Back to Journals » OncoTargets and Therapy » Volume 9
Intravenous chemotherapy combined with intravesical chemotherapy to treat T1G3 bladder urothelial carcinoma after transurethral resection of bladder tumor: results of a retrospective study
Authors Zhang Y, Xie L, Chen T, Xie W, Wu Z, Xu H, Xing C, Sha N, Shen Z, Qie Y, Liu X, Hu H, Wu C
Received 4 November 2015
Accepted for publication 14 December 2015
Published 28 January 2016 Volume 2016:9 Pages 605—611
DOI https://doi.org/10.2147/OTT.S99866
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Yu Zhang,1,* Linguo Xie,1,* Tao Chen,1,* Wanqin Xie,2 Zhouliang Wu,1 Hao Xu,1 Chen Xing,1 Nan Sha,1 Zhonghua Shen,1 Yunkai Qie,1 Xiaoteng Liu,1 Hailong Hu,1 Changli Wu1
1Department of Urology, The Second Hospital of Tianjin Medical University, Tianjin Institute of Urology, Tianjin, 2Key Laboratory of Genetics and Birth Health of Hunan Province, The Family Planning Research Institute of Hunan Province, Changsha, People’s Republic of China
*These authors contributed equally to this work
Objective: The management of stage 1 and grade 3 (T1G3) bladder cancer continues to be controversial. Although the transurethral resection of bladder tumor (TURBT) followed by intravesical chemotherapy is a conservative strategy for treatment of T1G3 bladder cancer, a relatively high risk of tumor recurrence and progression remains regarding the therapy. This study aimed to compare the efficacy of intravenous chemotherapy combined with intravesical chemotherapy versus intravesical chemotherapy alone for T1G3 bladder cancer after TURBT surgery.
Methods: We retrospectively reviewed the cases of 457 patients who were newly diagnosed with T1G3 bladder urothelial carcinoma between January 2009 and March 2014. After TURBT, 281 patients received intravesical chemotherapy alone, whereas 176 patients underwent intravesical chemotherapy in combination with intravenous chemotherapy. Tumor recurrence and progression were monitored periodically by urine cytology and cystoscopy in follow-up. Recurrence-free survival and progression-free survival of the two chemotherapy strategies following TURBT were analyzed. Univariable and multivariable Cox hazards analyses were performed to predict the prognostic factors for tumor recurrence and progression.
Results: The tumor recurrence rate was 36.7% for patients who received intravesical chemotherapy alone after TURBT, compared with 19.9% for patients who received intravenous chemotherapy combined with intravesical chemotherapy after TURBT (P<0.001). The progression rate was 10.6% for patients who underwent intravesical chemotherapy alone and 2.3% for patients who underwent the combined chemotherapies (P=0.003). Kaplan–Meier curves showed significant differences in recurrence-free survival and progression-free survival between the two treatment strategies, with a log-rank P-value of <0.001 and 0.003, respectively. Multivariable analyses revealed that intravenous chemotherapy was the independent prognostic factor for tumor recurrence and progression in the cohort.
Conclusion: Intravenous chemotherapy combined with intravesical chemotherapy offers a better oncologic outcome than the intravesical chemotherapy alone for patients with T1G3 bladder urothelial carcinoma after TURBT, and it may be considered as a new therapy strategy for T1G3 bladder cancer.
Keywords: bladder, intravenous chemotherapy, recurrence, progression
Introduction
Stage 1 and grade 3 (T1G3) bladder cancer is considered to be a category of nonmuscle invasive bladder cancer that is associated with a high risk of recurrence and progression after the treatment of transurethral resection alone, with a recurrence rate of 50%–70% and a tumor progression rate of 25%–50%.1,2
Although surgical urologists and oncologists are dedicating to improve and innovate the treatments for T1G3 bladder cancer, they have not reached an agreement on the optimal management of patients with the neoplasm. Currently, both aggressive therapy and conservative therapy are being adopted in clinic. Several studies have recommended the immediate radical cystectomy for patients with T1G3 bladder cancer and the radical cystectomy has proven an improvement in outcome of long-term tumor specific survival.3,4 However, the radical cystectomy is thought to be an excessive treatment for most patients and probably affects the patient’s quality of life.5 In light of this, conservative therapy sometimes becomes an option. It has been demonstrated that adjuvant intravesical therapy with bacillus Calmette–Guérin (BCG) may decrease the overall recurrence rate by ~30% compared with the transurethral resection of bladder tumor (TURBT) alone.6 However, 15%–40% of the patients who received BCG therapy will have progression of the disease within the first 5 years.7 Furthermore, BCG has not been approved for clinical use to bladder cancer by the Food and Drug Administration in the People’s Republic of China, over the past few years.
Recently, both the intra-arterial chemotherapy in combination with intravesical chemotherapy after transurethral resection and the radiation therapy with or without chemotherapy after organ preservation have shown efficacy in patients with T1G3 bladder cancer.8,9 Additionally, intravenous chemotherapy has been applied to the patients with advanced urothelial carcinoma.10 On the basis that intravesical chemotherapy has become a routine procedure after TURBT for the conservative treatment and the high risk of tumor recurrence and progression with T1G3 bladder cancer patients in our institute, and that intravenous chemotherapy may help to prevent high-grade tumor from recurrence and progression, we hypothesized that intravenous chemotherapy combined with intravesical chemotherapy following TURBT may offer plausible oncologic outcomes for T1G3 bladder cancer.
In this retrospective study, the tumor recurrence and progression outcomes of patients with T1G3 bladder cancer who underwent intravenous chemotherapy combined with intravesical chemotherapy after TURBT were compared with those of patients who received intravesical chemotherapy alone after TURBT. To the best of our knowledge, this is the first report to assess the impact of intravenous chemotherapy combined with intravesical chemotherapy in the outcomes of tumor recurrence and progression for patients with T1G3 bladder cancer.
Materials and methods
Patient characteristics
We retrospectively reviewed the medical records of 457 patients who were diagnosed with stage T1 (invading the lamina propria) grade 3 (poorly differentiated) urothelial carcinoma of bladder between January 2009 and March 2014 at the Second Hospital of Tianjin Medical University, Tianjin, People’s Republic of China. Pathological staging and grading of each tumor were determined according to the 2009 tumor, node, metastasis staging system and the International Society of Urological Pathology 1998/World Health Organization 2004 classification, respectively. Tumor stage and grade were re-evaluated by a single group of pathologists in the Department of Pathology in our institute. The inclusion criteria were: 1) TURBT was performed as initial treatment; 2) tumor was pathologically diagnosed as T1G3 and with no carcinoma in situ; 3) no residual tumor was detected in tumor base and edge; and 4) patient received adjuvant intravesical chemotherapy after TURBT. The exclusion criteria were: 1) patient had received any type of prior chemotherapy; 2) the Karnofsky score for Karnofsky Performance Scale Index for patient who underwent intravenous chemotherapy was less than 90%; 3) patient failed to receive further treatment because of severe complications; and 4) patient underwent intravenous chemotherapy for less than two cycles. The present study was approved by the Institutional Review Board of Tianjin Medical University and written informed consent was obtained from each patient. All data were anonymized and de-identified before being used in this study.
Treatment protocol
In this retrospective study, of the 457 patients who underwent complete resection of all visible tumors by TURBT and pathologically confirmed with no urothelial carcinoma in tumor base and edge after second transurethral resection, 281 patients received intravesical chemotherapy alone, whereas 176 patients underwent intravenous chemotherapy combined with intravesical chemotherapy. In addition to this, all patients received the first instillation of anticancer agents within 24 hours after the initial resection.
In terms of the adjuvant intravesical chemotherapy after TURBT, the chemotherapy drugs were the anthracycline antibiotics, including epirubicin and pirarubicin. The intravesical chemotherapy using epirubicin and pirarubicin had been reported in previous studies of Chinese populations.11,12 Intravesical chemotherapy began ~1–2 weeks after TURBT and proceeded once weekly for 8 weeks, then monthly for 12 months. Patients for the combined chemotherapies were treated with additional platinum-based intravenous chemotherapy after TURBT, including gemcitabine (1,200 mg/m2 on days 1 and 8) plus oxaliplatin (100 mg/m2 on day 2) for at least two cycles. The duration of a cycle was 21 days.
Follow-up and outcomes
Subsequent cystoscopy and urine cytology were performed at 3-month intervals for a period of 2 years. Then, cystoscopy was performed semiannually until the fifth year and annually thereafter. Bladder biopsy was performed when necessary. During the follow-up period, the end point for a patient in the study was the time when bladder cancer recurrence and tumor progression (recurrence with stage T2 or higher) had been found and histologically confirmed. Before and after intravenous chemotherapy, routine blood test, blood biochemical indexes including liver and kidney function test, and electrocardiogram were performed to assess the safety of the treatment.
Statistical analysis
The independent-sample Student’s t-test and chi-square test were used for continuous variables and categorical variables, respectively. Univariate and multivariate analyses using the Cox proportional hazards model were performed to identify independent variables that help to predict tumor recurrence and progression. Recurrence-free survival (RFS) and progression-free survival curves were plotted by the Kaplan–Meier method and differences of the curves were examined by log-rank test. All statistical analysis was performed using SPSS 19.0 statistical software (SPSS, IBM Corporation, Armonk, NY, USA) and a P-value <0.05 (two-sided) indicates a statistically significant difference.
Toxicity
Treatment toxic effects due to intravenous chemotherapy were evaluated for each patient. All adverse reactions were graded according to the Common Terminology Criteria for Adverse Events 3.0.
Results
Demographic and clinic characteristics of patients
As shown in Table 1, no significant differences in the demographic variables including age, sex, and smoking history were observed between patients who received intravesical chemotherapy alone and those who received intravenous chemotherapy combined with intravesical chemotherapy (all P>0.05). In addition, there were no significant imbalances in tumor size, number of lesions, and intravesical chemotherapy agents between the two groups (all P>0.05).
  | Table 1 Demographic and clinical characteristics of patient groups |
Recurrence of tumor
The mean length of follow-up for patients in this retrospective study was 33 months (range, 2–76 months). Tumor recurrence was reported in 103 of the 281 patients who received intravesical chemotherapy alone and in 35 of the 176 patients who received intravenous chemotherapy combined with intravesical chemotherapy. The recurrence rate differed significantly between the two groups (36.7% vs 19.9%, P<0.001) (Table 2). The recurrence-free curves for the two treatment strategies are shown in Figure 1. The RFS rate of patients who received the combined chemotherapies was significantly higher than that of patients who received intravesical chemotherapy alone after TURBT (log-rank test result: P<0.001).
Further, univariable and multivariable analyses using the Cox hazards regression model revealed that tumor size was significantly associated with tumor recurrence (hazard ratio [HR], 1.60, 95% confidence interval [CI], 1.13–2.27; and HR, 1.66, 95% CI, 1.17–2.36, respectively) (Table 3). Moreover, the results also show that intravenous chemotherapy was the independent prognostic factor for tumor recurrence in this cohort by multivariate Cox hazards regression model (HR, 2.07; 95% CI, 1.41–3.04).
  | Table 3 Univariable and multivariable analyses according to recurrence |
Progression of tumor
Progression was reported with 27 of the 281 patients who were treated by intravesical chemotherapy alone after TURBT and four of the 176 patients who were treated by intravenous chemotherapy combined with intravesical chemotherapy after TURBT. The patients in the latter group exhibited a disease progression rate of 2.3%, which was considerably lower than a rate of 10.6% in the former group (P=0.003) (Table 2). Accordingly, the progression-free survival rate of patients who underwent the combined chemotherapies was significantly higher than that of patients who received intravesical chemotherapy alone (log-rank test result: P=0.003) (Figure 2).
Similar to analysis for recurrence, the univariable analyses were performed by the Cox hazards regression model to predict prognostic factors for progression. Our analyses revealed that intravenous chemotherapy was the only prognostic factor for tumor progression (HR, 4.36; 95% CI, 1.53–12.47) (Table 4).
  | Table 4 Univariable analyses according to progression |
Toxicity
In these 176 patients, a total of 763 cycles of chemotherapy were given with a median of four cycles (range, two to six cycles) per patient. All patients under the combinatorial chemotherapies were assessable for toxicity. The related side effects are summarized in Table 5. The most common toxicity during the treatment was Grade 1/2 toxicity, including nausea/vomiting (30.2%), constipation/diarrhea (22.7%), hypoleukemia (13.0%), neutropenia (23.9%), increased alanine aminotransferase (7.95%), and increased creatinine (3.98%). Grade 3 toxicity was reported in 12 of 176 patients, including nausea/vomiting (1.14%), constipation/diarrhea (1.70%), and neutropenia (3.98%), which was easily managed by treatment of the symptoms. During the treatment, Grade 4/5 toxicity was not reported in patients.
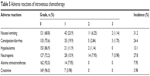  | Table 5 Adverse reactions of intravenous chemotherapy |
Discussion
The treatment of T1G3 bladder cancer continues to be debated by urologists, as it has a high risk of recurrence and progression. Currently, T1G3 bladder cancer is treated with early radical cystectomy or TURBT followed by adjuvant intravesical therapy with BCG,13,14 or followed by the intra-arterial chemotherapy combined with intravesical chemotherapy8 or radiation therapy with or without chemotherapy.9 Although intravesical therapy with BCG following TURBT is currently viewed as a gold standard for conservative therapy for T1G3 tumors,13,14 the BCG has not been approved for treatment of bladder cancer in the People’s Republic of China over the past few years. As quite a few patients with T1G3 bladder cancer do not accept radical cystectomy as the initial treatment, conservative therapies of high efficacy and safety are greatly anticipated. Here, our retrospective study reveals that intravenous chemotherapy combined with intravesical chemotherapy following TURBT could be a novel therapeutic strategy for T1G3 bladder cancer.
Our analysis shows that compared to intravesical chemotherapy alone after TURBT that had a recurrence rate of 36.7%, intravenous chemotherapy in combination with intravesical chemotherapy effectively decreased the tumor recurrence in patients with T1G3 bladder cancer, showing a lower rate of 19.9% (P<0.001). We find that the patients who received intravesical chemotherapy alone after TURBT in this study have a recurrence rate close to that in other studies reported previously. It showed that the recurrence rates for intermediate- and high-risk T1G3 patients who received adjuvant intravesical BCG after TURBT were 35.6% and 38.6%, respectively.15,16 For the patients who accepted the combined chemotherapies in this cohort, the recurrence rate apparently is higher than that in Chen’s study (19.9% vs 10.3%), where 31 patients underwent the intra-arterial combined with adjuvant mitomycin chemotherapy after bladder-preserving surgery.8 However, considering the differences in patient number and intravesical chemotherapy drugs between the two studies, it is not appropriate to make a direct comparison regarding the efficacy of the two treatment strategies.
Further, the prediction of prognostic factors for recurrence via univariable and multivariable analyses using the Cox hazards regression model demonstrated that a tumor size ≥3 cm was significantly associated with recurrence for tumor. This finding is consistent with that in the study by Gontero et al.17 Moreover, our study shows that intravenous chemotherapy is the most powerful prognostic factor for RFS for patients receiving intravesical chemotherapy with or without intravenous chemotherapy after TURBT. In other words, the patients who undergo the combined chemotherapies will have more favorable outcomes on RFS than the patients who only receive intravesical chemotherapy after TURBT.
It has been reported that patients with high-risk NMIBC have poor prognosis due to the tumor progression and ~5% of pT1 bladder tumors have regional nodal metastasis at presentation.18 With the progression to muscle-invasive disease, a relevant proportion of patients appear not to be cured even in cases of early cystectomy.19 Although conservative treatments for T1G3 bladder cancer have been shown to effectively prevent the progression in literatures,8,20 the relatively short duration of follow-up and the small size of patient cohort in the studies should be taken into account when interpreting the data. In one study, radiation therapy/chemotherapy after TURBT showed efficacy in 84 patients with T1G3 bladder cancer, with the progression rate of 16%.9 In our series of 457 T1G3 bladder cancer patients, tumor progression occurred in 10.6% of the patients treated with intravesical chemotherapy alone after TURBT and 2.3% of the patients treated with intravesical combined with intravenous chemotherapy after TURBT (P=0.003), suggesting that the combined chemotherapies have a considerably improved efficacy in preventing tumor from progression. We infer that the combined chemotherapies could decrease the risk of tumor progression by reaching the micrometastases and pelvic lymph nodes that usually are not treated adequately by intravesical therapy alone.
Some studies have shown that a tumor size >3 cm is a predictor for tumor recurrence and progression,17,21 whereas others cannot find the correlation.22,23 Here, we failed to show that a tumor size ≥3 cm is associated with tumor progression. However, our finding should be confirmed by studies with larger number of patients in the future.
Gemcitabine and cisplatin are the most commonly used chemotherapy agents for metastatic or locally advanced transitional cell carcinoma of the urothelium.24 Considering the advantage of oxaliplatin that it can be administered to patients with renal impairment,25 we adopted oxaliplatin for intravenous chemotherapy to treat patients with T1G3 bladder urothelial carcinoma. We notice that the active, safe chemotherapy strategy using gemcitabine and oxaliplatin for locally advanced or metastatic bladder urothelial carcinoma patients with reduced renal function has been reported in Phase II studies,26,27 and this strategy may also be considered for intravenous chemotherapy in the future. During the treatment, intravenous chemotherapy may encounter side effects caused by the chemotherapy drugs themselves. The major complications of intravenous chemotherapy are nausea/vomiting, hypoleukemia, and neutropenia, which can easily be managed by symptomatic treatment.
There are several limitations to our study. We should keep it in mind that this is a retrospective, nonrandomized case-control study where controls were matched to cases based on the pathological type of tumor but not by clinical features. Additionally, different intravesical chemotherapy agents were used for patients and we could not exclude the bias associated with the drugs themselves. In the future, a prospective and randomized case-control study should be conducted to validate our findings.
Conclusion
This study provides evidence-based data that intravenous chemotherapy combined with intravesical chemotherapy after TURBT is an effective treatment option for patients with T1G3 bladder cancer.
Acknowledgments
This work is funded by grants from the Natural Science Foundation of Tianjin (14JCYBJC26300) and National Key Specialty Construction of Clinical Projects, the Natural Science Foundation of Tianjin (15JCYBJC24600), and Tianjin Major Scientific and Technological special Project (12ZCDZSY16900). The authors wish to thank all of the patients included in this study
Disclosure
The authors report no conflicts of interest in this work.
References
Pham HT, Soloway MS. High-risk superficial bladder cancer: intravesical therapy for T1 G3 transitional cell carcinoma of the urinary bladder. Semin Urol Oncol. 1997;15(3):147–153. | ||
Jakse G, Loidl W, Seeber G, Hofstadter F. Stage T1, grade 3 transitional cell carcinoma of the bladder: an unfavorable tumor? J Urol. 1987;137(1):39–43. | ||
Denzinger S, Fritsche HM, Otto W, Blana A, Wieland WF, Burger M. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol. 2008;53(1):146–152. | ||
De Berardinis E, Busetto GM, Antonini G, Giovannone R, Gentile V. T1G3 high-risk NMIBC (non-muscle invasive bladder cancer): conservative treatment versus immediate cystectomy. Int Urol Nephrol. 2011;43(4):1047–1057. | ||
Kulkarni GS, Finelli A, Fleshner NE, Jewett MA, Lopushinsky SR, Alibhai SM. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007;4(9):e284. | ||
Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168(5):1964–1970. | ||
Smith JJ, Labasky RF, Cockett AT, Fracchia JA, Montie JE, Rowland RG. Bladder cancer clinical guidelines panel summary report on the management of nonmuscle invasive bladder cancer (stages Ta, T1 and TIS). The American Urological Association. J Urol. 1999;162(5):1697–1701. | ||
Chen J, Yao Z, Qiu S, et al. Comparing intra-arterial chemotherapy combined with intravesical chemotherapy versus intravesical chemotherapy alone: a randomised prospective pilot study for T1G3 bladder transitional cell carcinoma after bladder-preserving surgery. Cardiovasc Intervent Radiol. 2013;36(6):1521–1526. | ||
Weiss C, Wolze C, Engehausen DG, et al. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer: an alternative to intravesical therapy or early cystectomy? J Clin Oncol. 2006;24(15):2318–2324. | ||
von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–3077. | ||
Chen SY, Du LD, Zhang YH. Pilot study of intravesical instillation of two new generation anthracycline antibiotics in prevention of superficial bladder cancer recurrence. Chin Med J (Engl). 2010;123(23):3422–3426. | ||
Liu S, Hou J, Zhang H, et al. The evaluation of the risk factors for non-muscle invasive bladder cancer (NMIBC) recurrence after transurethral resection (TURBt) in Chinese population. PLoS One. 2015;10(4):e123617. | ||
Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639–653. | ||
Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178(6):2314–2330. | ||
Gohji K, Nomi M, Okamoto M, et al. Conservative therapy for stage T1b, grade 3 transitional cell carcinoma of the bladder. Urology. 1999;53(2):308–313. | ||
Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90–95. | ||
Gontero P, Sylvester R, Pisano F, et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guerin: results of a retrospective multicenter study of 2451 patients. Eur Urol. 2015;67(1):74–82. | ||
Ficarra V, Dalpiaz O, Alrabi N, Novara G, Galfano A, Artibani W. Correlation between clinical and pathological staging in a series of radical cystectomies for bladder carcinoma. BJU Int. 2005;95(6):786–790. | ||
van den Bosch S, Alfred WJ. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60(3):493–500. | ||
Inoue M, Ishioka J, Fukuda H, Kageyama Y, Saito Y, Higashi Y. Clinical outcome of chemoradiotherapy for T1G3 bladder cancer. Int J Urol. 2008;15(8):747–750. | ||
Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):465–466, 475–477. | ||
Palou J, Sylvester RJ, Faba OR, et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guerin. Eur Urol. 2012;62(1):118–125. | ||
Jancke G, Rosell J, Jahnson S. Impact of tumour size on recurrence and progression in Ta/T1 carcinoma of the urinary bladder. Scand J Urol Nephrol. 2011;45(6):388–392. | ||
Sonpavde G, Watson D, Tourtellott M, et al. Administration of cisplatin-based chemotherapy for advanced urothelial carcinoma in the community. Clin Genitourin Cancer. 2012;10(1):1–5. | ||
Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen panel. Biochem Pharmacol. 1996;52(12):1855–1865. | ||
Carles J, Esteban E, Climent M, et al. Gemcitabine and oxaliplatin combination: a multicenter phase II trial in unfit patients with locally advanced or metastatic urothelial cancer. Ann Oncol. 2007;18(8):1359–1362. | ||
Eroglu Z, Fruehauf JP. A phase II study of gemcitabine and oxaliplatin in advanced transitional cell carcinoma of the bladder. Cancer Chemother Pharmacol. 2013;72(1):263–267. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



