment, I refused it because of my family’s poor economic situation.
Acknowledgments
This study was approved by the ethics committee of The Third Hospital of Jilin University. Written, informed consent was obtained from the patient for publication of this case report and accompanying images.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors disclose no potential conflicts of interest.
References
1. Fisher ER, Palekar AS, Redmond C, Barton B, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol no. 4). VI. Invasive papillary cancer. Am J Clin Pathol. 1980;73(3):313–322. doi:10.1093/ajcp/73.3.313
2. Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6(6):660–662.
3. Luna-More S, Gonzalez B, Acedo C, Rodrigo I, Luna C. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract. 1994;190(7):668–674. doi:10.1016/S0344-0338(11)80745-4
4. Ellis IO, Cornelisse CJ, Schnitt SJ. Invasive Breast Carcinoma. Lyon: IARC Press; 2003.
5. Bartlett JM, Going JJ, Mallon EA, et al. Evaluating HER2 amplification and overexpression in breast cancer. J Pathol. 2001;195(4):422–428. doi:10.1002/path.971
6. Bartlett J, Mallon E, Cooke T. The clinical evaluation of HER-2 status: which test to use? J Pathol. 2003;199(4):411–417. doi:10.1002/path.1354
7. Ballard M, Jalikis F, Krings G, et al. ‘Non-classical’ HER2 FISH results in breast cancer: a multi-institutional study. Mod Pathol. 2017;30(2):227–235. doi:10.1038/modpathol.2016.175
8. Zare SY, Lin L, Alghamdi AG, et al. Breast cancers with HER2/CEP17 ratio >/= 2.0 and an average HER2 copy number <4.0 per cell: frequency, Immunohistochemical correlation, and clinicopathologic features. Hum Pathol. 2018.
9. Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235. doi:10.1016/j.jclinepi.2017.04.026
10. Stewart RL, Caron JE, Gulbahce EH, Factor RE, Geiersbach KB, Downs-Kelly E. HER2 immunohistochemical and fluorescence in situ hybridization discordances in invasive breast carcinoma with micropapillary features. Mod Pathol. 2017;30(11):1561–1566. doi:10.1038/modpathol.2017.65
11. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor Receptor 2 testing in breast cancer: american society of clinical oncology/College of American pathologists clinical practice guideline focused update. J clin oncol. 2018;36(20):2105–2122. doi:10.1200/JCO.2018.77.8738
12. Yang W, Wei B, Chen M, Bu H. [Evaluation of immunohistochemistry HER2 results interpretation in invasive micropapillary carcinoma of the breast]. Zhonghua Bing Li Xue Za Zhi = Chinese Journal of Pathology. 2015;44(1):48–52. Chinese.
13. Yu JI, Choi DH, Huh SJ, et al. Differences in prognostic factors and failure patterns between invasive micropapillary carcinoma and carcinoma with micropapillary component versus invasive ductal carcinoma of the breast: retrospective multicenter case-control Study (KROG 13-06). Clin Breast Cancer. 2015;15(5):
14. Hao S, Zhao YY, Peng JJ, et al. Invasive micropapillary carcinoma of the breast had no difference in prognosis compared with invasive ductal carcinoma: a propensity-matched analysis. Sci Rep. 2019;9(1):286. doi:10.1038/s41598-018-36362-8
15. Shi WB, Yang LJ, Hu X, Zhou J, Zhang Q, Shao ZM. Clinico-pathological features and prognosis of invasive micropapillary carcinoma compared to invasive ductal carcinoma: a population-based study from China. PLoS One. 2014;9(6):e101390. doi:10.1371/journal.pone.0101390
16. Bhargava R, Dabbs DJ. Interpretation of human epidermal growth factor receptor 2 (HER2) in situ hybridization assays using 2013 update of American Society of Clinical Oncology/College of American Pathologists HER2 Guidelines. J clin oncol. 2014;32(17):1855. doi:10.1200/JCO.2013.53.9213
17. Dowsett M, Procter M, McCaskill-Stevens W, et al. Disease-free survival according to degree of HER2 amplification for patients treated with adjuvant chemotherapy with or without 1 year of trastuzumab: the HERA trial. J clin oncol. 2009;27(18):2962–2969. doi:10.1200/JCO.2008.19.7939
18. Page DB, Wen H, Brogi E, et al. Monosomy 17 in potentially curable HER2-amplified breast cancer: prognostic and predictive impact. Breast Cancer Res Treat. 2018;167(2):547–554. doi:10.1007/s10549-017-4520-1
19. Donaldson AR, Shetty S, Wang Z, et al. Impact of an alternative chromosome 17 probe and the 2013 American Society of Clinical Oncology and College of American Pathologists guidelines on fluorescence in situ hybridization for the determination of HER2 gene amplification in breast cancer. Cancer. 2017;123(12):2230–2239. doi:10.1002/cncr.30592
20. Tse CH, Hwang HC, Goldstein LC, et al. Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J clin oncol. 2011;29(31):4168–4174. doi:10.1200/JCO.2011.36.0107
21. Hu X, Li Y, Yuan D, et al. Retrospective analysis of the association between human epidermal growth factor receptor 2 amplification and chromosome enumeration probe 17 status in patients with breast cancer. Oncol Lett. 2017;14(5):5265–5270. doi:10.3892/ol.2017.6897
22. Sneige N, Hess KR, Multani AS, Gong Y, Ibrahim NK. Prognostic significance of equivocal human epidermal growth factor receptor 2 results and clinical utility of alternative chromosome 17 genes in patients with invasive breast cancer: A cohort study. Cancer. 2017;123(7):1115–1123. doi:10.1002/cncr.30460
23. Jang MH, Kim EJ, Kim HJ, Chung YR, Park SY. Assessment of HER2 status in invasive breast cancers with increased centromere 17 copy number. Breast Cancer Res Treat. 2015;153(1):67–77. doi:10.1007/s10549-015-3522-0
24. Bieche I, Tomasetto C, Regnier CH, Moog-Lutz C, Rio MC, Lidereau R. Two distinct amplified regions at 17q11-q21 involved in human primary breast cancer. Cancer Res. 1996;56(17):3886–3890.
25. Hicks DG, Yoder BJ, Pettay J, et al. The incidence of topoisomerase II-alpha genomic alterations in adenocarcinoma of the breast and their relationship to human epidermal growth factor receptor-2 gene amplification: a fluorescence in situ hybridization study. Hum Pathol. 2005;36(4):348–356. doi:10.1016/j.humpath.2005.01.016
26. Heestand GM, Schwaederle M, Gatalica Z, Arguello D, Kurzrock R. Topoisomerase expression and amplification in solid tumours: analysis of 24,262 patients. Eur J Cancer. 2017;83:80–87. doi:10.1016/j.ejca.2017.06.019
27. Doublier S, Belisario DC, Polimeni M, et al. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer. 2012;12(1):4. doi:10.1186/1471-2407-12-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.