Back to Journals » International Medical Case Reports Journal » Volume 13
Invasive Aspergillosis After Non-Fatal Drowning
Authors Koide S, Hadano Y , Mizuochi S, Koga H , Yamashita H
Received 5 December 2019
Accepted for publication 12 February 2020
Published 9 March 2020 Volume 2020:13 Pages 77—83
DOI https://doi.org/10.2147/IMCRJ.S241234
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Shunichi Koide,1 Yoshiro Hadano,2,3 Shinji Mizuochi,4 Hitoshi Koga,1 Hisashi Yamashita1
1Department of Emergency Medicine, St. Mary’s Hospital, Kurume, Japan; 2Department of Infection Control and Prevention, Tokyo Medical and Dental University Medical Hospital, Tokyo, Japan; 3Biostatistics Center, Kurume University School of Medicine, Kurume, Japan; 4Department of Pathology, Kurume University School of Medicine, Kurume, Japan
Correspondence: Shunichi Koide
Department of Emergency Medicine, St. Mary’s Hospital, 422 Tsubukuhonmachi, Kurume, Fukuoka 830-8543, Japan
Tel +81-942-35-3322
Fax +81-942-34-3115
Email [email protected]
Background: Pneumonitis and pneumonia after non-fatal drowning are common and the pathogens involved are numerous. However, invasive aspergillosis after non-fatal drowning in immunocompetent individuals is relatively rare. Here, we report a case of invasive aspergillosis complicated by pulmonary embolism after non-fatal drowning that proved fatal.
Case Presentation: A 75-year-old Japanese man accidentally fell into a creek and was brought to a local hospital. His oxygenation steadily deteriorated to the point that he required intubation and mechanical ventilation. He was then transferred to the emergency department at our hospital. On arrival, he had severe respiratory dysfunction with diminished breath sounds. Radiography of the chest and computed tomography of the lungs showed diffuse bilateral infiltrates. The diagnosis was acute respiratory distress syndrome caused by aspiration pneumonitis as a result of non-fatal drowning and septic shock. Despite intensive care, the patient’s hypoxia continued to worsen and he died on day 7. Computed tomography scans obtained at autopsy showed that both lungs were extensively infiltrated with effusion. An embolus was also detected in the right pulmonary artery. Microscopic analysis revealed diffuse filamentous fungi throughout the lungs, heart, stomach, thyroid gland, and the pulmonary embolus, which were identified as Aspergillus fumigatus by culture.
Conclusion: Invasive aspergillosis should also be considered in immunocompetent patients with severe respiratory failure after non-fatal drowning who do not respond to broad-spectrum antibiotics. Angioinvasive aspergillosis can even result in fatal pulmonary embolism; hence, early targeted testing for Aspergillus species and empiric intravenous voriconazole should be considered in such cases.
Keywords: drowning, aspergillosis, pulmonary embolism, immunocompetent, acute respiratory distress syndrome
Background
Invasive aspergillosis is common in immunosuppressed patients with hematologic malignancies who have undergone hematopoietic cell transplantation or solid organ transplantation, but is uncommon in those who are not neutropenic. Rare cases of invasive aspergillosis have been described in immunocompetent individuals after non-fatal drowning.1–6 Given the diagnostic difficulties and rapidly progressive nature of this disease, the mortality rate is high at 50%.1 Here we report a fatal case of invasive aspergillosis with pulmonary embolism after non-fatal drowning.
Case Presentation
A 75-year-old Japanese man with hypertension and sequelae of ischemic stroke and mild right hemiplegia accidentally fell into a creek (depth of water about 0.3 m) on 2nd Dec 2016. He was found with his face submerged in the water. After being rescued, he was first brought to a local hospital that was not ours, near Kurume City, Fukuoka Prefecture, Japan. On arrival at that hospital, his Glasgow Coma Scale was E3V3M6, body temperature was 33.4°C, heart rate was 88 beats/min, blood pressure was 120/60 mmHg, respiratory rate was 26 breaths/min, and oxygen saturation was 92% on supplemental oxygen at a flow rate of 10 L/min. He had no obvious injury but showed bilateral coarse crackles and hypothermia. Rewarming using a warming blanket and antibiotic therapy (ampicillin and sulbactam) were started. Even after starting non-invasive ventilation, the patient’s oxygenation steadily deteriorated to oxygen saturation levels at 80% on 100% oxygen. He was intubated and mechanically ventilated, and transferred to the emergency department of our tertiary hospital in Kurume City on the same day.
On arrival at our hospital, he was sedated with propofol. His body temperature was 36.4°C, heart rate was 104 beats/min, blood pressure was 123/84 mmHg, respiratory rate was 30 breaths/min, and oxygen saturation level was 91% on supplemental oxygen at a flow rate of 10 L/min. The physical examination was unremarkable except for bilaterally diminished breath sounds. Arterial blood gas analysis showed a pH of 7.34, a PaCO2 of 42 mmHg, a PaO2 of 199 mmHg, and an HCO3 of 21.9 mmol/L under 100% oxygen.
Laboratory investigations on day 1 (2nd Dec) showed a white blood cell count of 2980/μL (normal range 3500-9000/μL) with 63.5% neutrophils (normal range 40-73%), a hemoglobin level of 15.3 g/dL (normal range 13–17 g/dL), a platelet level of 159,000/μL (normal range 140,000–340,000/μL), a C-reactive protein level of 0.6 mg/dL (normal range 0–0.2 mg/dL), and a highly increased procalcitonin level of 59.37 ng/mL (normal range <0.05 ng/mL). Ultrasonographic examination of the heart on the same day showed a normal ejection fraction with no valvular disease. Chest radiography and computed tomography (CT) of the lung showed diffuse infiltrates bilaterally with no pleural effusion (Figure 1).
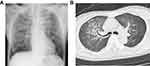 |
Figure 1 (A) A chest radiograph and (B) a computed tomography scan of the chest showing diffuse bilateral infiltrates. |
The diagnosis was acute respiratory distress syndrome (ARDS) caused by aspiration pneumonitis as a result of non-fatal drowning and septic shock. He was treated with isotonic crystalloids, an intravenous broad-spectrum antibiotic (meropenem 1.0 g every 12 hrs), vasopressors, and protective ventilation. Methylprednisolone 80 mg daily was started on day 3 for ARDS (Figure 2A). A beta-D-glucan assay (β-glucan test; FUJIFILM Wako Pure Chemical Corporation, Japan) on day 4 found it to be elevated at 37.6 pg/mL (normal range <11.0 pg/mL); serum galactomannan was not measured. The vasopressors could be stopped on day 5 but the hypoxia did not improve (Figure 2B). On day 6, the tracheal aspirate culture was found positive for Aeromonas hydrophila but the blood culture was negative. The patient was switched to piperacillin/tazobactam at a dosage of 4.5 g every 6 hrs based on the susceptibility test. The hypoxia continued to worsen and the patient died on day 7.
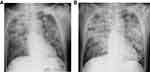 |
Figure 2 Chest radiographs obtained on day 3 (A) and 5 (B). |
CT scans obtained at autopsy did not reveal any focal lesion in the brain; however, both lungs were completely infiltrated with effusion (Figure 3), were severely congested, and each weighed over 1000 g (Figure 4A). An embolus was detected in the right pulmonary artery (Figure 5A) but not in either femoral vein. Microscopic analysis with Grocott staining revealed diffuse filamentous fungi throughout the lungs within necrotizing tissue and intravascular lesions (Figure 4B), as well as in the heart, stomach, and thyroid gland. The pulmonary embolus also contained filamentous fungi (Figure 5B), which might have been responsible for the patient’s acute deterioration. We cultured biopsies from both lungs taken from the histological preparations and the fungi were identified as Aspergillus fumigatus. Therefore, we diagnosed this case as invasive aspergillosis complicated by pulmonary embolism.
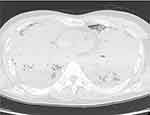 |
Figure 3 Computed tomography scans obtained at autopsy show that the lungs were completely infiltrated by effusion. |
 |
Figure 4 (A) Both lungs were massively congested. (B) Numerous filamentous fungi are seen in the lung after Grocott staining (×400). |
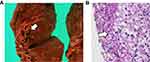 |
Figure 5 (A) The right pulmonary artery contained an embolus (arrow). (B) Filamentous fungi (arrow) were detected in the embolus by Periodic acid-Schiff staining (×400). |
Discussion
A 75-year-old immunocompetent Japanese man succumbed to severe respiratory failure after non-fatal drowning and was found at autopsy to have invasive aspergillosis complicated by pulmonary embolism. Pneumonitis and pneumonia after non-fatal drowning are often encountered in clinical practice and the causative pathogens are numerous. Aeromonas species, Burkholderia pseudomallei, Chromobacterium violaceum, and Streptococcus pneumoniae are frequently reported fresh-water pathogens, while C. violaceum, Pseudomonas aeruginosa, and Pseudallescheria boydii are common causative organisms in contaminated stagnant water.7 Fungi known to cause invasive disease after non-fatal drowning include P. boydii and, to a lesser degree, Aspergillus species. A search of the PubMed database using the search terms “aspergillosis” and “drowning” yielded 13 cases published between 1984 and 2016 (Table 1). These reports are from many countries and all patients involved were immunocompetent. The diagnosis of aspergillosis was made by microbiologic testing with or without galactomannan assay. The mortality rate was high at 62%, even though most cases were treated with antifungal agents. Furthermore, none of the survivors had an infection in their central nervous system.
 |
Table 1 Reports of Invasive Pulmonary Aspergillus Fumigatus After Non-Fatal Drowning (1984–2016) |
We did not consider that this patient could have a fungal infection and therefore did not implement the appropriate diagnostic test and targeted therapy, which may have contributed to the deterioration of his condition. There are several reasons why a fungal infection was missed in this case. First, although the patient had respiratory failure after non-fatal drowning, aspergillosis was not considered because he was fully immunocompetent. Moreover, although he showed mild right hemiplegia following a stroke, he had good swallowing function and no history of aspiration pneumonia. No other co-morbidities were observed in this case except hypertension. For these reasons, we misinterpreted the elevated β-glucan test result as a false positive and did not conduct any further tests. Second, fungi were not identified from the tracheal aspirate culture in our hospital. However, we later found out that an airway aspirate sample tested at the primary hospital was positive for A. fumigatus. Since a culture of any biological specimen is not very sensitive and takes about 5 days, histopathological findings and galactomannan levels from bronchial alveolar lavage (BAL) or lung biopsy are essential for early diagnosis of invasive aspergillosis. Unfortunately, the rapid progression of the disease, lasting only 7 days in the hospital, and severe hypoxia did not allow us to investigate the presence of opportunistic rare infections or perform any invasive procedures such as bronchoscopy and BAL or biopsy. Although serum galactomannan is not sensitive outside the hematology context, it is a non-invasive test and should have been measured in this case. Serial galactomannan measurements can, in fact, be helpful for early diagnosis of invasive aspergillosis.4
Invasive aspergillosis can occur in hosts who are not severely immunocompromised, particularly patients with chronic obstructive pulmonary disease (COPD). Airway colonization by Aspergillus species is common among COPD patients in intensive care units.8 Although there was no obvious history of COPD in our patient, he had a long history of smoking and, therefore, the impaired ciliary activity might have affected the defense mechanisms of the airways and allowed for fungal invasion. Moreover, as he had hypothermia his first presentation and his baseline white cell count was low, he may have had an impaired immune response. The use of methylprednisolone for ARDS could have also worsened his untreated aspergillosis. Most previously reported cases presented with ARDS but steroid usage varied between cases (Table 1). Steroids not only decrease the antifungal activity of alveolar macrophages but also enhance the growth of A. fumigatus in vitro.9 Furthermore, non-fatal drowning has been reported to be a risk factor for invasive pulmonary aspergillosis in critically-ill non-neutropenic patients.10
A right pulmonary artery embolus containing A. fumigatus was discovered in this patient despite thromboprophylaxis with unfractionated heparin and compression stockings. Given that no thrombus was found in the veins of the lower limbs at autopsy, it appears that the embolism was caused by the angioinvasiveness of Aspergillus species. This pulmonary embolism may have contributed to the rapid deterioration of this patient. Our case had the shortest length of hospital stay, only 7 days, compared with all previous fatal cases (Table 1). No other case reported invasive aspergillosis complicated by pulmonary embolism. Although we could not administer any antifungal therapy in this case, parenteral voriconazole is the first-line therapy for invasive aspergillosis. It has been reported to improve chances of survival and to have fewer side effects than amphotericin B as shown in a randomized control trial.16
Conclusions
We encountered a case of invasive aspergillosis complicated by pulmonary embolism after non-fatal drowning that was ultimately fatal. Invasive aspergillosis should be suspected in patients with severe respiratory failure who have been exposed to stagnant water and are unresponsive to broad-spectrum antibiotics, even if they are immunocompetent. Bronchoscopy should also be conducted in such patients to aid diagnosis as the serum galactomannan test alone is not sufficiently sensitive. If not treated correctly, angioinvasive aspergillosis can even result in fatal pulmonary embolism. Early targeted testing for Aspergillus species and treatment using empiric intravenous voriconazole should be considered in such cases.
Abbreviations
CT, computed tomography; ARDS, acute respiratory distress syndrome; BAL, bronchial alveolar lavage; COPD, chronic obstructive pulmonary disease.
Consent for Publication
Written informed consent was obtained from the patient’s next of kin for publication of this case report and any accompanying images.
Data Sharing Statement
The datasets generated during and/or analyzed during this research are available from the corresponding author on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jenks JD, Preziosi M. A challenging case of invasive pulmonary aspergillosis after near-drowning: a case report and literature review. Infect Dis Clin Pract (Baltim Md). 2015;23:227–230. doi:10.1097/IPC.0000000000000263
2. Munta K, Gopal PB, Vigg A. Invasive aspergillosis in near drowning nonneutropenic patient. Indian J Crit Care Med. 2015;19:739–742. doi:10.4103/0972-5229.171413
3. Kawakami Y, Tagami T, Kusakabe T, et al. Disseminated aspergillosis associated with tsunami lung. Respir Care. 2012;57:1674–1678. doi:10.4187/respcare.01701
4. Leroy P, Smismans A, Seute T. Invasive pulmonary and central nervous system aspergillosis after near-drowning of a child: case report and review of the literature. Pediatrics. 2006;118:e509–e513. doi:10.1542/peds.2005-2901
5. Kowacs PA, Monteiro de Almeida S, Pinheiro RL, et al. Central nervous system Aspergillus fumigatus infection after near drowning. J Clin Pathol. 2004;57:202–204. doi:10.1136/jcp.2003.010066
6. Mizukane R, Sawatari K, Araki J, et al. Invasive pulmonary aspergillosis caused by aspiration of polluted water after nearly drowning. Kansenshogaku Zasshi. 1996;70:1181–1185. doi:10.11150/kansenshogakuzasshi1970.70.1181
7. Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;25:896–907. doi:10.1086/515532
8. Shahi M, Ayatollahi Mousavi SA, Nabili M, Aliyali M, Khodavaisy S, Badali H. Aspergillus colonization in patients with chronic obstructive pulmonary disease. Curr Med Mycol. 2015;1:45–51. doi:10.18869/acadpub.cmm.1.3.45
9. Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology. 1994;140:2475–2479. doi:10.1099/13500872-140-9-2475
10. Trof RJ, Beishuizen A, Debets-Ossenkopp YJ, Girbes AR, Groeneveld AB. Management of invasive pulmonary aspergillosis in non-neutropenic critically ill patients. Intensive Care Med. 2007;33:1694–1703. doi:10.1007/s00134-007-0791-z
11. Vieira DF, Van Saene HK, Miranda DR. Invasive pulmonary aspergillosis after near-drowning. Intensive Care Med. 1984;10:203–204. doi:10.1007/bf00259439
12. Ter Maaten JC, Golding RP, Strack van Schijndel RJ, Thijs LG. Disseminated aspergillosis after near-drowning. Neth J Med. 1995;47:21–24. doi:10.1016/0300-2977(94)00102-f
13. Li P, Cao EH, Zhao BL, et al. Invasive aspergillosis after near-drowning: case reports and review of the literature. Zhonghua Jie He He Hu Xi Za Zhi. 2011;34:657–662.
14. Ratermann KL, Ereshefsky BJ, Fleishaker EL, Thornton AC, Buch KP, Martin CA. Fulminant invasive pulmonary aspergillosis after a near-drowning accident in an immunocompetent patient. Ann Pharmacother. 2014;48:1225–1229. doi:10.1177/1060028014537611
15. Tavazzi G, Via G, Marzani FC, Mojoli F. Invasive pulmonary aspergillosis after near-drowning. Lancet Infect Dis. 2016;16:1430. doi:10.1016/S1473-3099(16)30202-X
16. Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi:10.1056/NEJMoa020191
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
