Back to Journals » Infection and Drug Resistance » Volume 10
Invasive Aspergillus terreus morphological transitions and immunoadaptations mediating antifungal resistance
Authors Bengyella L, Yekwa EL, Subhani MN, Tambo E, Nawaz K, Hetsa BA , Iftikhar S, Waikhom SD, Roy P
Received 26 July 2017
Accepted for publication 25 August 2017
Published 7 November 2017 Volume 2017:10 Pages 425—436
DOI https://doi.org/10.2147/IDR.S147331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sahil Khanna
Louis Bengyella,1–3 Elsie Laban Yekwa,4 Muhammad Nasir Subhani,5 Ernest Tambo,6,7 Kiran Nawaz,5 Bakoena Ashton Hetsa,2 Sehrish Iftikhar,5 Sayanika Devi Waikhom,1 Pranab Roy8
1Department of Biomedical Science, The School of Basic and Biomedical Sciences, University of Health and Allied Sciences, Ho, Ghana; 2Department of Biotechnology, Faculty of Applied and Computer Sciences, Vaal University of Technology, Vanderbijlpark, South Africa; 3Department of Biotechnology, University of Burdwan, Bardhaman, India; 4Division of Medical Virology, Stellenbosch University, Stellenbosch, South Africa; 5Department of Plant Pathology, Institute of Agricultural Sciences, University of the Punjab, Lahore, Pakistan; 6Department of Biochemistry and Pharmaceutical Sciences, Université des Montagnes, Bangangté, 7Department of Communications, Africa Disease Intelligence and Surveillance, Communication and Response Institute, Yaoundé, Cameroon; 8Department of Biotechnology, Haldia Institute of Technology, Haldia, India
Background and aims: Aspergillus terreus Thom is a pathogen of public health and agricultural importance for its seamless abilities to expand its ecological niche. The aim of this study was holistically to investigate A. terreus morphological and immunoadaptations and their implication in antifungal resistance and proliferation during infection.
Materials and methods: In-depth unstructured mining of relevant peer-reviewed literature was performed for A. terreus morphological, immune, resistance, and genetic diversity based on the sequenced calmodulin-like gene.
Results: Accessory conidia and phialidic conidia produced by A. terreus confer discrete antifungal resistance that ensures survivability during therapies. Interestingly, by producing unique metabolites such as Asp–melanin and terretonin, A. terreus is capable of hijacking macrophages and scavenging iron, respectively. As such, A. terreus has established a rare mechanism to mitigate phagocytosis and swing the interaction dynamics in favor of its proliferation and survival in hosts.
Conclusion: It is further unraveled that besides A. terreus genetic diversity, morphological, biochemical, and immunologic adaptations associated with conidia germination and discharge of chemical signals during infection enable masking of the host defense as an integral part of its strategy to survive and rapidly colonize hosts.
Keywords: HSP70, terrein, terretonin, Asp–melanin, virulence, stomata atropism
Introduction
Aspergillus terreus is a medically important pathogen associated with life-threatening states, ranging from cystic fibrosis, aspergillomas, spondylodiscitis, and periprosthetic hip-joint infection in immunocompetent hosts and lethal invasive disease in immunocompromised individuals.1–4 An upsurge in the mortality rate of 74%–92% in transplant recipients suffering from invasive aspergillosis has been reported.5,6 It is now understood that invasive aspergillosis sufferers often have higher incidence of cancer and organ transplantation,7 an indication that Aspergillus infections could lead to sequential development of other diseases. Other than the harmful effect in medicine, A. terreus is the only source of the valuable drug lovastatin, used for reduction of serum cholesterol.7 Regardless of modern therapies, ~30%–50% of invasive aspergillosis patients die for such reasons as late diagnosis, infection of the brain not efficiently treated with antimycotics, and drug-resistance emergence.8,9 One possible reason could be the partial understanding of the survival mechanism of A. terreus conidia and intertwining with host defense. Another limitation has been how A. terreus is transported from the common site of infection, often the lungs, to distant sites, such as the central nervous system, in immunocompetent patients, associated with fungal meningitis.10–12 Given successful germination of A. terreus conidia is the key step to invasive aspergillosis, a recent proteomic study uncovered virulent proteins, such as Hog1 and MPKC, and high-level transcripts, such as the DHO and TER genes, associated with the transition stages that are a hallmark of conidia germination.13
Economically, A. terreus has influenced agriculture negatively and positively. Positively, A. terreus acts as a biocontrol agent against Biomphalaria alexandrina snails,14 acts as a promoter of plant growth, and mitigates the adverse effects of stem-rot disease caused by Sclerotium rolfsii.15 Contrary to the beneficial aspects in agriculture, A. terreus and other filamentous fungi are mainly responsible for severe loss of crops worldwide, damaging over 125 million tons of food grains annually.16–19 Whether inside the seed, on the seed, or as a contaminant, evidence shows that A. terreus is part of the cohort of fungi that adversely affects seed viability, germination, emergence, and plant-growth vigor, and eventually hampers agricultural productivity.20 The saprophytic nature of A. terreus and ability to colonize important new vegetables, such as sugar beet (Beta vulgaris), Jew’s mallow (Corchorus olitorius), cucumber (Cucumis sativus), purslane (Portulaca oleracea), and eggplant (Solanum melongena),20 is an indication that its current impact in agriculture is underestimated. The successful nature of A. terreus to cause diseases in plants and animals and/or jump hosts qualifies it as a cross-kingdom pathogen.
Each fungal infection starts with germination of conidia.21 Because of the ease with which A. terreus colonizes novel niches, one could question whether there exist rare strategies based on morphological changes and adaptability during active invasion of hosts. Nonetheless, the current literature has scanty information on the mechanism by which A. terreus infects plant hosts. Capturing infection mechanisms in plants and drawing parallels with infection mechanisms of animals could provide new insights on the control of A. terreus in animals and plants. This ignored gap poses a serious public health problem, since on such plant hosts as food crops, A. terreus infects and liberates mycotoxins that are of public health concern. While plant and animal immunity are different, we draw parallels between the two systems and provide analysis of how A. terreus successfully thrives on hosts. This study provides a holistic analysis of the invasion strategies explored by A. terreus to mask host defenses, focusing on morphological adaptability and chemical arsenals. It is revealed that morphological changes associated with conidia germination and discharge of unique chemical signals, such as terrein, terretonin, Asp–melanin, overexpression of catalase, and HSP70, in A. terreus are integral arsenals employed in outwitting host defenses and mounting resistance to antimycotics. The study aims to unravel the morphological, immune, and susceptibility changes associated with conidia proliferation and chemical signal impact on plants and animals of public health concern.
Materials and methods
Database search, extraction, and synthesis
An unstructured review was performed on A. terreus infections, morphology, virulence, immunoresponse, and resistance based on available data in peer-reviewed literature (from Medline, Scopus, Google Scholar, and CINAHL) and gray literature in book chapters and unindexed sources, including local and global agencies, eg, the UN Food and Agriculture Organization and World Health Organization in the months of June–August 2017 (Table S1). Prevention and early-warning websites were searched using such keywords as “Aspergillus species”, “A. terreus”, “A. terreus – morphology”, A. terreus – resistance”, “A. terreus – pathogenicity, and “A. terreus – virulence”. Key MeSH terms used included “A. terreus health morphological adaptability”, “A. terreus chemical metabolites and agriculture”, and “A. terreus resistance to antimycotics”. Abstract (or résumés) were read for relevance, then the full articles or documents reviewed.
A total of 30 studies were referred to for this study. We extracted A. terreus morphological and susceptibility changes to survival data as reported in various studies according to the criteria and methods used in each study. Further evaluation was based on susceptibility testing to antimycotics, conidia-germination strategies, and chemical signal impact on plants and animals of public health concern. The overarching data set and information generated were analyzed.
DNA extraction, PCR sequencing, and phylogeny
Comparing clinical A. terreus strains with that of plant origin could provide insights on their evolutionary pattern in different habitats and allow for prediction of the challenges that could be encountered should a strain from a plant infect an animal. For this course, the genomic DNA of virulent A. terreus (GenBank accession GI|JX155853) of potato origin19 grown in nutrient broth (HiMedia, Mumbai, India) was isolated. The total genomic DNA was extracted from mycelium mats using UltraClean microbial DNA-isolation kits (Mo Bio Laboratories, Carlsbad, CA, USA) as described by the manufacturer. The quality and quantity of the DNA were determined using 1.0% agarose-gel electrophoresis and nanodrop spectrophotometry (BioSpec-nano; Shimadzu, Kyoto, Japan), respectively.
For polymerase chain reaction (PCR), specific primers (forward 5'-ccgagtacaaggaggccttc-3', reverse 5'-ccgatagaggtcataacgtgg-3') were designed for the CAD gene using reference A. terreus (GenBank EF669543.1) in Integrated DNA Technology (IDT) primer designer software. The PCR mixture was composed of 1 µL of 100 ng genomic DNA, 5 µL 10× high-fidelity standard PCR buffer, 0.5 µL deoxynucleotide triphosphate (dNTP) mix (10 mM), 1.25 µL MgCl2 (50 mM), 0.5 µL of each primer, and 0.2 µL Phusion DNA polymerase (5 U/µL; Thermo Fisher Scientific, Waltham, MA, USA), resulting in a final volume of 25 µL. The cycle conditions used initially were 98°C for 30 seconds for denaturing, followed by 35 cycles of 98°C for 10 seconds, 56°C for 30 seconds, 72°C for 1 minute, and a final extension of 72°C for 7 minutes in a MasterCycler gradient thermal cycler (Eppendorf, Hamburg, Germany) targeting the 559 bp region. The amplicon was purified with a QIAquick PCR purification kit (Qiagen, Venlo, Netherlands) and sequenced with reverse and forward primers using a BigDye Terminator 3.1 cycle-sequencing kit (Thermo Fisher Scientific), coupled with an ABI Prism 3100 genetic analyzer sequencer following the manufacturer’s instructions (Thermo Fisher Scientific). The sequenced CAD gene was analyzed in DNA Baser and submitted to the National Center for Biotechnology Information database as accession KC305600, based on 100% similarity with type isolates. Phylogenetic analysis was performed using a Mega 6.1 server.
Results and discussion
Significance of A. terreus conidia morphological adaptability during infection
A. terreus falls within the group of fungi whose conidia display unique morphological changes during infection. Inherently, A. terreus produces asexual conidia, also called phialidic conidia (Figure 1), at the tips of broom-like conidiophores.19,22,23 In addition to phialidic conidia (PC; 1.5–2 µm diameter), A. terreus produces globular accessory conidia (AC; 3–6 µm diameter) emanating from colonizing hyphae.19,22 Important factors affecting A. terreus virulence are inoculum size, host characteristics,24 and the expression of terrelysin (a hemolytic protein), CgrA, CREB, PepP, MyoA, Mep, Hog1, and MPKC, secreted during germination of A. terreus conidia.13 Remarkably, the rate of A. terreus infection increases proportionally with the pathogen inoculum, but inoculum size does not affect intrinsic host characteristics.24,25 Therefore, it is important to understand key morphological aspects of PC and AC, and how they influence host colonization.
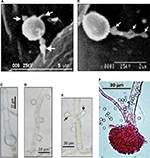  | Figure 1 Morphological differentiation of Aspergillus terreus (GenBank accession KC305600) conidia during infection of potato leaf. Notes: (A) A germinating phialidic conidium infecting abaxial leaf of potato produces multipolar hyphae (tagged with arrows); (B) hyphae from phialidic conidium could produce irregular protuberance (tagged with arrows); (C) irregular protuberance is pigmented, probably melanin, which plays a protective role; (D) thorn-like pattern of accessory conidia; (E) opposing accessory conidia; (F) conidiophores stained with rose Bengal as previously described by Louis et al.19 Images reproduced from Louis B, Sayanika DW, Pranab R, et al. Invasion of Solanum tuberosum L. by Aspergillus terreus: a microscopic and proteomics insight on pathogenicity. BMC Res Notes. 2014;7:350.28 |
High-resolution staining has revealed that AC have a uniform ring-like pattern of the β-glucan layer compared to PC, whereby a nonuniform contour of the β-glucan layer was observed26 in accordance with a previous thesis that AC and PC could have different cell-wall composition.23 Equally, AC are multinucleated and undergo multipolarization, which leads to several hyphae capable of inducing heightened inflammatory responses in a pulmonary model of aspergillosis.25 Based on the uniqueness of AC and PC, it has been suggested that there exist differential immunoresponses to distinct conidia types in an animal model,27 opening other avenues for investigation.
A. terreus produces different invasive structures on plant host
As most studies on A. terreus have focused on animal models, it remains unknown whether PC and AC exhibit similar germination strategies to invade all hosts. More recently, phytopathology data revealed that PC also undergo multipolar germination (Figure 1), marked by three to four delicate hyphae, to invade potato-leaf tissue.28 Interestingly, it was noted that by 8 hours after germination of PC, hyphae had differentiated into pigmented irregular protuberances (0.2–0.5 μm in diameter) and failed to penetrate epidermal cells and stomata28 thus exhibiting stomata atropism (ie, inability to penetrate via the stomata). Although the role of irregular protuberances in infection is unknown, the authors indicated that irregular protuberances form recursive hubs for efficient colonization of potato leaf. While the infection process in plants and animals are different, commonalities lie in the ability of A. terreus to produce PC and AC, which are potent virulence factors.
Spatial distribution pattern of AC on colonizing hyphae and host sensing
Little investigation has been performed on morphological changes in PC and AC in host sensing during germination and infection. Two patterns of distribution of AC on colonizing hyphae have been identified. Based on animal models, AC arise on the surface of colonizing hyphae in an alternating thorn-like pattern23,26 and irregularly spaced. Interestingly, it has been found serendipitously that AC can arise in an opposing pattern perpendicularly to one another and irregularly spaced on colonizing hyphae during infection of potato-leaf epidermis, but not on potato slices in vitro28 (Figure 1). The authors suggested that a specific host-tissue signal could be responsible for the production and alignment of AC on infection hyphae. With comparison of patterns of AC development on colonizing hyphae in animal26 and plant28 models, it is not unreasonable to hypothesize that AC could be playing a sensing role that aids in recognizing a suitable host during infection.
To gain insight into the role of AC in host sensing, it could be interesting to profile the whole proteome of AC and predict glycosylphosphatidylinositol-anchored proteins, which often act as effectors and participate in cell recognition, and compare it with those of PC, firstly to understand the amplitude of PC in inflammatory response in animals. Secondly, such investigation could provide novel insights into how AC and PC subdue the host during infection. The proposed proteomic approach could help shed light into why A. terreus AC elicit a superior immunoresponse, as observed via ex vivo alveolar macrophages and in vivo intratracheally challenged mice.26 It has been found that clinical isolates of A. terreus produce morphologically diverse AC and relatively similar AC morphologies at the strain level,23 which could be a function of genetic diversity. Recently, it was shown that isolates of A. terreus specifically from dried grape showed significant genomic diversity based on random amplification of polymorphic DNA–PCR data, although morphological differences were not significant.29
Using the sequenced CAD gene from A. terreus (GenBank accession KC305600; 559 bp), which causes foliar potato blight, and by comparing the sequence with those of clinical strains, we found evidence of divergent evolution due to point mutations (Figure 2). Morphological features based on conidia, conidiophores, and universal rDNA help in identification of A. terreus, but cannot effectively discriminate isolates of A. terreus, because of overlapping morphological traits and genome plasticity at rDNA region.28,37 We used the CAD locus, because it has been shown to discriminate different isolates of A. terreus accurately.37 From the 31 sequences computed, 188 patterns were found from a total of 763 sites, and 313 sites were without polymorphisms (41.02%). The nucleotide frequencies were A 22.73%, T/U 25.17%, C 28.07%, and G 24.03% at a discrete γ-distribution of 23.5. The best substitution-model parameters were determined based on the Akaike information criterion, corrected (1,642.868) and Bayesian information criterion (3,864.291). The generated phylogenetic tree had the highest log likelihood: –1,640.72. The estimated average evolutionary divergence overall in A. terreus strains in the data set was 1.47±1.1. Based on the high-level clustering pattern in clade II, and considering that only one clinical strain clustered with KC305600 indicated that A. terreus strains were highly diverse, this diversity was able to be translated at the morphological level during host colonization (Figure 1). This genetic variability could explain in part the differences in the spatial distribution pattern of opposing AC on colonizing hyphae of A. terreus KC305600, not yet observed in clinical isolates (Figure 1). Therefore, comparative proteomics could delineate whether the different morphological forms of AC bear similar effector proteins during infection.
  | Figure 2 Molecular phylogenetic analysis by maximum likelihood (ML) based on the K2 substitution model55 using calmodulin gene. Notes: Evolutionary analyses were conducted in MEGA6.56 Regions of significant point mutations in the shown sequence alignment are highlighted, and main clades I and II are shown. The ML tree indicated that clade II containing Aspergillus terreus KC305600 encompasses highly diverse strains, represented by subclusters IIA, IIB, IIC, and IIC1. The highlighted taxa showed a close relationship between a clinical strain (ie, EU147536) and strain KC305600, which causes foliar blight of potato. |
Impact of A. terreus use of chemical signals to mask infectious activity
A. terreus produces terretonin, a meroterpenoid that acts as an iron scavenger,30 thus affecting vital physiological processes in the hosts. Often, pathogenic fungi receive morphological and chemical signals from plant cells that are direct consequences of fungal invasion,31 but this may not be true for animal cells. Nonetheless, A. terreus is known to produce a vast number of metabolites.32 Because chemical signals are often metabolites used for defense against predators and competitors, for chemical communication, and to manipulate host cells,28 A. terreus is consequently a formidable pathogen. Importantly, A. terreus produces terrein, a metabolite expressed under the influence of environmental stimuli33–35 that confers an added advantage for invading diverse hosts. For example, in mammals, terrein reduces tyrosinase protein levels and induces a decrease in melanin synthesis via the extracellular signal-regulated protein-kinase pathway and microphthalmia-associated transcription factor.36 A previous study elucidated that terrein inhibited the growth of bacteria, fungi, and mammalians cells,34 hence signifying that A. terreus senses the host or immediate environment. Irrespective of the inflammatory potential of AC, terrein also acts as an antioxidant and anti-inflammatory agent,33 indicating that a given A. terreus strain could infect animals yet mask inflammation, regardless of the presence of AC on colonizing hyphae.
Overproduction of AC and PC during infection is an effective survival strategy
Aspergillus conidia display high tolerance to heat (~85°C),37 germinate at low water activity (<0.6), show high tenacity for pressure and chaotropic agents, and can survive for over 60 years.38 While strong superficial colonization of potato leaves is marked by abundant production of PC and AC,22 the scenario is different in invasive aspergillosis. For instance, in severe invasive aspergillosis characterized by angioinvasion, colonizing hyphae can spread hematogenously and equally penetrate deep organs.12,39 It is evident such a colonization process encounters resistance and immunoresponses that can alter the survival of conidia or affect their production. One important aspect to consider is how strong and weak host responses could influence survival of A. terreus vis-à-vis PC and AC. Hypothetically, when one considers that strong host response leads to cellular restriction of infection, then there is a high chance of abundant production of PC. This is because PC is the principal form of survival in adverse conditions, and such a scenario could lead to subsequent fatal invasive aspergillosis in those associated with long-term persistence of PC and liver degeneration.33 On the other hand, if host response is weak at the onset of colonization, PC germinates and produces colonizing hyphae that bear AC, which enhance virulence and severity of the infection. This intertwining in the developmental stages associated with infection that gives rise to PC and AC (Figure 3) offers a highly successful means of survival under adverse conditions.
Using vacuum ultraviolet irradiation (100–180 nm), low temperature, and a combination thereof to evaluate A. terreus conidia survival under simulated interplanetary conditions led to the deduction that interplanetary survival of conidia depends mainly on dehydration stress in vacuum.40 Nonetheless, PC-survival probability extended to 4 days in vacuum (~34%), due to exudation of proteins on the external surface membrane, which prevents excessive conidia dehydration.40 Results from this interplanetary simulative study provided clues that only drastic sterilization procedures would eliminate A. terreus under clinical settings.
Mitigating antimycotic effect through overexpressed A. terreus catalase and HSP
Research has shown that A. terreus inherently displays resistance to frontline polyene–amphotericin B, and in some cases 98% of clinical isolates showed resistance.25 The A. terreus-resistance mechanism to amphotericin B is not clearly understood, but it has been suggested that overproduction of catalase may contribute to amphotericin B resistance.41 Aspergillus spp. have the ability to overexpress catalase and hyperoxide dismutase during infection and lower intracellular reactive oxygen species, thus increasing amphotericin B resistance.42,43 It worth mentioning that colonization of potato tubers is marked by differential expression of catalase–peroxidase complexes, often involved in signal-transduction pathways and cross talk in pathogenic responses.28 Another line of evidence indicates that the main cause of amphotericin B resistance in A. terreus is cytosolic HSPs, which naturally play a protective role against various stress responses (Figure 3). Experimentally, an HSP70 inhibitor (eg, pifithrin-µ) considerably enhances the susceptibility of resistant A. terreus strains to amphotericin B, but nonetheless has little impact on susceptible strains.44 By considering these resistance mechanisms,41,43,44 it is suggested that A. terreus may switch between catalase overexpression and HSP chaperones to mount strong resistance to amphotericin B in immunocompromised patients under therapy. It could be possible that A. terreus uses diverse chaperones that nullify antifungal effects as part of its strategies to counter and survive adverse conditions during infection.
Assessing AC ability to confer higher resistance to A. terreus than PC
At this point, one could be tempted to determine whether AC and PC exhibit equal level of resistance against elite antifungal drugs to ensure survival during infection. To investigate amphotericin B and azole (eg, voriconazole) resistance further in A. terreus, Lass-Flörl et al44 used PC and AC from 31 strains and determined the minimum inhibitory concentration (MIC) of amphotericin B and voriconazole vis-à-vis the strains. It was found that 90% of the 31 strains had an MIC of 2.5 µg/mL of amphotericin B as it relates to PC, but 5 µg/mL amphotericin B for AC was needed.45 It was also found that an MIC of 1 µg/mL of voriconazole was required for PC, while the MIC of voriconazole for AC stood at 2 µg/mL.45 Even though the relationships among amphotericin B, PC, and AC were not statistically significant, striking disparities in MIC were slightly higher than for hyphae, thus suggesting that AC and PC confer discrete antifungal resistance to A. terreus that could ensure survivability during treatment. Furthermore, since invasive aspergillosis leads to tissue necrosis at points of infection and reduces leukocyte penetration, as well as the effectiveness of antimycotics,39 all these attributes enhance A. terreus resistance. Importantly, AC have a unique surface architecture (smooth, thick, and apparently unpigmented cell surface), cell-wall composition (less cell membrane ergosterol), and are multinucleated (three to seven nuclei) in contrast to PC,23,26 and that could be an underlying cause of dissimilarities in MIC values. Intriguingly, cases of A. terreus resistance continue to rise, and may be intrinsic, acquired from exposure to azole fungicides or antifungal drug exposure, or in antifungal drug-naïve patients.46 Therefore, treatment of invasive aspergillosis could require personalized drugs. The rationale behind this is that most antifungal drugs are primarily designed for fungicidal activity, and consequently it is not clear whether commercial antifungal drugs target AC or PC more. Firstly, antifungal drugs that primarily target PC more could be of great medical importance, because only PC are airborne, while AC are formed only as accessories on infection hyphae during colonization. Secondly, it could allow for deducing best-treatment regimens based on combination therapy. Thirdly, this investigation could lead to repurposing of some antifungal drugs to effectively counter antifungal drug resistance. Repurposing and personalizing antifungal drugs could shed light on the existing concept that antifungal-combination therapy offers no definite advantage over montherapy.47–49
Understanding of systemic colonization of A. terreus production of pigmented and acidic conidia
While most fungi associated with lung disease remain localized in the disease area, invasive aspergillosis caused by A. terreus has been detected in the central nervous system,12 challenging minds as to the means of transport to distant organs. It has been found that A. terreus produces pigmented PC that partially protects it from predators, such as macrophages. Conceptually, innate immunity (ie, neutrophils and macrophages) engulfs and eliminates harmful microbes. Conidia of some Aspergillus spp. produce certain types of dihydroxynaphthalene (DHN)–melanin, which inhibits phagolysosome acidification.50 A. terreus, on the other hand, lacks genes for the synthesis of DHN–melanin, but produces a different kind of pigment called Asp–melanin, which partially favors acidification.51 Interestingly, Asp–melanin plays a key role in A. terreus survival by conferring resistance against ultraviolet light, as well as hampers phagocytosis by soil amoeba.50 Remarkably, the failure of Asp–melanin to inhibit acidification of phagolysosomes suggests it contributes to the survival of A. terreus conidia in acidic environments.51 Most fungi conidia are usually neutral pH-loving, contrary to conidia of A. terreus, which are acid-loving. It has been suggested that the acid-loving property allows conidia of A. terreus to survive distant transport by hitching on immune cells as transport vehicles throughout the body.51 By hijacking the host macrophages (Figure 3), A. terreus has established a rare strategy to mitigate phagocytosis, and readily induces thrombosis and swings interaction dynamics in favor of its proliferation.
Outlook on A. terreus pathogenicity and invasiveness
Morphological changes during germination in cross-kingdom fungi during infection are an intrinsic strategy to colonize hosts rapidly, and improve their fitness on new hosts. A. terreus and A. fumigatus are the leading causes of hard-to-treat invasive aspergillosis. Recent studies have aimed to identify pathogenicity factors in Aspergillus spp. For instance, it was shown that A. fumigatus produces galactosaminogalactan (Gal), which is immunosuppressive, induces apoptotic death of neutrophils, and favors A. fumigatus infection.53 Also, hydrophobin a virulence factor on spores of Aspergillus spp., masks dectin 1/2 recognition of conidia, impairs neutrophil recruitment, increases fungal survival,52,53 and hence prevents immunoresponse. More recently, conidial melanin, hydrophobin, and Gal were revealed to be important pathogenicity factors that modulate platelet activity and impact immunoresponse, inflammation, and thrombosis in infected patients.54 It could be interesting to evaluate the effect of terrein on melanin, Gal, and hydrophobin mutants of A. terreus to gain insights into the extent to which terrein overproduction might mask PC and AC detection by immune cells. Finally, it will be fascinating to identify stimuli that trigger differentiation of colonizing hyphae into AC and irregular protuberance, and investigate whether terrein can inhibit or stimulate the production of AC during infection.
Conclusion
We found that A. terreus adopts diverse strategies to cause invasive aspergillosis, which results in morbidity and mortality in patients with hematological malignancies and blight diseases in plant hosts. The strategies identified include divergent evolution between isolates and adoption of different morphological forms during infection. Importantly, the interlocking relationship between AC, PC, and delicate colonizing hyphae confers a discrete level of resistance to antifungal drugs. Remarkably, the potential of A. terreus conidia to produce chemical signals that mask infectious activity enable the fungus to be highly successful.
Acknowledgments
This research was jointly supported by the World Academy of Sciences (TWAS), Trieste, Italy and the Department of Biotechnology, Government of India (DBT/TWAS PG fellowship 3240223450) and postdoctoral funding from Vaal University of Technology and the Institute of Agricultural Sciences, University of the Punjab, Lahore, Pakistan.
Disclosure
The authors report no conflicts of interest in this work.
References
sVincken W, Schandevul W, Roels P. Allergic bronchopulmonary aspergillosis caused by Aspergillus terreus. Am Rev Respir Dis. 1983;127:388–389. | ||
Dunne K, Prior AR, Murphy K, et al. Emergence of persistent Aspergillus terreus colonization in a child with cystic fibrosis. Med Mycol Case Rep. 2015;9:26–30. | ||
Comacle P, Govic YL, Hoche-Delchet C, et al. Spondylodiscitis due to Aspergillus terreus in an immunocompetent host: case report and literature review. Mycopathologia. 2016;181:575–581. | ||
Bartash R, Guo Y, Pope JB, et al. Periprosthetic hip joint infection with Aspergillus terreus: a clinical case and a review of the literature. Med Mycol Case Rep. 2017;18:24–27. | ||
Paterson DL, Singh N. Invasive aspergillosis in transplant recipients. Medicine (Baltimore). 1999;78:123–138. | ||
Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 2009;12:310–350. | ||
Boruta T, Bizukojc M. Production of lovastatin and itaconic acid by Aspergillus terreus: a comparative perspective. World J Microbiol Biotechnol. 2017;33:34. | ||
Taccone FS, van den Abeele AM, Bulpa P, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. | ||
Denning DW, Bromley MJ. How to bolster the antifungal pipeline. Science. 2015;347:1414–1416. | ||
Carrazana EJ, Rossitch E Jr, Morris J. Isolated central nervous system aspergillosis in the acquired immunodeficiency syndrome. Clin Neurol Neurosurg. 1991;93:227–230. | ||
Scully EP, Baden LR, Katz JT. Fungal brain infections. Curr Opinion Neurol. 2008;21:347–352. | ||
Elsawy A, Faidah H, Ahmed A, Mostafa A, Mohamed F. Aspergillus terreus meningitis in immunocompetent patient: a case report. Front Microbiol. 2015;6:1353. | ||
Thakur R, Shankar J. Proteome profile of Aspergillus terreus conidia at germinating stage: identification of probable virulent factors and enzymes from mycotoxin pathways. Mycopathologia. Epub 2017 Jun 24. | ||
Saad AE, Khalil MT, Ragab FM, Mekawey AA, El-Wareth MT. Efficacy of the fungi Aspergillus terreus and Penicillium janthinellum as biological control agents against Biomphalaria alexandrina snails. Int J Environ Sci Eng. 2014;5:25–37. | ||
Waqas M, Khan AL, Hamayun M, Shahzad R, et al. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrinum and Aspergillus terreus. J Plant Interact. 2015;10:280–287. | ||
Kozakiewicz Z. Aspergillus species on stored products. Aberystwyth, UK: Cambrian Printers; 1989. | ||
Dewan MM, Sivasithamparam K. Occurrence of species of Aspergillus and Penicillium in roots of wheat and rye grass and their effects on root rot caused by Gaeumannomyces graminis var. tritici. Aust J Bot. 1988;36:701–710. | ||
De Lucca AJ. Harmful fungi in both agriculture and medicine. Rev Iberoam Micol. 2007;24:3–13. | ||
Louis B, Roy P, Sayanika DW, et al. Aspergillus terreus Thom a new pathogen that causes foliar blight of potato. Plant Pathol Quar. 2013;3(1):29–33. | ||
Abdelwehab SA, El-Nagerabi SA. Mycobiota associated with imported seeds of vegetable crops in Sudan. Open Mycol J. 2014;8:156–173. | ||
Louis B, Waikhom SD, Jose RC, Goyari S, Talukdar NC, Roy P. Cochliobolus lunatus colonizes potato by adopting different invasion strategies on cultivars: new insights on temperature dependent-virulence. Microb Pathog. 2015;87(2015):30–39. | ||
Balajee SA. Aspergillus terreus complex. Med Mycol. 2009;47:42–46. | ||
Deak E, Wilson SD, White E, et al. Aspergillus terreus accessory conidia are unique in surface architecture, cell wall composition and germination kinetics. PLoS One. 2009;4:e7673. | ||
Lass-Flörl C. Aspergillus terreus: how inoculum size and host characteristics affect virulence. J Infect Dis. 2012;205:1192–1194. | ||
Slesiona S, Ibrahim-Granet O, Olias P, Brock M, Jacobsen ID. Murine infection models for Aspergillus terreus pulmonary aspergillosis reveal long-term persistence of conidia and liver degeneration. J Infect Dis. 2012;205:1268–1277. | ||
Deak E, Nelson M, Hernández-Rodríguez Y, et al. Aspergillus terreus accessory conidia are multinucleated, hyperpolarizing structures that display differential dectin staining and can induce heightened inflammatory responses in a pulmonary model of aspergillosis. Virulence. 2011;2:200–207. | ||
Vyas JM. The duality of Aspergillus terreus: differential immune responses to distinct conidia. Virulence. 2011;2:181–184. | ||
Louis B, Sayanika DW, Pranab R, et al. Invasion of Solanum tuberosum L. by Aspergillus terreus: a microscopic and proteomics insight on pathogenicity. BMC Res Notes. 2014;7:350. | ||
Narasimhan B, Asokan M. Genetic variability of Aspergillus terreus from dried grapes using RAPD-PCR. Adv Biosci Biotechnol. 2010;1:345–353. | ||
Guo CJ, Knox BP, Chiang YM, et al. Molecular genetic characterization of a cluster in A. terreus for biosynthesis of the meroterpenoid terretonin. Org Lett. 2012;14:5684–5687. | ||
Wilson RA, Gardner HW, Keller NP. Cultivar-dependent expression of a maize lipoxygenase responsive to seed infesting fungi. Mol Plant Microbe Interact. 2001;14:980–987. | ||
Haas H. How to trigger a fungal weapon. Elife. 2015;4:e10504. | ||
Zaehle C, Gressler M, Shelest E, Geib E, Hertweck C, Brock M. Terrein biosynthesis in Aspergillus terreus and its impact on phytotoxicity. Chem Biol. 2014;21:719–731. | ||
Gressler M, Meyer F, Heine D, Hortschansky P, Hertweck C, Brock M. Phytotoxin production in Aspergillus terreus is regulated by independent environmental signals. Elife. 2015;4:e07861. | ||
Park SH, Kim DS, Kim WG, et al. Terrein: a new melanogenesis inhibitor and its mechanism. Cell Mol Life Sci. 2004;61:2878–2885. | ||
Wyatt TT, Golovina EA, van Leeuwen R, Hallsworth JE, Wösten HA, Dijksterhuis J. A decrease in bulk water and mannitol and accumulation of trehalose and trehalose-based oligosaccharides define a two-stage maturation process towards extreme stress resistance in ascospores of Neosartorya fischeri (Aspergillus fischeri). Environ Microbiol. 2015;17: 383–394. | ||
Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus: what makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013;9:e1003743. | ||
Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006;2:e129. | ||
Sarantopoulou E, Gomoiu I, Kollia Z, Cefalas AC. Interplanetary survival probability of Aspergillus terreus spores under simulated solar vacuum ultraviolet irradiation. Planet Space Sci. 2011;59:63–78. | ||
Blum G, Perkhofer S, Haas H, et al. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob Agents Chemother. 2008;52:1553–1555. | ||
Takasuka T, Sayers NM, Anderson MJ, Benbow EW, Denning DW. Aspergillus fumigatus catalases: cloning of an Aspergillus nidulans catalase B homologue and evidence for at least three catalases. FEMS Immunol Med Microbiol. 1999;23:125–133. | ||
Moore CB, Sayers N, Mosquera J, Slaven J, Denning DW. Antifungal drug resistance in Aspergillus. J Infect. 2000;41:203–220. | ||
Blatzer M, Blum G, Jukic E, et al. Blocking HSP70 enhances the efficiency of amphotericin B treatment against resistant Aspergillus terreus strains. Antimicrob Agents Chemother. 2015;59:3778–3788. | ||
Lass-Flörl C, Rief A, Leitner S, et al. In vitro activities of amphotericin B and voriconazole against aleurioconidia from Aspergillus terreus. Antimicrob Agents Chemother. 2005;49:2539–2540. | ||
Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect. 2014;20:42–48. | ||
Sutton DA, Sanche SE, Revankar SG, Fothergill AW, Rinaldi MG. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison with voriconazole. J Clin Microbiol. 1999;37:2343–2345. | ||
Hatipoglu N, Hatipoglu H. Combination antifungal therapy for invasive fungal infections in children and adults. Expert Rev Anti Infect Ther. 2013;11:523–525. | ||
Louis B, Waikhom SD, Atadja PW. Current trends in outwitting resistance development in Candida infections through photodynamic and short peptide therapies: a strategic-shift from conventional antifungal agents. Expert Rev Anti Infect Ther. 2016;14:345–352. | ||
Thywiben A, Heinekamp T, Dahse HM, et al. Conidial dihydroxynaphthalene melanin of the human pathogenic fungus Aspergillus fumigatus interferes with the host endocytosis pathway. Front Microbiol. 2011;2:96. | ||
Geib E, Gressler M, Viediermikova I, et al. A non-canonical melanin biosynthesis pathway protects Aspergillus terreus conidia from environmental stress. Cell Chem Biol. 2015;23:587–597. | ||
Aimanianda V, Bayry J, Bozza S, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. | ||
Carrion SJ, Leal SM Jr, Ghannoum MA, Aimanianda V, Latgé JP, Pearlman E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1 and dectin-2 dependent responses and enhances fungal survival in vivo. J Immunol. 2013;191:2581–2588. | ||
Fontaine T, Delangle A, Simenel C, et al. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011;7:e1002372. | ||
Rambach G, Blum G, Latgé JP, et al. Identification of Aspergillus fumigatus surface components that mediate interaction of conidia and hyphae with human platelets. J Infect Dis. 2015;212:1140–1149. | ||
Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A. 2004;101:11030–11035. | ||
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2014;30:2725–2729. |
Supplementary material
  | Table S1 An unstructured review performed on Aspergillus terreus infections, morphology, virulence, immune response, resistance, and combination-therapy in databases up to August 2017 |
References
Vincken W, Schandevul W, Roels P. Allergic bronchopulmonary aspergillosis caused by Aspergillus terreus. Am Rev Respir Dis. 1983;127:388–389. | ||
Dunne K, Prior AR, Murphy K, et al. Emergence of persistent Aspergillus terreus colonization in a child with cystic fibrosis. Med Mycol Case Rep. 2015;9:26–30. | ||
Comacle P, Govic YL, Hoche-Delchet C, et al. Spondylodiscitis due to Aspergillus terreus in an immunocompetent host: case report and literature review. Mycopathologia. 2016;181:575–581. | ||
Bartash R, Guo Y, Pope JB, et al. Periprosthetic hip joint infection with Aspergillus terreus: a clinical case and a review of the literature. Med Mycol Case Rep. 2017;18:24–27. | ||
Paterson DL, Singh N. Invasive aspergillosis in transplant recipients.Medicine (Baltimore). 1999;78:123–138. | ||
Taccone FS, van den Abeele AM, Bulpa P, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. | ||
Elsawy A, Faidah H, Ahmed A, Mostafa A, Mohamed F. Aspergillus terreus meningitis in immunocompetent patient: a case report. Front Microbiol. 2015;6:1353. | ||
Thakur R, Shankar J. Proteome profile of Aspergillus terreus conidia at germinating stage: identification of probable virulent factors and enzymes from mycotoxin pathways. Mycopathologia. Epub 2017 Jun 24. | ||
Saad AE, Khalil MT, Ragab FM, Mekawey AA, El-Wareth MT. Efficacy of the fungi Aspergillus terreus and Penicillium janthinellum as biological control agents against Biomphalaria alexandrina snails. Int J Environ Sci Eng. 2014;5:25–37. | ||
Kozakiewicz Z. Aspergillus species on stored products. Aberystwyth, UK: Cambrian Printers; 1989. | ||
Louis B, Roy P, Sayanika DW, et al. Aspergillus terreus Thom a new pathogen that causes foliar blight of potato. Plant Pathol Quar. 2013;3(1):29–33. | ||
Abdelwehab SA, El-Nagerabi SA. Mycobiota associated with imported seeds of vegetable crops in Sudan. Open Mycol J. 2014;8:156–173. | ||
Balajee SA. Aspergillus terreus complex. Med Mycol. 2009;47:42–46. | ||
Deak E, Wilson SD, White E, et al. Aspergillus terreus accessory conidia are unique in surface architecture, cell wall composition and germination kinetics. PLoS One. 2009;4:e7673. | ||
Lass-Flörl C. Aspergillus terreus: how inoculum size and host characteristics affect virulence. J Infect Dis. 2012;205:1192–1194. | ||
Slesiona S, Ibrahim-Granet O, Olias P, Brock M, Jacobsen ID. Murine infection models for Aspergillus terreus pulmonary aspergillosis reveal long-term persistence of conidia and liver degeneration. J Infect Dis. 2012;205:1268–1277. | ||
Deak E, Nelson M, Hernández-Rodríguez Y, et al. Aspergillus terreus accessory conidia are multinucleated, hyperpolarizing structures that display differential dectin staining and can induce heightened inflammatory responses in a pulmonary model of aspergillosis. Virulence. 2011;2:200–207. | ||
Vyas JM. The duality of Aspergillus terreus: differential immune responses to distinct conidia. Virulence. 2011;2:181–184. | ||
Louis B, Sayanika DW, Pranab R, et al. Invasion of Solanum tuberosum L. by Aspergillus terreus: a microscopic and proteomics insight on pathogenicity. BMC Res Notes. 2014;7:350. | ||
Haas H. How to trigger a fungal weapon. Elife. 2015;4:e10504. | ||
Zaehle C, Gressler M, Shelest E, Geib E, Hertweck C, Brock M. Terrein biosynthesis in Aspergillus terreus and its impact on phytotoxicity. Chem Biol. 2014;21:719–731. | ||
Park SH, Kim DS, Kim WG, et al. Terrein: a new melanogenesis inhibitor and its mechanism. Cell Mol Life Sci. 2004;61:2878–2885. | ||
Sarantopoulou E, Gomoiu I, Kollia Z, Cefalas AC. Interplanetary survival probability of Aspergillus terreus spores under simulated solar vacuum ultraviolet irradiation. Planet Space Sci. 2011;59:63–78. | ||
Blum G, Perkhofer S, Haas H, et al. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob Agents Chemother. 2008;52:1553–1555. | ||
Moore CB, Sayers N, Mosquera J, Slaven J, Denning DW. Antifungal drug resistance in Aspergillus. J Infect. 2000;41:203–220. | ||
Takasuka T, Sayers NM, Anderson MJ, Benbow EW, Denning DW. Aspergillus fumigatus catalases: cloning of an Aspergillus nidulans catalase B homologue and evidence for at least three catalases. FEMS Immunol Med Microbiol. 1999;23:125–133. | ||
Lass-Flörl C, Rief A, Leitner S, et al. In vitro activities of amphotericin B and voriconazole against aleurioconidia from Aspergillus terreus. Antimicrob Agents Chemother. 2005;49:2539–2540. | ||
Sutton DA, Sanche SE, Revankar SG, Fothergill AW, Rinaldi MG. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison with voriconazole. J Clin Microbiol. 1999;37:2343–2345. | ||
Geib E, Gressler M, Viediermikova I, et al. A non-canonical melanin biosynthesis pathway protects Aspergillus terreus conidia from environmental stress. Cell Chem Biol. 2015;23:587–597. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.