Back to Journals » Breast Cancer: Targets and Therapy » Volume 9
Invasive lobular carcinoma of the male breast – a systematic review with an illustrative case study
Authors Senger JL, Adams SJ, Kanthan R
Received 1 November 2016
Accepted for publication 3 March 2017
Published 17 May 2017 Volume 2017:9 Pages 337—345
DOI https://doi.org/10.2147/BCTT.S126341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Jenna-Lynn Senger,1 Scott J Adams,2 Rani Kanthan3
1Division of Plastic Surgery, University of Alberta, Edmonton, AB, Canada; 2College of Medicine, 3Department of Pathology and Laboratory Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Abstract: Male breast cancer is rare, comprising only 1% of all mammary cancers; invasive ductal carcinoma is by far the commonest subtype in both men and women. Though lobular breast cancer is the second most common subtype seen in women, such cancers are extremely uncommon in men, and this is likely related to the lack of lobular development in the male breast. Thus, due to the rarity of this subtype among breast cancers, compounded by the overall rarity of breast cancer in men, current understanding of the pathogenesis of this disease and its management is largely derived from case series and extrapolation of information from the larger cohort of female patients. This paper provides a systematic review on invasive lobular carcinoma of the male breast in the context of an illustrative case study. A comprehensive analysis of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Data 1973–2013 leading to an exploration of the pathogenesis, epidemiology, clinical presentation, diagnosis, tumor characteristics, and management of lobular breast carcinoma in men is also discussed. Lobular subtype of breast cancer remains an enigmatic elusive disease that needs additional research to unravel its overall pathogenesis and molecular profile to provide insight for improved therapeutic management options.
Keywords: male breast cancer, lobular breast carcinoma, e-cadherin
Introduction
Carcinoma of the male breast is rare, representing only 1% of all breast cancers, with infiltrating ductal carcinoma compromising the majority of cases (74%–95%).1 Lobular breast carcinoma (LBC) is exceptionally rare, comprising only 1% of all male breast malignancies.2 Due to its extremely uncommon occurrence, we are greatly limited in our understanding of the natural history of disease progression, clinical presentation, best treatment management, and prognosis in men.
Methodology
Case report
We herein report the case of a 63-year-old male presenting with unilateral gynecomastia due to an underlying invasive lobular carcinoma (ILC).
Literature review
A systematic review of the published literature using MEDLINE/PubMed and Google Scholar using the search terms “lobular breast cancer”, “lobular carcinoma”, “mammary”, “breast”, “male”, “males”, and “men”, was carried out for all publications of ILC since 2000 with particular emphasis on reports in male patients. Secondary references obtained from these publications were identified by a manual search and reviewed as relevant. Cases with mixed morphology or lobular carcinoma in situ were excluded. Additionally, as Zahir et al3 recently published a complete review of pleomorphic lobular carcinoma in the male breast, this subtype was also excluded from the present study. Eighteen cases of ILC in men were identified in the published literature since 2000; these are tabulated in Table 1, including age, presentation, risk factors, laterality, e-cadherin status, metastatic disease, treatment, and outcome.2,4–15 We have also reviewed the current published English literature in relation to the pathogenesis, epidemiology clinical presentation, investigations, treatment, and prognosis of this rare entity.
  | Table 1 Composite table of cases of invasive lobular breast carcinoma in men published since 2000 listed in descending chronological order Notes: aIncludes: Klinefelter’s, infertility, BRCA+, hormonal therapy, liver disease. A summary of reported cases of invasive lobular breast carcinoma in men up to year 2000 is tabulated by Scheidbach et al.15 Abbreviations: NR, not reported; CA, cancer. |
SEER data analysis
Cases of LBC in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) 1973–2013 dataset were retrieved using SEER*Stat version 8.2.1 (National Cancer Institute, Bethesda, MD, USA). Cases were limited to the International Classification of Diseases for Oncology 3rd edition (ICD-O-3) code 8520/3 (lobular carcinoma, not otherwise specified (NOS); infiltrating lobular carcinoma, NOS; and lobular adenocarcinoma). Variables abstracted were mean age at diagnosis; mean survival; grade; stage; estrogen, progesterone, and HER-2 status; race; surgical treatment; and radiation treatment. Descriptive statistical analysis was performed using Statistical Package for the Social Sciences (SPSS), version 23 (IBM, Chicago, IL, USA). A total of 88 cases in men and 96,609 cases of infiltrating lobular carcinoma in female patients were retrieved for analysis (Table 2).
Case study
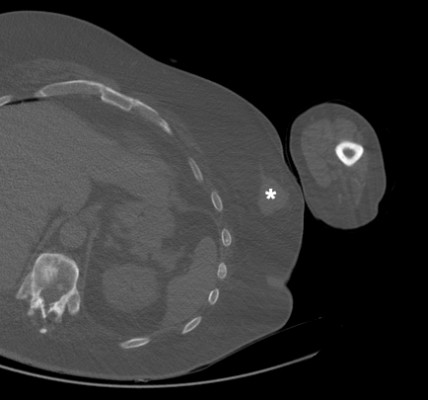
A 63-year-old obese man presented with unilateral left gynecomastia and a palpable mass in the left breast. His past medical history was significant for dyslipidemia, hypertension, noninsulin-dependent diabetes, ankylosing spondylitis, and a traumatic neck injury requiring anterior cervical discectomy and plating with upper extremity weakness sequelae. computed tomography (CT) imaging revealed a well-circumscribed density of the left breast measuring 3.1×3.7 cm consistent with asymmetrical gynecomastia vs carcinoma, as seen in Figure 1. Core biopsies were undertaken.
1  | Figure 1 Axial cut of CT-chest demonstrates a well-circumscribed soft tissue density (*) in the left breast measuring 3.1×3.7 cm. Abbreviation: CT, computed tomography. |
Histopathological examination of the core biopsy revealed the presence of diffusely infiltrating neoplastic cells with small nuclei and no significant pleomorphism or evidence of tubule formation (Figure 2A). On immunohistochemical analysis, the lesional cells were positive for LMWK, HMWK (Figure 2B), and with focal expression of GCDFP. Hormone receptor immunostains showed overexpression of the lesional cells to ER (Figure 2C) and PR (Figure 2D) and negative for ErbB2. Cells showed no expression of e-cadherin, PSA, PAPH, TTF1, CDX2, CD56, NSE, CK7, CK20, and chromogranin. Based on these features, the final diagnosis of ILC was confirmed. Genetic analysis revealed a normal male karyotype. Upon staging by whole-body skeletal scan, multiple metastases in the thoracolumbar spine, pelvis, bilateral femurs, and right 11th rib were identified. Abdominal CT imaging confirmed the involvement of liver as well.
Per the recommendation of medical oncology, the patient began treatment with tamoxifen. Four months after diagnosis, he presented with failure to thrive and lower extremity weakness and pain. CT spine and chest demonstrated progressive metastatic disease and a right-sided pleural effusion with a single pulmonary nodule. Thoracentesis sampling of right pleural fluid demonstrated a dual population of cells (Figure 3A) with the malignant cells expressing BerEP4 (Figure 3B) and negative to staining with calretinin, which was strongly expressed in the background mesothelial cells (Figure 3C), consistent with metastatic breast carcinoma. As he was considered unsuitable for active chemotherapy, he received ten fractions of palliative radiation to his lumbar spine and was transferred for hospice care.
Preoperative written consent at our institution includes the use of patient information for research purposes, and deidentified case reports are exempt from our institutional ethics board review the University of Saskatchewan Research Ethics Board.
Historical perspectives
In 1986, Sanchez et al16 were the first to report “lobular carcinoma” of the male breast in a patient with Klinefelter’s syndrome, though reports of “small cell infiltrating carcinoma” of the male breast had been previously reported. In 2000, Scheidback et al15 reported a case of ILC in an 85-year-old man together with a comprehensive review of the literature and summarized a total of 20 cases including the previously described histology of so called “small cell carcinoma”.
Pathogenesis
In females, invasive LBC arises from lobular and terminal duct epithelium and is usually accompanied by in situ lobular neoplasia; however, lobular development does not occur in the male breast, and the exact origin of this subtype of breast carcinoma in men remains largely unexplained.12,17,18 Endogenous or exogenous stimulation of estrogen is thought to promote formation of acini and lobules, explaining why estrogen/androgen imbalance increases the risk of this malignancy.6 Identified risk factors include 1) genetic events such as Klinefelter’s syndrome or BRCA1/2 mutations, 2) endocrine risk factors with hyperestrogenism related to obesity, exogenous estrogens, and testicular dystrophic lesions, 3) environmental exposures to radiation, high temperatures or electromagnetic fields, and 4) sociodemographic factors such as increasing age, race, African or Ashkenazi Jewish ancestry, and excessive alcohol use.19 Whereas invasive ductal carcinoma (IDC) is commonly associated with mutations of BRCA1 and PT53, BRCA2 mutations are common in both IDC and LBC, and CDH1 are more common in LBC.20 Geographic variation in rates of male LBC, with an increased prevalence in Africa/Egypt, is hypothesized to be secondary to higher prevalence of liver disease (schistosomiasis and malnutrition) causing an increased milieu of endogenous estrogen.9 In the current case, the patient had a normal male karyotype, yet was obese, and thus a high estrogen/androgen was likely a contributing risk factor. Among published case reports, approximately half (10/18) of all patients had one or more known risk factors for breast cancer (Table 1).
Near universal staining loss of the cell adhesion protein e-cadherin, present in 95% of cases, is a unique and defining feature of ILC and is responsible for the discohesive histomorphological characteristics of these tumors.21,22 E-cadherin dysfunction is the master regulator of the lobular phenotype of breast cancer; the e-cadherin calcium-dependent transmembrane protein encoded by the CDH1 gene located on chromosome 16q22 is a tumor-suppressor gene that mediates cell–cell adhesion while maintaining tissue integrity.21,22 The loss of e-cadherin staining may be due to hypermethylation of the CDH1 gene promoter, deletion, or mutation. This mutation is associated with heterozygous loss of chromosome 16q, the locus for CDH1, in just less than 90% of cases.21 Epigenetic silencing of the CDH1 promoter rather than downregulation has been proposed; however, insufficient evidence is available and further investigation is required to prove or disprove this hypothesis. Before its identification in LBC, loss of CDH1 was first implicated in the tumorigenesis of diffuse gastric cancer; these two cancers share histopathological characteristics including individual or small clusters of signet-ring cells in an infiltrative growth pattern. It has also been recognized that patients with genetic CDH1 mutations are at an increased risk for both types of malignancies, and women who harbor this mutation have a similar risk of developing breast cancer as women with a BRCA1/2 mutation.20
In addition to E-cadherin loss, other molecular markers for LBC have been described. FOXA1, a transcriptional modulator of the endoplasmic reticulum, is elevated secondary to somatic mutations in 3%–4% of LBCs, whereas in IDC, mutations are not specific to this chromosomal region. By contrast, IDC more often shows mutation of GATA3, a mutually exclusive event to FOXA1 mutations. PTEN protein expression is significantly lower in LBC compared with IDC; therefore, it follows that LBC tumors have increased upregulation of upstream EGFR and STAT3, a similarity observed in the more aggressive HER2+ and triple-negative breast cancers that typically have high PI3K/Akt signaling.21
ILC also differs from IDC on a cellular level. Shortened telomeres in breast cancers are most common in HER-2-positive carcinomas and triple-negative breast cancers and are independently predictive of poor clinical outcomes. Recently, Heaphy et al23 demonstrated a unique telomere length and phenotype in ILC leading to the concept of a “telomere signature” for ILC. The author reported that ILCs have fewer short telomeres compared with IDC (48% vs 85%, respectively), which may explain why these tumors are typically HER-2 negative and hormone receptor positive.
Recently, two independent research groups have described different methods of classifying ILC, describing genomically similar groups with different nomenclature. The “immune-like” tumors of Nakagawa24 express modulators of immunogenic signaling (interleukins, chemokine receptors, major histocompatibility complex, tumor necrosis factor) and increased levels of macrophage-associated signaling (CD68, macrophage-associated colony stimulating factor, macrophage-associated TH1, T-cell receptor). These tumors are similar to the “immune-related subtype” of Michaut et al,25 which is similarly characterized by upregulation of immune, cytokine, and chemokine signaling with severe lymphocytic infiltration, upregulation of T-cell markers, and higher messenger RNA (mRNA) levels of negative regulators of the immune response (PD-L1, PD-1, CTLA-4) with higher sensitivity to DNA-damage. Michaut’s second group is termed a “hormone-related subtype” associated with epithelial-to-mesenchymal transition, with estrogen/progesterone receptor upregulation. This group is characterized by chromosomal gains of 1q and 8q and loss of 11q.25
This group is similar to Nakagawa’s reactive-like tumors that express epithelial/stromal-associated-signaling elements including keratin, kallikrein, and claudin genes, as well as the oncogenes EGFR, MET, PDGFRA, and KIT.24 Nakagawa describes a third group not represented by Michaut’s classification system, termed “proliferative-type”, which have low expression of all these genes.
Though poorly represented in breast cancer literature, the role of the tumor microenvironment on carcinogenesis is likely significantly implicated in tumor progression, invasion, and metastases.24 Differences in the stromal microenvironment of LBC compared with invasive ductal carcinoma likely contribute to differences in the histomorphology and natural history of the disease. Cancer-associated fibroblasts (CAFs) of the tumor microenvironment represent the most abundant component of breast cancer stroma, associated with tumorigenesis, angiogenesis, and invasion as well as conferring therapeutic resistance and are more abundant in ILC compared with invasive ductal carcinoma.24 Similarly, compared to IDC, invasive LBC has more pronounced intratumoral neovascularization and greater expression of insulin-like growth factor (IGF-1), a major factor that promotes progression and metastases and confers a poorer prognosis.24
Despite all the above insights, large gaps continue to persist regarding the initiation and development of LBCs, particularly in men.
Epidemiology
The incidence of the lobular subtype among male breast cancer is approximately 1.5%.11 The highest reported rate of lobular carcinoma of the male breast is in a manuscript published in 2012 that used information collected from the National Cancer Database (1998–2007) in which the rate of lobular carcinoma among male breast cancer patients was 10%; however, original pathology could not be reviewed to confirm the diagnosis.26 The relative incidence of lobular carcinoma in males is significantly lower (1%–2%) than in females (10%–15%).11 Based on our analysis of the SEER database 1973–2012, the mean age at diagnosis of LBC in males is comparable to females, at 66.8 and 64.4 years respectively; these ages are older than in lobular carcinoma in situ, in which the mean age of diagnosis is 53.5 and 54.6 years. From the SEER database, the majority of men affected with LBC are Caucasian (88.6%) followed by African American (6.8%) with low rates among Asians (3.4%) and First Nations (0%); similar trends are seen among women. At presentation, most men present with grade II (moderately differentiated, 55.1% of reported cases) or grade III (poorly differentiated, 30.6% of reported cases) disease; most patients are stage I (37.9% of reported cases) or stage IIA (27.3% of reported cases) on diagnosis; however, a larger proportion of men present with stage IV compared with women (13.6% vs 5.9% of reported cases). In keeping with the literature, the majority of men are ER (95% of reported cases) and PR (73.2%) positive, which are similar to the female population (94.8% and 78.1% of reported cases) (Table 2).
Clinical presentation
Lobular carcinoma of the breast presents similar to invasive ductal carcinoma in men. Male breast cancer patients typically present with a palpable mass with or without nipple changes, as seen in Table 1, and is usually diagnosed at a late stage as seen in our case which is due, in part, to low patient and clinical suspicion. Lymphadenopathy may thus be palpable at presentation, as was found in 6/18 cases in the published literature (Table 1). Similar to women, male breast cancer is most prevalent among older patients, in their sixth decade of life.2 Based on our review of the literature, there appears to be no preferred laterality to these tumors, with approximately equal distribution to the right and left breast (Table 1). As LBC has a greater propensity for gastric metastases than IDC, gastric presentations as mimics of primary gastric cancer have also been reported. This relationship is likely related to shared loss of e-cadherin expression in LBC and gastric malignancy;21 an isolated incidence of clinical presentation with carcinomatosis from an unknown primary has been reported.10 Invasive LBC has a higher frequency of bilaterality and multicentricity compared with invasive ductal carcinoma.6
Investigations
The growth pattern of these tumors, likely due to the poor cell–cell contacts associated with loss of e-cadherin, creates a diffuse tumor mass that precludes clinical and imaging detection.27 Mammographic detection of LBC is hindered by a diffuse growth pattern and lesions of opacity similar to normal breast parenchyma on imaging in men.2 On magnetic resonance imaging (MRI), invasive LBC appears as a well-circumscribed focal mass (Figure 1) with regional or segmental enhancement and/or multiple small lesions. Lesions may have enhancement patterns similar to breast tissue, thereby making detection difficult.28
Fine needle aspiration biopsy (FNAB) has been used in the diagnosis of invasive LBC; however, false-negative rates are as high as 25% and up to 25% can be misdiagnosed as invasive ductal carcinoma. The difficulty in accurate diagnosis on FNAB arises from ill-defined cytological features of invasive LBC that include minimal cytological and nuclear pleormophism, indistinct nucleoli, and variable amounts of cytoplasm as seen in Figure 3A.6
On histopathological evaluation, cells of LBC are small and monomorphic, lacking cohesion, with a round/notched ovoid nuclei; some cells can have a signet ring cell-type appearance. These cells are classified as type A (classic) or type B (vesicular nuclei). These cells infiltrate the stroma in a single-file pattern.29 Invading tumor cells minimally disrupt the surrounding architecture and are arranged concentrically around normal ducts.22 Additional distinct architectural patterns – alveolar, solid, trabecular – and cytological features – pleomorphic, apocrine, histiocytoid, signet ring – are described ILC subtypes that are collectively termed “mixed nonclassic ILC”.29
Pleomorphic lobular carcinoma is a recognized variant of LBC characterized by greater cellular atypia, higher mitotic rate, and pleomorphism.3,30 This variant additionally has increased chromatin clumping, prominent nucleoli, and abundant cytoplasm.17 Such features lead to a more aggressive clinical course. This subtype of male LBC is exceedingly rare, with only scattered reports in the available literature, and these were recently summarized by Zahir et al.3
Invasive LBC is more likely to be estrogen and progesterone positive compared with IDC and are usually HER-2, p53, and EGFR negative as seen in the index case.28 This is in keeping with the results of the SEER database review in which 64.8% and 41.6% of male LBCs were positive for ER and PR, respectively, but only 2.3% expressed HER-2 receptor (Table 2). As previously discussed, the majority of these tumors have negative staining for e-cadherin, and many authors advocate this as a diagnostic criteria for LBC;12 however, other suggest that negative e-cadherin staining is not mandatory for diagnosis.30 By contrast, 85% of invasive ductal carcinomas express e-cadherin.8
Treatment
There remain no evidence-based guidelines for the management of any type of male breast cancer, and therefore treatment modalities are based on the female counterpart, with surgical, medical, and radiotherapeutic treatments.
Surgery with simple, radical, or modified radical mastectomy is the mainstay of treatment with sentinel node or axillary dissection in the instance of lymph node involvement, as seen in Table 3.
Medical treatment of LCC includes chemotherapeutics and hormone therapy. Clinical and pathological responses to preoperative chemotherapy are worse than IDC.28,29 A higher proportion of male breast cancers, up to 80%–85%, are hormone receptor positive, and thus adjuvant chemotherapy and tamoxifen have some proven benefit; however, evidence is limited.2 New results suggest Letrozole improves disease-free survival in LBC compared with tamoxifen therapy.29 Nevertheless, the majority of patients reported in the literature (8/18), as well as our illustrative case, were treated with tamoxifen. Though only 3%–5% of LBCs are HER-2 positive, within this group, trastuzumab therapy for one year is recommended, showing similar outcomes as patients with IDC.20
As reported in the literature, men have a higher likelihood of requiring postoperative radiation therapy than women due to the higher risk of skin and nipple involvement. However, based on SEER database, a higher proportion of men (64.8%) do not receive radiation therapy compared to women (57.6%) (Table 2). Palliative radiotherapy may play a role in metastatic disease in men.2 Perhaps this discrepancy is due to a failure of physicians to report palliative radiation, and the SEER data reflects predominately therapeutic results.
Prognosis
As seen in the females, prognostic factors of LBC are similar to IDC and include tumor size, axillary node status, hormone receptors, synthesis (S)-phase, and age.28 While LBC is usually of low histological grade, low Ki67/mitotic index, and hormone receptor positive with a positive response to endocrine therapy, patient outcomes have been reported as poorer than in men with IDC given the invasive nature and tendency for widespread metastatic disease.24 This paradoxical behavior of a good prognostic tumor phenotype is the perplexing aspect of ILC that continues to pose serious clinical challenges. ILC has a very invasive nature, with a greater propensity for widespread metastatic colonization including in uncommon sites such as skin, pleura, peritoneum, ovaries, and gastrointestinal tract, and most specifically the stomach.31 Gastric metastases may occur years after the initial diagnosis and can clinically, endoscopically, radiologically, and histopathologically mimic primary gastric cancer.31 Gastric metastases from a breast primary is significantly more common with a lobular subtype than other breast primaries. Immunohistochemical biomarkers to help identify a breast lineage of origin in metastatic lesions include positive expression of GCDFP15, GATA3, ER/PR, and CK7.31
Poor prognosis of male LBC compared to its female counterpart is likely attributable to factors similar to other forms of male breast cancer including late diagnosis, scarcity of breast tissue between the skin and areola, and rich dermal lymphatic vessels resulting in early metastases. Over sixty percent of men have lymph node involvement at the time of initial diagnosis.8 Even patients who present with early stage disease – review of the SEER database suggesting 54.6% of patients are diagnosed with stage I or II disease – will often develop highly aggressive metastatic disease; as such the traditional belief that lobular carcinoma confers an improved prognosis over IDC has been called into question.27 Review of the SEER database reports a mean survival of 76.4 months following diagnosis in men diagnosed with LBC, which is lower than the 88.7 months reported in women (Table 2). Similar trends were observed in the characterization of lobular carcinoma of the male breast using the SEER database 1988–2008 as reported by Moten et al.32
Conclusion
Lobular carcinoma of the male breast is an exceedingly rare entity for which we have little evidence to guide clinical work-up and patient management. Current available evidence is based on isolated case reports, with accepted management protocols based on the female counterpart of this diagnosis. The difficulty of detecting Male LBC, leads to challenges in diagnosis, and finding the appropriate treatment as there are no consensus guidelines for managing these lesions that have a propensity for diffusion and delayed metastases, resulting in poor long-term outcomes. Development of a central registry of such rare mammary malignancies in males is necessary to collate multi-institutional international data to better understand the epidemiology and pathophysiology of this rare disease that will guide clinical management for improved patient outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
National Breast Cancer Foundation, Inc [homepage on the Internet]. Male breast cancer; 2015. Available from: http://www.nationalbreastcancer.org/male-breast-cancer. Accessed October 31, 2016. | ||
Upadhyay R, Kumar P, Sharma DN, et al. Invasive lobular carcinoma of the male breast: a rare histology of an uncommon disease. J Egypt Natl Canc Inst. 2016;28:55–58. | ||
Zahir MN, Minhas K, Shabbir-Moosajee M. Pleomorphic lobular carcinoma of the male breast with axillary lymph node involvement: a case report and review of the literature. BMC Clin Pathol. 2014;14:16. | ||
Abreu M, Pereira P, Marques JC, Esteves G. Invasive lobular carcinoma: a rare presentation in the male breast. BMJ Case Rep. 2016;2016: pii: bcr2016215665. | ||
Gogoi G, Duara LM, Borgohain M, Kaur D, Konwar U, Sarma D. Invasive lobular carcinoma of male breast: what we don’t know – a report of 5 cases. Indian J Pathol Oncol. 2016;3(2):315–319. | ||
Ghosh A, Kanan A. Lobular carcinoma of male breast diagnosed on fine needle aspiration cytology – a case report. Cytopathology. 2013;25:205–212. | ||
Mariolis-Sapsakos T, Theodoropoulos G, Flessas II, et al. Lobular breast cancer in men: case report and review of the literature. Onkologie. 2013;33(12):698–700. | ||
Ninkovic S, Azanjac G, Knezevic M, et al. Lobular breast cancer in a male patient with a history of irradiation due to Hodgkin’s disease. Breast Care. 2012;7:315–318. | ||
Shah P, Shah A, Khursheed S, Naaz I. Lobular carcinoma of the male breast. JK Science. 2010;12(1):33–34. | ||
Spencer JT, Shutter J. Synchronous bilateral invasive lobular breast cancer presenting as carcinomatosis in a male. Am J Surg Pathol. 2009;33(3):470–474. | ||
Briest S, Vang R, Terrell K, Emens L, Lange JR. Invasive lobular carcinoma of the male breast: a rare histology in an uncommon disease. Breast Care. 2009;4:36–38. | ||
Erhan Y, Zekioglu O, Erhan Y. Invasive lobular carcinoma of the male breast. Can J Surg. 2006;49(5):365–366. | ||
Koc M, Öztas S, Erem MT, Ciftcioglu MA, Onuk MD. Invasive lobular carcinoma of the male breast: a case report. Jpn J Clin Oncol. 2001;31(9):444–446. | ||
Chandrasekharan S, Fasanya C, Macneill FA. Invasive lobular carcinoma of the male breast: do we need to think of Klinefelter’s syndrome? Breast. 2001;10:176–178. | ||
Scheidbach H, Dworak O, Schmucker B, Hohenberger W. Lobular carcinoma of the breast in an 85-year-old man. Eur J Surg Oncol. 2000;26(3):319–321. | ||
Sanchez AG, Villanueva AG, Redondo C. Lobular carcinoma of the breast in a patient with Klinefelter’s syndrome. A case with bilateral, synchronous, histologically different breast tumors. Cancer. 1986;57:1181–1183. | ||
Rohini B, Singh PA, Vatsala M, Vishal D, Mitali S, Nishant S. Pleomorphic lobular carcinoma in a male breast: a rare occurrence. Patholog Res Int. 2010;2010:871369. | ||
Michaels BM, Nunn CR, Roses DF. Lobular carcinoma of the male breast. Surgery. 1994;115(3):402–405. | ||
Senger JL, Chandran G, Kanthan R. Is routine pathological evaluation of tissue from gynecomastia necessary? A 15-year retrospective pathological and literature review. Plast Surg. 2014;22(2):112–116 | ||
Dossus L, Benusiglio PR. Lobular breast cancer: incidence and genetic and non-genetic risk factors. Breast Cancer Res. 2015;17:37. | ||
Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506–519. | ||
Reed AEM, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and ‘omics. Breast Cancer Res. 2015;17:12. | ||
Heaphy CM, Asch-Kendrick R, Argani P, Meeker AK, Cimino-Mathews A. Telomere length alterations unique to invasive lobular carcinoma. Hum Pathol. 2015;46:1197–1203. | ||
Nakagawa S, Miki Y, Miyashita M, Hata S, et al. Tumor microenvironment in invasive lobular carcinoma: possible therapeutic targets. Breast Cancer Res Treat. 2016;155:65–75. | ||
Michaut M, Chin SF, Majewski I, et al. Integration of genomic, transcriptomic and proteomic data identifies two biologically distinct subtypes of invasive lobular breast cancer. Sci Rep. 2016;5(6):18517. | ||
Greif JM, Pezzi CM, Klimberg VS, Bailey L, Zuraek M. Gender differences in breast cancer: analysis of 13,000 cancers in men from the National Cancer Data Base. Ann Surg Oncol. 2012;19:3199–3204. | ||
Lehmann U. Lobular breast cancer – the most common special subtype or a most special common subtype? Breast Cancer Res. 2015;17:99. | ||
Cocquyt V, Van Belle S. Lobular carcinoma in situ and invasive lobular cancer of the breast. Curr Opin Obstet Gynecol. 2005;17:55–60. | ||
Barroso-Sousa R, Metzger-Filho O. Differences between invasive lobular and invasive ductal carcinoma of the breast: results and therapeutic implications. Ther Adv Med Oncol. 2016;8(4):261–266. | ||
Ishida M, Mori T, Umeda T, et al. Pleomorphic lobular carcinoma in a male breast: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6(7):1441–1444. | ||
Riccuiti B, Leonardi GC, Ravaioli N, et al. Ductal breast carcinoma metastatic to the stomach resembling primary linitis plastic in a male patient. J Breast Cancer. 2016;19(3):324–329. | ||
Moten A, Obirieze A, Wilson LL. Characterizing lobular carcinoma of the male breast using the SEER database. J Surg Res. 2013;185:e71–e76. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




