Back to Journals » Open Access Emergency Medicine » Volume 14
Intravenous Fluid Bolus Rates Associated with Outcomes in Pediatric Sepsis: A Multi-Center Analysis
Authors Mullan PC , Pruitt CM, Levasseur KA, Macias CG, Paul R, Depinet H, Nguyen ATH, Melendez E
Received 29 March 2022
Accepted for publication 16 July 2022
Published 28 July 2022 Volume 2022:14 Pages 375—384
DOI https://doi.org/10.2147/OAEM.S368442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Paul C Mullan,1 Christopher M Pruitt,2 Kelly A Levasseur,3 Charles G Macias,4 Raina Paul,5 Holly Depinet,6 Anh Thy H Nguyen,7 Elliot Melendez8
1Department of Pediatrics, Division of Emergency Medicine, Eastern Virginia Medical School, Children’s Hospital of the King’s Daughters, Norfolk, VA, USA; 2Department of Pediatrics, Division of Pediatric Emergency Medicine, Medical University of South Carolina, Charleston, SC, USA; 3Pediatric Emergency Medicine, Beaumont Children’s Hospital, Royal Oak, MI, USA; 4Division of Pediatric Emergency Medicine, University Hospitals Rainbow Babies and Children’s, Case Western Reserve University School of Medicine, Cleveland, OH, USA; 5Department of Emergency Medicine, Advocate Children’s Hospital, Park Ridge, IL, USA; 6Division of Emergency Medicine, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati School of Medicine, Cincinnati, OH, USA; 7Johns Hopkins All Children’s Institute for Clinical and Translational Research, St. Petersburg, FL, USA; 8Division of Pediatric Critical Care, Connecticut Children’s Medical Center, University of Connecticut, Hartford, CT, USA
Correspondence: Paul C Mullan, Email [email protected]
Purpose: Pediatric sepsis guidelines recommend rapid intravenous fluid (IVF) bolus administration rates (BAR). Recent sepsis studies suggest that rapid BAR may be associated with increased morbidity. We aimed to describe the association between emergency department (ED) IVF BAR and clinical outcomes in pediatric sepsis.
Patients and Methods: Secondary post-hoc analysis of retrospective cohort data from 19 hospitals in the Pediatric Septic Shock Collaborative (PSSC) database. Patients with presumed septic shock were defined by severe sepsis/septic shock diagnostic codes, receipt of septic shock therapies, or floor-to-ICU transfers within 12 hours from ED admission for septic shock. Patients (2 months– 21 years) with complete data on weight, antibiotic receipt, bolus timing, and bolus volumes were included. The primary outcome was 30-day mortality. Associations between BAR and mortality and secondary (intubation or non-invasive positive pressure ventilation = NIPPV) outcomes were assessed using unadjusted and adjusted logistic regression.
Results: The PSSC database included 6731 patients; 3969 met inclusion and received a median ED volume of 40.2 mL/kg. Seventy-six (1.9%) patients died, 151 (3.8%) were intubated, and 235 (5.9%) had NIPPV administered. The median BAR was 25.7 mL/kg/hr. For each 20 mL/kg/hr increase in BAR, the adjusted odds ratio (aOR) for 30-day mortality [aOR = 1.11 (95% CI 1.01, 1.23)], intubation [aOR = 1.25 (95% CI 1.09, 1.44)], and NIPPV [aOR = 1.20 (95% CI 1.05, 1.38)] significantly increased.
Conclusion: Faster ED IVF bolus administration rates in this pediatric sepsis database were associated with higher adjusted odds of death, intubation and NIPPV. Controlled trials are needed to determine if these associations are replicable.
Keywords: emergency department, mortality, intubation, collaborative
Plain Language Summary
When children get an infection, with symptoms like a fever or vomiting, they are usually able to recover from the illness within a few days without any medical treatment. Some of these children have a more severe form of infection called septic shock that causes organ failure and can be fatal. Septic shock treatment typically starts in the emergency department (ED) where children receive therapeutic interventions such as intravenous fluid (IVF) boluses and antibiotics. An IVF bolus is a volume of fluids that is given rapidly into the bloodstream. The speed at which these IVF bouses go into the bloodstream may be associated with better or worse outcomes for children with septic shock. A group of nineteen EDs compiled a database that included the timing of various therapeutic interventions, as well as several clinical outcomes, in a population of their pediatric patients with presumed septic shock. Our research team retrospectively analyzed 3969 patients in this database. We discovered that patients who had received a higher amount of IVF volumes over a shorter amount of time were more likely to die in the first 30 days after ED arrival and more likely to need a ventilator to help them breathe effectively. This association of faster fluids with worse clinical outcomes requires further research to determine if the same findings would be found in other settings. If future research found similar findings, it would help clinicians determine the safest speed to administer IVF to children with septic shock.
Introduction
Background
Pediatric sepsis is associated with significant morbidity and mortality, and recent evidence suggests that its prevalence is rising.1–3 Severe sepsis and septic shock account for 4% of pediatric hospitalizations and 8% of intensive care unit (ICU) admissions. Rates of in-hospital mortality in pediatric severe sepsis range from 9% to 21%.2–6 Initiation of timely, goal-directed therapies for sepsis and septic shock has been shown to decrease hospital length of stay, organ dysfunction, and death.4,7–10
While sepsis and septic shock cause high mortality rates in both adult and pediatric populations, treatment guidelines differ between the age groups. Prior treatment guidelines for pediatric severe sepsis, at the time of this study’s design, had recommended rapidly administering 20 mL/kg of an isotonic crystalloid or colloid fluids and repeating boluses, up to a total of 60 mL/kg, over the first 10–15 minutes if hypoperfusion persists.11,12 Recent pediatric sepsis guidelines are more fluid-restrictive but continue to recommend multiple boluses in the first hour, depending on whether there is access to intensive care resources (up to 60 mL/kg) or no access to such resources (up to 40 mL/kg).13–16 In contrast, recent adult guidelines advocate administering a more modest 30 mL/kg of crystalloid fluids over the first 3 hours of resuscitation.17
Importance
While prospective, outcome-based research into septic shock has fueled more conservative fluid administration for adults, such evidence is largely lacking for pediatric severe sepsis.18,19 Though guidelines exist on how much fluid children should receive within the first hour, there is a paucity of literature to support these recommendations.14,15 There are added concerns about the generalizability of these guidelines, as there is evidence of increased mortality with fluid boluses in pediatric sepsis in resource-limited settings.20–22 While studies have demonstrated that overall fluid overload is associated with increased mortality in pediatric sepsis, there is a lack of equipoise on fluid volume in the early therapeutic window, or the optimal time to infuse the first bolus of volume, especially in resource-rich settings with access to intensive care resources.23–28 Only one single-center study has specifically examined rates of early fluid bolus administration in pediatric septic shock, with mixed results as to clinical outcomes.29 An investigation that assesses the association between fluid administration rates and mortality will provide guidance for prospective studies on pediatric sepsis.
Goals of This Investigation
Using pediatric sepsis data from a multi-center, United States-based, pediatric emergency department quality improvement collaborative, the objective of our study is to examine the relationship between early isotonic fluid bolus administration rates (BAR) and 30-day mortality (primary outcome), and the relationship between early isotonic fluid BAR and the need for intubation or non-invasive positive pressure ventilation (NIPPV) (secondary outcomes).
Materials and Methods
Study Design and Setting
The Pediatric Septic Shock Collaborative (PSSC) was formed by a group of investigators within the Section of Emergency Medicine in the American Academy of Pediatrics (AAP) in collaboration with the Children’s Hospital Association.30 Experts in pediatric sepsis care with diverse, multi-disciplinary backgrounds developed a comprehensive change package that was consistent with evidence-based guidelines that included sepsis screening tools, educational resources, shared sepsis definitions, and recommended consistent data definitions, although individual sites could implement identification as their resources dictated.12,31 The key drivers of care included rapid recognition, escalation of care, first hour resuscitation goals, and transfer of care.
The PSSC was comprised of nineteen United States-based pediatric emergency departments. The ED sites had a median of 52,300 (IQR 33000, 69,100) annual visits. The affiliated hospitals, the majority (63%) of which were freestanding children’s hospitals, had a median of 289 (IQR 159, 329) inpatient beds and 36 (IQR 23, 43) ICU beds. Physician and nursing champions led the efforts at each local site. These leaders from across the collaborative used established models to collaborate in monthly virtual webinar sessions and twice annually at half-day, in-person sessions at national conferences.32
Selection of Participants
The study period was from November 1, 2013, until May 31, 2016. The PSSC defined cases at collaborative sites with any of the following a priori criteria: 1) any ICD codes consistent with severe sepsis or septic shock, 2) a positive sepsis screening tool plus treatment with a sepsis care bundle (defined as ≥1 parenteral antibiotic, ≥2 boluses of isotonic fluid, and obtainment of a blood culture), 3) sepsis care bundle treatment plus any of the following: use of vasoactive agent in the ED, lactate assessment, or ICU admission (directly from the ED or any floor-to-ICU transfers within 12 hours of ED admission); or 4) use of a septic shock order set. Patients were excluded from the collaborative database if they had been transferred from another ED and had already received antibiotics or two fluid boluses prior to ED arrival.
For this secondary, post hoc analysis of an existing database of patients ages 2 months–21 years old, we excluded any patients with missing weight data, missing bolus administration timestamp data, lack of having at least two documented bolus volumes, or inappropriately large single bolus volumes (ie, we excluded a priori >60 mL/kg single documented bolus volumes, as this amount is not a common clinical standard of care and likely represented several individual boluses combined into one data entry). We also excluded any patients with an interval duration of <3 minutes between the initiation times of sequential boluses as this was deemed not feasible.33,34 In addition, patients with an interval time of >120 minutes between bolus volumes were also excluded based on the assumption that these patients were not being actively resuscitated.
Interventions and Measurements
Site leaders screened their electronic health record for included ED cases and entered data for all included patients into a central, password-protected, data portal. The collaborative identified a priori data elements to be collected and standardized the definition of data elements, required submission of all data related to the primary outcomes, and had quality assurance logic checks to ensure appropriate data entry. Additional data on the collaborative’s secondary outcomes were also collected, as resources for data entry allowed, at each site. Data related to IVF administration included bolus initiation timestamps and bolus volume amounts. Given the post hoc nature of this secondary analysis, site investigators were unaware of the objectives of this present study at the time of data abstraction. The PSSC and participating sites obtained institutional review board approvals and/or waivers (Baylor College of Medicine–H23511).30 The database complied with all relevant data protection and privacy regulations.
The BAR for each patient was calculated as Total Bolus Volume/Patient Weight/Elapsed Time expressed in milliliters/kilogram/hour (mL/kg/hr). Elapsed time is defined as the time difference from the first bolus recorded time to the last bolus recorded time. Therefore, Total Bolus Volume is defined as the sum of all ED boluses administered for the patient less the volume of the last bolus administered. The primary outcome was 30-day aggregate mortality during the index admission from any cause. Secondary outcomes were intubation and use of non-invasive positive pressure ventilation (NIPPV) such as Bi-level Positive Airway Pressure or Continuous Positive Airway Pressure. NIPPV usage included initiation of new or above baseline ventilatory support. We adhered to the Strengthening the Reporting of Observational Students in Epidemiology (STROBE) guidelines.35
Analysis
All statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC). Demographic and clinical characteristics were summarized for the overall population and stratified for each outcome using median and interquartile range for continuous variables and counts and percentages for categorical variables. The association between bolus rates and each outcome was evaluated using unadjusted (bivariate) and adjusted (multiple) logistic regression models. Random intercepts to adjust for hospital variations in outcome rates were included in the models if statistically significant at the 0.05 level. Age, sex, and total bolus volumes were planned a priori to be included in the adjusted models, while the other clinical variables (see Table 1) were included if statistical significance was achieved in unadjusted analyses.36–38 Intubation and NIPPV were additionally assessed as potential confounders for mortality. Hypotension was assessed in the analyses as a potential confounder in the adjusted models and it was defined as the presence of a low systolic blood pressure in the ED (defined as less than ‘70 + 2 times the age in years’ or 90 mm Hg for patients ≥10 years old).31 Non-linear associations between BAR and each outcome were also assessed by evaluating the statistical significance of quadratic terms for the bolus rate. Adjusted models used patients with complete information for all variables. P-values less than 0.05 were considered statistically significant.
 |
Table 1 Descriptive Statistics of the Study Population |
Two sensitivity analyses were conducted to evaluate the influence of extreme bolus values and missing values. In the first sensitivity analysis, patients were excluded if their BAR was above 280 mL/kg/hr, which corresponded to the mean + (3 × standard deviation) of the log-transformed values. For the second sensitivity analysis, estimates were calculated following the multiple imputation process using the dataset from the first sensitivity analysis. Twenty imputed datasets were created using fully conditional specification (due to non-monotone missing data).39 Missing hemoglobin values were imputed using predictive mean matching while missing categorical variables (sex, hypotension, vasoactive agent started, intubated, NIPPV) were imputed using logistic regression.
Results
Characteristics of Study Subjects
The PSSC database contained 6731 pediatric sepsis patients between 2 months and 21 years of age. ED sites had a median of 208 sepsis cases (IQR 95, 427) and the percent admitted to the ICU was 56% (IQR 44, 64). A total of 3969 patients were included for analysis (Figure 1, Table 1). Included patients received a total median ED volume of 40.2 mL/kg (IQR 40, 60) with a BAR of 25.7 mL/kg/hr (IQR 16.3, 46.2). Of the included patients, seventy-six (1.9%) patients died, 151 (3.8%) were intubated, and 235 (5.9%) had NIPPV administered. There was significant variation in NIPPV rates across hospitals but no variation in intubation or mortality.
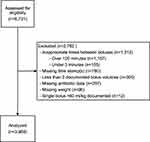 |
Figure 1 Flow diagram for subjects. |
Main Results
Unadjusted analyses revealed that for every 20 mL/kg/hr increase in the BAR, the odds of death increased by 19% (odds ratio [OR]=1.19; 95% confidence interval [CI]=1.11–1.27) (Table 2). In univariate analysis, four additional factors were significantly associated (p < 0.05) with mortality and were included in the adjusted model: ICU admission, hypotension, use of vasoactive agents, and NIPPV utilization. In the adjusted model, for each 20 mL/kg/hr increase in the BAR, the adjusted odds ratio (aOR) for 30-day mortality increased by 11% (OR = 1.11; 95% CI = 1.01–1.23). These results remained significant in the sensitivity analysis.(Supplementary Table 1).
 |
Table 2 Patient Characteristics and Analyses for Primary Outcome (30-Day Mortality) |
Secondary Outcomes
For our secondary outcomes of intubation and NIPPV, unadjusted analyses revealed that for each 20 mL/kg/hg increase in the BAR, there was a significant increased odds for both outcomes (Tables 3 and 4). In univariate analysis of the outcome of intubation, five factors were associated with the outcome and were included in the adjusted model: age, sex, total bolus volume, ICU admission, hypotension, vasoactive agent, and quadratic term for bolus rate. In the univariate analysis for the outcome of NIPPV, the same five additional factors, as well as hemoglobin level, were included in the adjusted model. In the adjusted models, the increased odds for both secondary outcomes remained significant (Tables 3 and 4). These results also remained significant in the sensitivity analysis (Supplementary Table 1).
 |
Table 3 Patient Characteristics and Analyses for the Secondary Outcome of Endotracheal Intubation |
 |
Table 4 Patient Characteristics and Analyses for the Secondary Outcome of Non-Invasive Positive Pressure Ventilation Utilization |
Discussion
This study aimed to describe the association between intravenous fluid bolus administration rates and pediatric sepsis outcomes using a multi-center database of patients that had presented to pediatric emergency departments with access to intensive care resources. In this dataset of almost 4000 cases of presumed sepsis, faster intravenous fluid bolus administration rates were associated with a significant increase in the odds of 30-day mortality, which was sustained in the adjusted model. In the adult emergency department setting, a 149-center study of 49,331 patients with sepsis and septic shock showed that despite lower sepsis bundle care compliance being associated with higher risk-adjusted mortality, there was no effect on mortality with longer times of completion of the intravenous fluid boluses.40 In a randomized controlled study of adults in Zambia with septic shock, those who received an early fluid bolus in a resuscitation protocol, versus usual care therapy, had a greater use of vasopressor agents and significantly higher mortality.41 The literature on pediatric septic shock mortality comparing different volume administrations has been controversial with significant heterogeneity among the study populations and interventions tested. While the pediatric FEAST trial found an association with increased mortality and fluid bolus administration in the first hour of treatment, other pediatric studies found no differences in mortality using variable volumes.20,26,42
None of these aforementioned studies examined the effect of specific IVF bolus administration rates on mortality. The Sankar et al study in an emergency department in India was the closest to examining fluid administration rates by comparing 20 mL/kg boluses given over 5–10-minute or 15–20-minute time periods. While they found no change in mortality between the two groups, the lack of precision of the intervals for each treatment arm might have biased their results towards a null finding for mortality.29 Multi-center, prospectively designed studies are necessary to determine whether specific IVF bolus administration rates are associated with increases in mortality. If such an association exists, sepsis care guidelines would need to consider whether slower bolus administration rates might be warranted during the early provision of care. Currently, the Surviving Sepsis guidelines recommend 40–60 mL/kg in the first hour, depending on intensive care resource availability.13 Our study provides an important glimpse, as to the potential association between BAR and mortality in pediatric sepsis, which will require prospective research to further investigate.
In our adjusted model, we also found that patients who had a faster bolus administration rate had a higher risk of intubation and NIPPV. These findings are consistent with the findings of the aforementioned Sankar et al study, which found that patients with septic shock who had received fluid boluses over 5–10 minutes, rather than over 15–20 minutes, were more likely to need mechanical ventilation and had a greater increase in the oxygenation index in the first six hours of care.29 In another prospective randomized controlled study of pediatric septic shock patients in an emergency department in India, the group that received two fluid boluses over one hour (versus over 20 minutes) had significantly increased hepatomegaly rates but did not find a significant increase in intubation (46.5% control vs 55% study group, p = 0.28).42
Limitations
There were several limitations in our study. First, our study design did not allow us to determine the specific duration of the administration of each bolus as the end times were not recorded. As a result, we used the start of a subsequent bolus as a surrogate for the end of the prior fluid bolus. As the rate of fluid administration was our primary predictor, this inherent imprecision of our study design should have biased the results towards the null hypothesis for each of our outcomes. A second limitation was that the collaborative did not collect data on the total amount of fluids given to patients in the first 24 hours of care. Since early fluid overload in this time period has been associated with worse outcomes in pediatric sepsis, our study was focused on bolus volumes received during initial ED-based resuscitation.23,25,43 As the focus of the study was fluid administration in the ED context, we did not abstract certain clinical elements (eg, vasoactive agent receipt) subsequent to the ED encounter.
Additional data limitations included that data on source of infection, pathogen of infection, lactate levels, presence of chronic condition comorbidities, presence of organ dysfunction, traditional sepsis risk stratification scores (eg, pediatric index of mortality or pediatric risk of mortality) and timing of antibiotics were not included in the regression models as these data were inconsistently recorded across the nineteen sites. As a retrospective study, the decision as to when someone is intubated or gets started or advanced on NIPPV was not standardized, so local institutional practices, and not necessarily volume overload, might have driven the need for these interventions. Additionally, we did not abstract data on specific NIPPV modalities. While we adjusted for various factors (eg, hypotension, gender, age) in our multi-variable models that had previously been associated with increases in sepsis-related mortality, and included them in the regression analysis, these factors may be insufficient to capture all elements of adjusting for severity. Despite the adjusted factors in our model, it is possible that confounding by indication contributed to the association of faster BAR with worse outcomes due to an unmeasured severity of illness characteristic(s) that prompted clinicians to deliver more aggressive fluid management. Unfortunately, there are no validated severity of illness scoring tools to predict the risk of worse outcomes for pediatric sepsis, and the use of pediatric severity of illness scores has not been validated outside the pediatric intensive care setting.44–46 Lastly, with only 214 children presenting with hypotension, this study cannot separately analyze the impact of fluid administration rates in hypotensive children. Our findings highlight the need for further prospective studies that focus on BAR for pediatric sepsis in the ED setting.
Conclusion
In summary, this analysis of a multi-center database of pediatric sepsis patients, who presented to various emergency departments with access to intensive care resources, found that higher intravenous fluid bolus administration rates were associated with higher adjusted odds of mortality, intubation and NIPPV. Future studies are needed to further understand the impact of bolus administration rates on determining the optimal management of pediatric sepsis patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med. 2014;15(9):798–805. doi:10.1097/PCC.0000000000000225
2. Ruth A, McCracken CE, Fortenberry JD, Hall M, Simon HK, Hebbar KB. Pediatric severe sepsis: current trends and outcomes from the pediatric health information systems database. Pediatr Crit Care Med. 2014;15(9):828–838. doi:10.1097/PCC.0000000000000254
3. Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med. 2013;14(7):686–693. doi:10.1097/PCC.0b013e3182917fad
4. Balamuth F, Weiss SL, Fitzgerald JC, et al. Protocolized treatment is associated with decreased organ dysfunction in pediatric severe sepsis. Pediatr Crit Care Med. 2016;17(9):817–822. doi:10.1097/PCC.0000000000000858
5. Kutko MC, Calarco MP, Flaherty MB, et al. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med. 2003;4(3):333–337. doi:10.1097/01.PCC.0000074266.10576.9B
6. Jaramillo-Bustamante JC, Marín-Agudelo A, Fernández-Laverde M, Bareño-Silva J. Epidemiology of sepsis in pediatric intensive care units: first Colombian multicenter study. Pediatr Crit Care Med. 2012;13(5):501–508. doi:10.1097/PCC.0b013e31823c980f
7. Long E, Duke T. Fluid resuscitation therapy for paediatric sepsis. J Paediatr Child Health. 2016;52(2):141–146. doi:10.1111/jpc.13085
8. Paul R, Neuman MI, Monuteaux MC, Melendez E. Adherence to PALS sepsis guidelines and hospital length of stay. Pediatrics. 2012;130(2):e273–280. doi:10.1542/peds.2012-0094
9. Lane RD, Funai T, Reeder R, Larsen GY. High reliability pediatric septic shock quality improvement initiative and decreasing mortality. Pediatrics. 2016;138(4):e20154153. doi:10.1542/peds.2015-4153
10. Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. JAMA. 2018;320(4):358–367. doi:10.1001/jama.2018.9071
11. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi:10.1097/CCM.0b013e31827e83af
12. Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666–688. doi:10.1097/CCM.0b013e31819323c6
13. Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(2):e52–e106. doi:10.1097/PCC.0000000000002198
14. Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017;45(6):1061–1093. doi:10.1097/CCM.0000000000002425
15. Maconochie IK, Aickin R, Hazinski MF, et al. Pediatric Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142(16_suppl_1):S140–S184. doi:10.1161/CIR.0000000000000894
16. Van de Voorde P, Turner NM, Djakow J, et al. European Resuscitation Council Guidelines 2021: paediatric life support. Resuscitation. 2021;161:327–387. doi:10.1016/j.resuscitation.2021.02.015
17. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi:10.1097/CCM.0000000000002255
18. Gelbart B, Glassford NJ, Bellomo R. Fluid bolus therapy-based resuscitation for severe sepsis in hospitalized children: a systematic review. Pediatr Crit Care Med. 2015;16(8):e297–307. doi:10.1097/PCC.0000000000000507
19. Ford N, Hargreaves S, Shanks L. Mortality after fluid bolus in children with shock due to sepsis or severe infection: a systematic review and meta-analysis. PLoS One. 2012;7(8):e43953. doi:10.1371/journal.pone.0043953
20. Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495. doi:10.1056/NEJMoa1101549
21. Duke T. What the African fluid-bolus trial means. Lancet. 2011;378(9804):1685–1687.
22. Yue J, Zheng R, Wei H, et al. Childhood mortality after fluid bolus with septic or severe infection shock: a systematic review and meta-analysis. Shock. 2021;56(2):158–166. doi:10.1097/SHK.0000000000001657
23. Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(3):257–268. doi:10.1001/jamapediatrics.2017.4540
24. Chen J, Li X, Bai Z, et al. Association of fluid accumulation with clinical outcomes in critically ill children with severe sepsis. PLoS One. 2016;11(7):e0160093. doi:10.1371/journal.pone.0160093
25. Wong JJ, Ho SX, Lee AOC, et al. Positive fluid balance is associated with poor clinical outcomes in paediatric severe sepsis and septic shock. Ann Acad Med Singap. 2019;48(9):290–297. doi:10.47102/annals-acadmedsg.V48N9p290
26. Inwald DP, Canter R, Woolfall K, et al. Restricted fluid bolus volume in early septic shock: results of the fluids in shock pilot trial. Arch Dis Child. 2019;104(5):426–431. doi:10.1136/archdischild-2018-314924
27. Parker MJ, Thabane L, Fox-Robichaud A, Liaw P, Choong K; Canadian Critical Care Trials Group and the Canadian Critical Care Translational Biology Group. A trial to determine whether septic shock-reversal is quicker in pediatric patients randomized to an early goal-directed fluid-sparing strategy versus usual care (SQUEEZE): study protocol for a pilot randomized controlled trial. Trials. 2016;17(1):556. doi:10.1186/s13063-016-1689-2
28. van Paridon BM, Sheppard C, Joffe AR, Joffe AR; Alberta Sepsis Network. Timing of antibiotics, volume, and vasoactive infusions in children with sepsis admitted to intensive care. Crit Care. 2015;19:293. doi:10.1186/s13054-015-1010-x
29. Sankar J, Ismail J, Sankar MJ, Meena RS, Meena RS. Fluid bolus over 15–20 versus 5–10 minutes each in the first hour of resuscitation in children with septic shock: a randomized controlled trial. Pediatr Crit Care Med. 2017;18(10):e435–e445. doi:10.1097/PCC.0000000000001269
30. Depinet H, Macias CG, Balamuth F, et al. Pediatric Septic Shock Collaborative improves emergency department sepsis care in children. Pediatrics. 2022;149(3):e2020007369. doi:10.1542/peds.2020-007369
31. Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: Pediatric Advanced Life Support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18Suppl 3):S876–908. doi:10.1161/CIRCULATIONAHA.110.971101
32. IHI - Institute for Healthcare Improvement. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement. Available from: http://www.ihi.org/resources/Pages/IHIWhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchievingBreakthroughImprovement.aspx?PostAuthRed=/resources/_layouts/download.aspx?SourceURL=/resources/Knowledge%20Center%20Assets/364ac260-175b-498d-ae21-8670db1fbc92/IHIBreakthroughSerieswhitepaper2003.pdf.
33. Stoner MJ, Goodman DG, Cohen DM, Fernandez SA, Hall MW. Rapid fluid resuscitation in pediatrics: testing the American College of Critical Care Medicine guideline. Ann Emerg Med. 2007;50(5):601–607. doi:10.1016/j.annemergmed.2007.06.482
34. Toshniwal G, Ahmed Z, Sengstock D. Simulated fluid resuscitation for toddlers and young children: effect of syringe size and hand fatigue. Paediatr Anaesth. 2015;25(3):288–293. doi:10.1111/pan.12573
35. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi:10.1016/j.ijsu.2014.07.013
36. Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi:10.1097/01.PCC.0000149131.72248.E6
37. Tan B, Wong JJM, Sultana R, et al. Global case-fatality rates in pediatric severe sepsis and septic shock: a systematic review and meta-analysis. JAMA Pediatr. 2019;173(4):352–362. doi:10.1001/jamapediatrics.2018.4839
38. Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701. doi:10.1164/rccm.200207-682OC
39. Raghunathan T, Berglund PA, Solenberger P. Multiple Imputation in Practice: With Examples Using IVEware. CRC Press, Taylor & Francis Group; 2018.
40. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. doi:10.1056/NEJMoa1703058
41. Andrews B, Semler MW, Muchemwa L, et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA. 2017;318(13):1233–1240. doi:10.1001/jama.2017.10913
42. Santhanam I, Sangareddi S, Venkataraman S, Kissoon N, Thiruvengadamudayan V, Kasthuri RK. A prospective randomized controlled study of two fluid regimens in the initial management of septic shock in the emergency department. Pediatr Emerg Care. 2008;24(10):647–655. doi:10.1097/PEC.0b013e31818844cf
43. Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13(3):253–258. doi:10.1097/PCC.0b013e31822882a3
44. Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–197. doi:10.1016/S0140-6736(03)13908-6
45. Straney L, Clements A, Parslow RC, et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med. 2013;14(7):673–681. doi:10.1097/PCC.0b013e31829760cf
46. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi:10.1097/00003246-199605000-00004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
