Back to Journals » Infection and Drug Resistance » Volume 15
Intrauterine Infection and Mother-to-Child Transmission of Hepatitis B Virus: Route and Molecular Mechanism
Received 19 January 2022
Accepted for publication 26 March 2022
Published 12 April 2022 Volume 2022:15 Pages 1743—1751
DOI https://doi.org/10.2147/IDR.S359113
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xianlei Zhao,1,2 Xiaoxia Bai,1 Yongmei Xi1,2
1Division of Human Reproduction and Developmental Genetics, the Women’s Hospital, Zhejiang University School of Medicine, Hangzhou City, Zhejiang Province, 310006, People’s Republic of China; 2Institute of Genetics and Department of Human Genetics, Zhejiang Provincial Key Laboratory of Genetic & Developmental Disorders, Zhejiang University School of Medicine, Hangzhou City, Zhejiang Province, 310058, People’s Republic of China
Correspondence: Yongmei Xi, Email [email protected]
Abstract: In high prevalence settings, mother-to-child transmission is responsible for more than 50% of chronic Hepatitis B Virus (HBV) infections with 1– 9% of newborns of HBV-carrying mothers acquiring HBV in early life. Little is known about the routes and cellular mechanisms by which HBV intrauterine transmission occurs. Clinical studies indicate that placental trophoblasts can be infected with HBV. In vitro studies using primary trophoblast and cell lines support this hypothesis. Several cellular parameters, including the differentiation state of the trophoblasts, cytokine secretion, and the surface molecules involved in virus entry, may influence the receptivity of trophoblastic cells to HBV. In HBV-infected trophoblastic cells, a reduction of apoptosis and increased production of antiviral cytokines has been observed, presumably via an HBx antigen-Akt or TLRs-MyD88-NF-kB pathway. Trophoblast HBV infection occurrence involves complex pathological processes with little currently known of the related mechanisms within infected cells. Whilst much focus has been on the placental routes of infection, through trophoblasts in particular, other routes have also been suggested. In this article, we review the models for HBV mother-to-child transmission and discuss the possible mechanisms of HBV intrauterine transmission with particular emphasis upon the involvement of placental trophoblast infection.
Keywords: hepatitis B virus, intrauterine infection, trophoblastic cell, mother-to-child transmission
Introduction
Chronic hepatitis B virus infection remains a serious global health issue affecting approximately 240 million people worldwide.1 In high prevalence/endemic areas, HBV transmission is usually either horizontally, through shared needles or contaminated blood transfusion; or vertically, where mother-to-child transmission accounts for more than 50% of chronic HBV infections.2,3 More than 80% of those infected perinatally or during early childhood will become chronic viral carriers, leading to significantly increased risk of diseases associated with liver injury in later adulthood.4 Mother-to-child transmission of HBV can occur prenatally during pregnancy, natally during labor, or postnatally through close contact such as in breastfeeding. Most vertical transmissions occurring perinatally are to newborns without prior HBV vaccination. However, numerous reports have shown that, despite timely immunoprophylaxis with vaccines and HB immunoglobulin (HBIG), 1–9% of newborns of mothers with HBeAg and/or HBV DNA seropositivity still go on to acquire HBV in early childhood.5,6
Prevention of transmission during pregnancy presents a difficult challenge as little is known about the routes or intracellular mechanisms utilized by HBV in its transport from mother to fetus. Intrauterine infection can also occur at different times and stages during gestation ranging from gamete infection,7–9 to occurrence associated with the transport of contaminated maternal blood and peripheral blood mononuclear cells (PBMCs) occurring as early as second trimester10,11 or via the placenta. In these, accumulating evidence12–15 seems to implicate the primacy of HBV transplacental infection and propose diverse models of intrauterine infection. The overall objective of this study is to summarize the current evidence supporting the primacy of HBV transplacental transmission with an emphasis on trophoblast infection given its strategic value in the placental barrier.
Epidemiology
Though a 20-year effort in childhood vaccination in China has resulted in a 97% reduction of HBV infections, China still retains the largest pool of HBV infection in Asia with a prevalence of more than 80 million estimated chronic infections in 2018.1 The application of HBV vaccines in combination with HBIG has greatly reduced the incidence of perinatal HBV transmission. The prevalence of HBsAg in the Chinese 1–29 age group has dropped dramatically from 10.13% in 1997 to 2.64% in 2014.16 Nevertheless, these strategies do not completely block the mother-to-child transmission of HBV17–19 and immunoprophylaxis failure occurs in 1–9% of newborns,5,6 in which case infants born to HBV-carriers still suffer HBV infection. Conversely, the likelihood for adults to become chronic carriers upon exposure is much lower, being from 5% to 10%.3,20 In other words, infants and children are more prone to chronic HBV infections than adults. This sharp contrast highlights the strong requirement to place our primary focus for this disease on the prevention of mother to child transmission during pregnancy and delivery.
Criteria for Mother-to-Child Transmission of HBV
The transmission from the infected mother to the offspring is traditionally termed as mother-to-child transmission (MTCT), including transmission before birth, during birth and in early childhood. In this article, we mainly focus on transmission during pregnancy, which is referred to as intrauterine transmission. The definition of HBV intrauterine transmission is still controversial with various sources offering differing diagnostic criteria including: 1) Serum HBsAg and/or HBeAg positivity or HBV DNA positivity after birth;11,21,22 2) Serum HBsAg and/or HBeAg positivity or HBV DNA positivity after birth, lasting >2 months;18,23 3) Persistent serum anti-HBc IgM-positive after birth.24 The lack of consistency in such criteria inevitably leads to varying rates of mother-to-child transmission, depending on which definition is used. The surprisingly high rate of MTCT in some studies can be attributed to the fact that the authors had only tested from neonate HBV seromarkers at birth, with no follow-up visit. In such cases, HBsAg and HBV DNA seropositivity in the infant directly after birth could be a result of transient antigenemia and could disappear with 8–12 months. Such serological indexes could also be removed via host immunity and/or vaccination.25,26 Such positive markers that are no longer detectable during follow-up visits are often considered as the positive result having been transferred from the infected mother, rather than showing an established HBV infection in the infant.25,26 However, a small proportion of new-borns still exhibited seropositivity during follow-ups, unprevented by HBV immunoprophylaxis, representing true cases of transmission during pregnancy.
Models of HBV Vertical Intrauterine Transmission
The vertical transmissions of HBV occur mainly during delivery or by intrauterine transmission. Several models of intrauterine transmission of HBV have been proposed. These include infection via paracellular routes (transplacental leakage and PBMCs), germline infection, and placental infection.
Transplacental Leakage
Transplacental leakage has been viewed as the most common route for intrauterine transmission when preterm labor is threatened or where abortion causes uterine muscle contraction, consequently leading to rupture of the placental barrier and allowing direct exchange between maternal blood and fetal blood.5 Transplacental leakage can also occur during early pregnancy due to placental immaturity, or during invasive procedures such as chorionic sampling and amniocentesis. In such cases, the mix of fetal peripheral blood with maternal blood, as a consequence of partial breakdown in placental barrier, introduces HBV-containing blood into fetal circulation causing in utero infant HBV infection. A number of studies have been published regarding this route of transmission. However, conflicting results have been presented where Ohto et al27 and Lin et al28 suggested that HBV intrauterine transmission is associated with a history of preterm labor or preterm abortion, whereas Tang et al29 indicated otherwise. This discrepancy may be due to the small sample sizes represented by these three papers. A fuller case-control study of 402 newborns recruited from 402 HBsAg positive mothers showed that maternal HBeAg seropositivity (OR = 14.46 and 17.07 from univariate and multivariate analysis, respectively) and threatened preterm labor (OR = 6.66 and 5.44) were significantly associated with HBV intrauterine transmission and identified as major risk factors.30 This was further verified by a recent study conducted among 96 HBs-Ag positive women and their infants.31 Notably, many studies, including those covering cases of c-section, showed that even where there was no evidence of transplacental leakage, HBeAg was still able to cross the placenta and induce infant T-cell tolerance for HBV in the infant.12,32,33
PBMCs
HBV may not be strictly hepatotropic. Studies have confirmed its presence in PBMCs.34,35 As an extrahepatic reservoir for HBV, PBMCs have been closely associated with HBV chronic infection or HBV reoccurrence with various forms of HBV DNA detected within PBMCs.34,36 The significant association between HBV intrauterine transmission and HBV DNA in PBMCs suggests that HBV-infected PBMCs may serve as a vector for HBV transfer from mother to fetus.10,11,21,22 Xu et al conducted a population-based nested case-control study with 312 HBsAg seropositive mothers and their 312 newborns. Among these, 26% of neonates were detected with HBV DNA in PBMCs and a 67% rate of infant infection was noted from mothers with HBV DNA in PBMCs.21 Infants born to HBV DNA-seronegative but HBV DNA-positive PBMCs mothers were at a 5 times higher risk of HBV infection than those born to mothers with HBV DNA negativity in PBMCs.21 This indicates that HBV-infected PBMCs contribute to intrauterine transmission. However, any further conclusions relating to the significance of PBMC infection remain limited by the lack of follow-up studies for such HBV-infected infants.
Germline Infection
The germline infection model depicts HBV infection at the time of conception via either oocyte or sperm where HBV has been shown to invade and replicate within ova.7,37 The presence and expression of HBV within the oocyte is closely associated with the HBV DNA level and infection status of the mother.7 Additional information is required for this phenomenon due to conflicting results as to whether infected oocytes can transmit the virus vertically to the offspring. In addition to blood and liver, HBV can also be detected in semen. Evidences that HBV can integrate its DNA into the male spermatozoa genome have been presented.8,38 It remains controversial as to whether HBV DNA sequences can be passed onto the embryos through infected spermatozoa. In spite of various studies using animal models supporting this theory, it is unclear whether this occurs in humans, with conflicting results from a number of studies.8,9,37
Placental Infection
The placenta is a highly specialized organ that not only generates a nourishing environment for fetal development, but also acts as a barrier against the transmission of blood-borne pathogens.39,40 The placental barrier is composed of four elements. From the fetal to the maternal side these are the fetal endothelial cells, the connective tissues that surround the fetal capillaries, the cytotrophoblasts and the syncytiotrophoblasts.41 Pathogens within maternal blood need to cross those layers of cells in order to reach fetal circulation and consequently infect the fetus.
Various studies have confirmed the presence of HBV, including HBsAg and HBV DNA, in each of these layers of placental tissue. A study by Bai et al examined such placental tissues for HBV infection by RT-PCR and immunohistochemistry. The seromarker HBsAg was present in 5 cases out of 6 infants with positive cord blood, and HBV DNA was noted in the placental tissues, although at a relatively low level of 5.0×102–3.0 × 103 copy/mL.14 In 20 women whose serum tested positive for HBV DNA, 6 cases showed positive immunohistochemical staining of HBsAg in their placental tissues, 5 of which exhibited positive staining of villous trophoblasts and mesenchymal cells, and with one case of positive staining of villous capillary endothelial cells.14 Xu et al13 found that the rate of HBV infection in different cell layers of placental tissues gradually decreased from the maternal side to fetal side, with the highest positivity rate of HBsAg, HBcAg, and HBV DNA in decidual cells, followed by trophoblasts, villous mesenchymal cells and villous capillary endothelial cells. These results were consistent with those from a research study by Chen et al,12 which confirmed that the rate of HBV infection differed among the cell layers of placental tissues. A recent study also unveiled the positive correlation between HBV intrauterine transmission and placental cell infection, with OR 4.6 (95% CI 2.29–9.4; p = 0.002).42 These articles concluded that the closer to the fetus the HBV infection occurred, the higher the risk of HBV intrauterine transmission.
An added complication is that the composition of the placental barrier changes as pregnancy progresses. In early pregnancy, cytotrophoblasts predominate within the placental villi, whereas late in gestation mononucleated cytotrophoblasts differentiate and fuse into a continuous layer of syncytiotrophoblasts, forming the primary barrier between the maternal and fetal circulation. One in vitro study has suggested that the formation of this continuous barrier is associated with the reduction of the potential to transmit a virus. Evidence for this was presented in that the monolayer of differentiated BeWo cells did not transcytose HBV as effectively as forskolin-untreated cells where the former exhibited a reduction rate of 24%.15 However, a contrasting clinical study by Chang et al43 challenged this, where in 25 examined cases of first-trimester villi tissues from HBsAg-seropositive women, 8 cases were detected with positive HBV signals. This inconsistency may result from the small sample size and the somewhat descriptive nature of these two clinical studies.
Despite the conflicting results pertaining to the role of placenta differentiation status on its susceptibility to HBV, different gestation stages at the time of infection with different infection outcomes have been reported for other viruses, such as HIV, B19, and CMV. For example, it has been proposed that cytotrophoblasts isolated from first-trimester villi are susceptible to HIV-1, while syncytiotrophoblasts from third-trimester villi develop potent infections.44,45 Maternal exposure to B19 at the second-trimester stage has also been associated with a higher risk of vertical transmission.46,47 Furthermore, early and mid-gestation trophoblasts are noted as prone to CMV infection, whereas late term trophoblasts cannot be infected with CMV.48–50 In addition to in vivo evidence for the presence of HBV within placental trophoblasts, there are also numerous in vitro studies investigating the infection mechanism of trophoblasts with HBV, which will be discussed in the next section.
Molecular Mechanism of Trophoblast Infection with HBV
Transcytosis of HBV in Trophoblastic Cell Lines
An in vitro system using the human trophoblast-derived BeWo cell line, a choriocarcinoma cell line grown as polarized monolayers on semipermeable membranes, showed that BeWo was able to transcytose infectious HBV particles in an endosome-dependent manner.15 Our recent study demonstrated the transcytosis of HBV across trophoblasts using trophoblastic cell line Swan71 and primary trophoblasts.51 BeWo cells could actively transcytose HBIG and the reduced virus transcytosis rate was thought to be due to the formation of HBV-IgG complexes which were internalized via the Fc γ receptor.15,52 Additionally, HBV transcytosis efficiency was reduced with the fusion of cytotrophoblasts into syncytiotrophoblasts and with the addition of HBIG. As previously mentioned, HBV transcytosis was noted as inhibited by mononucleated trophoblast differentiation into multinucleated syncytiotrophoblasts,15 and infection efficiency and outcomes are seen to vary with gestation stages.43 This may be attributed to the different protein expression profiles of the different stages of the trophoblasts. In human liver cells and other hepatic cell lines, HBV’s specific binding to the cell surface is known to be mediated by the hepatotropic receptor NTCP, a process in which many other surface molecules also contribute as co-receptors, such as FTL, SCCA-1, and HSPGs.53 However, HBV infections are not restricted to hepatocytes.38 As far as receptors on trophoblasts are concerned, the Fc γ receptor family has been studied extensively and its distribution across the placenta is well understood.
Fc γ Receptor-Mediated HBsAg-Anti-HBs Uptake into Trophoblasts
Consistent with previous findings,54 the Fc γ receptor III was found to be expressed on placental trophoblasts and villous mesenchymal cells, whereas the Fc γ receptor II was found solely on villous mesenchymal cells.55 In the placenta, trophoblasts are particularly known to transfer IgG from maternal circulation to the fetus, through Fc γ receptor-mediated entry of immune complexes of infectious origins. The presence of maternal HBIG has been correlated with a lower level of HBV intrauterine transmission. This is supported by a meta-analysis of research data derived from 37 individual studies.23 In addition, postnatal HBIG injection of the infants was effective and prevented HBV horizontal transmission after birth. Bhat & Anderson15 discovered that beyond the addition of anti-HBV IgG at 100mg/L, HBV transcytosis decreased by a modest but significant degree. It was supposed that this was due to the formation of complexes between HBV anti-HBV IgG.
However, Xu et al13 in their immunohistochemistry study examined 2 placental tissues from 2 HBV-seropositive women for detection of HBsAg and anti-HBs. They observed the presence of HBsAg-anti-HBs complex in the cytoplasm and on the surface of trophoblastic cells and in the mesenchymal cells of both areas. Thus, the presence of maternal IgG in the placenta against HBV may be double-edged in that IgG coupled with HBV may be internalized into the trophoblasts through Fc γ receptor-mediated entry.56 Further studies are required to elucidate whether transcytosed HBV occurs in a free form or to investigate the infectivity of transcytosed HBV particles.
Toll-Like Receptors-Mediated HBV Infection in Trophoblasts
Toll-like receptors are a family of molecules that recognize pathogen-associated molecular patterns (PAMP) and activate downstream antiviral pathways. In addition to surface molecules that facilitate HBV transmission across trophoblasts, the expression of Toll-like receptors (TLR) in trophoblasts has been reported to be involved in preventing intrauterine transmission57 where the placental expression level of TLR7 is significantly higher in cases of intrauterine transmission. In Swan71, an immortalized trophoblastic cell line, exposure to HBV greatly induced the expression of TLR7, TLR8, MyD88, and some intracellular antiviral cytokines, such as interferon-α (IFN-α), IFN-β, and interleukin-8 (IL-8).57 As a universal adaptor for TLRs in hepatic cells, knockdown of MyD88 in trophoblastic cells significantly increased the amount of transcytosed HBV.57 It was hypothesized that TLRs in trophoblasts are likely involved in the prevention of HBV intrauterine transmission via the recruitment of the effector protein MyD88, which then triggers the activation of NF-kappa B and subsequently generates an inflammatory response against HBV.
Cytokines May Promote HBV Infection in Trophoblasts
The placenta is a complex environment where a variety of soluble cytokines play key roles in gestation and are necessary for successful pregnancy. These include IL-1,3,4,6, transforming growth factor-β (TGF-β), IFN-γ, and tumor necrosis factor-α (TNF-α). Some cytokines in the placental microenvironment may also play a regulatory role in protecting the fetus or conversely, in driving viral expression.58 In support of this theory, two studies have provided evidence of enhanced HBV uptake into choriocarcinoma cell lines such as JAR, JEGIII and primary trophoblasts in the presence of TNF-α.58,59 The susceptibility to HBV of JAR cells was significantly enhanced in the presence of the cytokine TNF-α.58 Similar results showed that in vitro infection with HBV induces the accumulation of HBV DNA and HBsAg and leads to an increase in the secretion of HBsAg within JEGIII, all of which were significantly enhanced by the addition of TNF-α.59
Suppressed Apoptosis in HBV-Infected Trophoblasts via Smad and PI3K/pAKT Signaling
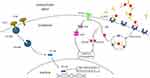
HBV was first reported to be involved in the reduction of apoptosis in trophoblasts via the stimulation of PI3K/pAKT signaling.60 Using the choriocarcinoma cell line JEGIII as a model for in vitro infection, they successfully detected intracellular HBV DNA with expressions of HBx mRNA and HBxAg exhibiting decreases in their proportions in cells at both early and late apoptosis, and with corresponding increases in the levels of PI3K and PAKT.60 Overexpression of the HBx protein in the trophoblastic cell line HTR-8/SVneo inhibited apoptosis and increased the invasive ability of HBx-transfected cells. An increased mRNA level of inflammatory factors IL-6 and IL-10 was also detected with Western blot indicating higher levels of pSmad2 and pSmad3 in the transfected cells.61 This increased inflammatory response and invasion activity may have resulted from the activation of the Smad signaling pathway. Therefore, the inhibition of apoptosis induced by HBx may prolong the survival of HBV-infected trophoblasts and thus provide an opportunity for a latent infection and the subsequent infection of surrounding cells (Figure 1). This coincides with the results of Bai et al,62 showing a higher clinical expression of HBxAg and PI3K, and a decrease in the apoptotic index in placental tissues from women of the high replication group (serum HBV DNA >1x103 copies/mL), in comparison with those of the low replication group (serum HBV DNA <1x103 copies/mL). In a more recent study,63 a constructed plasmid to express HBx was transfected into JEGIII cells where similar results were achieved. This supported the understanding that HBV induces a reduction in apoptosis via HBxAg.
HBx Reduces Apoptosis in Trophoblasts via EGFR/Akt Signaling Pathways
The intracellular EGFR/Akt signaling pathway has been recently identified to be involved in trophoblastic infection with HBV.63 A lower degree of apoptosis was observed in HBx-expressing JEGIII cells, while a higher level of pEGFR was detected both in placental tissues from HBV carriers and in HBx-transfected JEGIII cells.63 Both the upregulation of pAkt and reduced apoptosis could be offset by the EGFR inhibitor gefitinib. In addition, the knockdown of HBx suppressed the activation of the EGFR/Akt pathway and increased cellular apoptosis, whereas EGF stimulation counteracted this effect. This supports the hypothesis that HBx reduces apoptosis in trophoblasts, possibly through the activation of the EGFR/Akt signaling pathway. This suggested that HBx inhibits trophoblast cell apoptosis by activating the EGFR/Akt signaling pathway. In addition, a recent study demonstrates that HBx promotes HBV replication in trophoblasts via downregulation of Smc5/6, activates the EGFR promoter and inhibits trophoblast apoptosis via the PI3K/p-AKT downstream signaling pathway.64 Therefore, the anti-apoptotic pathways induced by HBx might contribute to the intrauterine transmission of HBV (Figure 1).
Conclusion
Mother-to-child transmission (MTCT) is the major cause of chronic infection of hepatitis B virus (HBV) in patients. Clarification of the routes and cellular mechanisms by which HBV intrauterine transmission occurs is of high importance. Clinical studies have verified that placental cells, trophoblasts in particular, can be infected with HBV. A number of in vitro studies using isolated primary trophoblasts and trophoblastic cell lines have suggested that trophoblast infection with HBV depends on trophoblastic surface receptors such as Fc γ and TLRs and is correlated with reduced apoptosis via various pathways. We have recently proposed as an unconventional protein secretion pathway, autophagy may be hijacked by HBV to complete the process of intracellular transport and exocytosis. In the HBV infected trophoblasts, AnxA2–S100A10 complex-mediated exocytosis could result in HBV intrauterine transmission. However, exactly how HBV enters trophoblasts, the intracellular transport mechanism of the virus, and how HBV egresses out of trophoblasts still awaits clarification. In this way more effort is required to clarify the intracellular events responsible for trophoblast infection with HBV.
Acknowledgments
This work was funded by the Fundamental Research Funds for the Central Universities (17221012101) and National Natural Science Foundation of China (81671478). We thank Chris Wood for English language support.
Author Contributions
X.Z and Y.X. took part in drafting, revising or critically reviewing the article. Y.X. and X.B gave final approval of the version to be published. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest.
References
1. Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 2019;4(2):135–184. doi:10.1016/S2468-1253(18)30270-X
2. Sokal EM, Paganelli M, Wirth S, et al. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European society of pediatric gastroenterology, hepatology and nutrition. J Hepatol. 2013;59(4):814–829. doi:10.1016/j.jhep.2013.05.016
3. Chang M-H. Hepatitis B virus infection. Semin Fetal Neonatal Med. 2007;12(3):160–167. doi:10.1016/j.siny.2007.01.013
4. Lai CL, Ratziu V, Yuen M-F, et al. Viral hepatitis B. Lancet. 2003;362(9401):2089–2094. doi:10.1016/S0140-6736(03)15108-2
5. Ma L, Alla NR, Li X, et al. Mother-to-child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014;24:396–406.
6. Schillie S, Walker T, Veselsky S, et al. Outcomes of infants born to women infected with hepatitis B. Pediatrics. 2015;135(5):e1141. doi:10.1542/peds.2014-3213
7. Hu XL, Zhou XP, Qian YL, et al. The presence and expression of the hepatitis B virus in human oocytes and embryos. Hum Reprod. 2011;26(7):1860–1867. doi:10.1093/humrep/der103
8. Huang JM, Huang T-H, Qiu H-Y, et al. Studies on the integration of hepatitis B virus DNA sequence in human sperm chromosomes. Asian J Androl. 2002;4(3):209–212.
9. Hadchouel M, Scotto J, Huret JL, et al. Presence of HBV DNA in spermatozoa: a possible vertical transmission of HBV via the germ line. J Med Virol. 1985;16(1):61–66. doi:10.1002/jmv.1890160109
10. Shao Q, Zhao X, Yao MD. Role of peripheral blood mononuclear cell transportation from mother to baby in HBV intrauterine infection. Arch Gynecol Obstet. 2013;288(6):1257–1261. doi:10.1007/s00404-013-2893-x
11. Wang DD, Yi LZ, Wu LN, et al. Relationship between maternal PBMC HBV cccDNA and HBV serological markers and its effect on HBV intrauterine transmission. Biomed Environ Sci. 2019;32(5):315–323. doi:10.3967/bes2019.043
12. Chen Y, Wang L, Xu Y, et al. Role of maternal viremia and placental infection in hepatitis B virus intrauterine transmission. Microbes Infect. 2013;15(5):409–415. doi:10.1016/j.micinf.2013.02.008
13. Xu D-Z, Yan Y-P, Zou S, et al. Role of placental tissues in the intrauterine transmission of hepatitis B virus. Am J Obstet Gynecol. 2001;185(4):981–987. doi:10.1067/mob.2001.117968
14. Bai H, Zhang L, Ma L, et al. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J Gastroenterol. 2007;13(26):3625–3630. doi:10.3748/wjg.v13.i26.3625
15. Bhat P, Anderson DA. Hepatitis B virus translocates across a trophoblastic barrier. J Virol. 2007;81(13):7200–7207. doi:10.1128/JVI.02371-06
16. Cui F, Shen L, Li L, et al. Prevention of chronic hepatitis b after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23(5):765–772. doi:10.3201/eid2305.161477
17. Cheung KW, Seto MTY, Kan ASY, et al. Immunoprophylaxis failure of infants born to hepatitis B carrier mothers following routine vaccination. Clin Gastroenterol Hepatol. 2018;16(1):144–145. doi:10.1016/j.cgh.2017.07.013
18. Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190(9):489–492. doi:10.5694/j.1326-5377.2009.tb02524.x
19. Liu C-P, Zeng Y-L, Zhou M, et al. Factors associated with mother-to-child transmission of hepatitis B virus despite immunoprophylaxis. Intern Med. 2015;54(7):711–716. doi:10.2169/internalmedicine.54.3514
20. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi:10.1002/hep.29800
21. Xu Y-Y, Liu H-H, Zhong Y-W, et al. Peripheral blood mononuclear cell traffic plays a crucial role in mother-to-infant transmission of hepatitis B virus. Int J Biol Sci. 2015;11(3):266–273. doi:10.7150/ijbs.10813
22. Shi X, Wang X, Xu X, et al. Impact of HBV replication in peripheral blood mononuclear cell on HBV intrauterine transmission. Front Med. 2017;11(4):548–553. doi:10.1007/s11684-017-0597-5
23. Shi Z, Li X, Ma L, et al. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother-to-child transmission-a meta-analysis. Int J Infect Dis. 2010;14(7):e622–34. doi:10.1016/j.ijid.2009.09.008
24. Chau KH, Hargie MP, Decker RH, et al. Serodiagnosis of recent hepatitis B infection by IgM class anti-HBc. Hepatology. 1983;3(2):142–149. doi:10.1002/hep.1840030202
25. Zhang L, Gui X-E, Wang B, et al. Serological positive markers of hepatitis B virus in femoral venous blood or umbilical cord blood should not be evidence of in-utero infection among neonates. BMC Infect Dis. 2016;16(1):408. doi:10.1186/s12879-016-1754-1
26. Cheung KW, Lao TT-H. Hepatitis B – vertical transmission and the prevention of mother-to-child transmission. Best Pract Res Clin Obstet Gynaecol. 2020;68:78–88. doi:10.1016/j.bpobgyn.2020.02.014
27. Ohto H, Tohyama H, Lin -H-H, et al. Intrauterine transmission of hepatitis B virus is closely related to placental leakage. J Med Virol. 1987;21(1):1–6. doi:10.1002/jmv.1890210102
28. Lin HH, Lee T-Y, Chen D-S, et al. Transplacental leakage of HBeAg-positive maternal blood as the most likely route in causing intrauterine infection with hepatitis B virus. J Pediatr. 1987;111(6 Pt 1):877–881. doi:10.1016/S0022-3476(87)80210-X
29. Tang S. [Study on the mechanisms and influential factors of intrauterine infection of hepatitis B virus]. Zhonghua Liu Xing Bing Xue Za Zhi. 1991;12(6):325–326. Chinese.
30. Xu DZ, Yan Y-P, Choi BCK, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67(1):20–26. doi:10.1002/jmv.2187
31. Sirilert S, Khamrin P, Kumthip K, et al. Placental infection of hepatitis B virus among Thai pregnant women: clinical risk factors and its association with fetal infection. Prenat Diagn. 2020;40(3):380–386. doi:10.1002/pd.5628
32. Wang J-S, Zhu Q-R. Infection of the fetus with hepatitis B e antigen via the placenta. Lancet. 2000;355(9208):989. doi:10.1016/S0140-6736(00)90021-7
33. Mavilia MG, Wu GY. Mechanisms and prevention of vertical transmission in chronic viral hepatitis. J Clin Transl Hepatol. 2017;5(2):119–129. doi:10.14218/JCTH.2016.00067
34. Joshi SS, Coffin CS. Hepatitis B virus lymphotropism: emerging details and challenges. Biotechnol Genet Eng Rev. 2018;34(1):139–151. doi:10.1080/02648725.2018.1474324
35. Gatta A, Giannini C, Lampertico P, et al. Hepatotropic viruses: new insights in pathogenesis and treatment. Clin Exp Rheumatol. 2008;26(1 Suppl 48):S33–8.
36. Michalak TI, Pasquinelli C, Guilhot S, et al. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest. 1994;94(2):907. doi:10.1172/JCI116950C1
37. Jin L, Nie R, Li Y, et al. Hepatitis B surface antigen in oocytes and embryos may not result in vertical transmission to offspring of hepatitis B virus carriers. Fertil Steril. 2016;105(4):1010–1013. doi:10.1016/j.fertnstert.2015.12.008
38. Zhong Y, Liu D-L, Ahmed MM, et al. Transcription and regulation of hepatitis B virus genes in host sperm cells. Asian J Androl. 2018;20(3):284–289. doi:10.4103/aja.aja_46_17
39. León-Juárez M, Martínez–Castillo M, González-García LD, et al. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog Dis. 2017;75(7):ftx093. doi:10.1093/femspd/ftx093
40. Cornish EF, Filipovic I, Åsenius F, et al. Innate immune responses to acute viral infection during pregnancy. Front Immunol. 2020;11:572567. doi:10.3389/fimmu.2020.572567
41. Huppertz B. The anatomy of the normal placenta. J Clin Pathol. 2008;61(12):1296–1302. doi:10.1136/jcp.2008.055277
42. Dachlan EG, Nugraheni C, Rahniayu A, et al. Quantitative HBsAg and qualitative HBeAg predicts intrauterine placental infection and umbilical blood cord in pregnant women. J Family Reprod Health. 2020;14(2):106–115. doi:10.18502/jfrh.v14i2.4353
43. Chang WH, Xu D-Z, Yan Y-P, et al. [Study on the presence of hepatitis B virus in first-trimester villi in pregnant women with hepatitis B surface antigen positive]. Zhonghua Fu Chan Ke Za Zhi. 2005;40(6):376–379. Chinese.
44. Sheikh AU, Polliotti BM, Miller RK. Human immunodeficiency virus infection: in situ polymerase chain reaction localization in human placentas after in utero and in vitro infection. Am J Obstet Gynecol. 2000;182(1 Pt 1):207–213. doi:10.1016/S0002-9378(00)70514-X
45. Kala S, Dunk C, Acosta S, et al. Periconceptional exposure to lopinavir, but not darunavir, impairs decidualization: a potential mechanism leading to poor birth outcomes in HIV-positive pregnancies. Hum Reprod. 2020;35(8):1781–1796. doi:10.1093/humrep/deaa151
46. Gigi CE, Anumba DOC. Parvovirus b19 infection in pregnancy - A review. Eur J Obstet Gynecol Reprod Biol. 2021;264:358–362. doi:10.1016/j.ejogrb.2021.07.046
47. Morey AL, Keeling JW, Porter HJ, et al. Clinical and histopathological features of parvovirus B19 infection in the human fetus. Br J Obstet Gynaecol. 1992;99(7):566–574. doi:10.1111/j.1471-0528.1992.tb13822.x
48. Kekkou K, Kavatha D, Karalexi M, et al. Risk of congenital cytomegalovirus infection in children born to women with IgG avidity in the grey zone during first trimester of pregnancy. J Matern Fetal Neonatal Med. 2021;34(12):2025–2029. doi:10.1080/14767058.2019.1651277
49. Parry S, Holder J, Strauss JF 3rd. Mechanisms of trophoblast-virus interaction. J Reprod Immunol. 1997;37(1):25–34. doi:10.1016/S0165-0378(97)00071-5
50. Enders G, Daiminger A, Bäder U, et al. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol. 2011;52(3):244–246. doi:10.1016/j.jcv.2011.07.005
51. Bai X, Ran J, Zhao X, et al. The S100A10-AnxA2 complex is associated with the exocytosis of hepatitis B virus in intrauterine infection. Lab Invest. 2022;102(1):57–68. doi:10.1038/s41374-021-00681-8
52. Ellinger I, Schwab M, Stefanescu A, et al. IgG transport across trophoblast-derived BeWo cells: a model system to study IgG transport in the placenta. Eur J Immunol. 1999;29(3):733–744. doi:10.1002/(SICI)1521-4141(199903)29:03<733::AID-IMMU733>3.0.CO;2-C
53. Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;3. doi:10.7554/eLife.00049
54. Bright NA, Ockleford CD, Anwar M. Ontogeny and distribution of Fc gamma receptors in the human placenta. Transport or immune surveillance? J Anat. 1994;184(Pt 2):297–308.
55. Ishikawa T, Takizawa T, Iwaki J, et al. Fc gamma receptor IIb participates in maternal IgG trafficking of human placental endothelial cells. Int J Mol Med. 2015;35(5):1273–1289. doi:10.3892/ijmm.2015.2141
56. Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020;20(10):633–643. doi:10.1038/s41577-020-00410-0
57. Tian T, Sun D, Wang P, et al. Roles of toll-like receptor 7 and 8 in prevention of intrauterine transmission of hepatitis B virus. Cell Physiol Biochem. 2015;37(2):445–453. doi:10.1159/000430367
58. Wang XP, Li F-J, Xu D-Z, et al. Uptake of hepatitis B virus into choriocarcinoma cells in the presence of proinflammatory cytokine tumor necrosis factor-alpha. Am J Obstet Gynecol. 2004;191(6):1971–1978. doi:10.1016/j.ajog.2004.06.038
59. Li F, Wang X, Men K, et al. Receptivity of human choriocarcinoma JEGIII cells and isolated trophoblast cells to hepatitis B virus infection and enhancement by tumor necrosis factor alpha. Jpn J Infect Dis. 2007;60(4):167–172.
60. Bai G, et al. Effect of hepatitis B virus infection on apoptosis of a human choriocarcinoma cell line in vitro. J Obstet Gynaecol Res. 2013;39(6):1200–1211. doi:10.1111/jog.12046
61. Cui H, Fu F, Tang Y, et al. Hepatitis B virus X protein modifies invasion, proliferation and the inflammatory response in an HTR-8/SVneo cell model. Oncol Rep. 2015;34(4):2090–2098. doi:10.3892/or.2015.4172
62. Bai G, Wang Y, Zhang L, et al. The study on the role of hepatitis B virus X protein and apoptosis in HBV intrauterine infection. Arch Gynecol Obstet. 2012;285(4):943–949. doi:10.1007/s00404-011-2096-2
63. Wang W, Bai G, Zhang Y, et al. HBxAg suppresses cell apoptosis and promotes the secretion of placental hormones in human placental trophoblasts via activation of the EGFR/Akt pathway. Cell Biol Int. 2018;42(2):237–247. doi:10.1002/cbin.10891
64. Lin Y, Liu Y, Xu D, et al. HBxAg promotes HBV replication and EGFR activation in human placental trophoblasts. Exp Ther Med. 2021;22(5):1211. doi:10.3892/etm.2021.10645
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.