Back to Journals » Journal of Pain Research » Volume 12
Intrathecal TRPM8 blocking attenuates cold hyperalgesia via PKC and NF-κB signaling in the dorsal root ganglion of rats with neuropathic pain
Authors Cao S, Li Q, Hou J, Li Z, Cao X, Liu X, Qin B
Received 5 December 2018
Accepted for publication 27 February 2019
Published 18 April 2019 Volume 2019:12 Pages 1287—1296
DOI https://doi.org/10.2147/JPR.S197168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Song Cao,1,2* Qingmei Li,3* Jingfeng Hou,1 Zhourui Li,1 Xinya Cao,1 Xiaohong Liu,4 Bangyong Qin1
1Department of Pain Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, People’s Republic of China; 2Guizhou Key Laboratory of Anesthesia and Organ Protection, Zunyi Medical University, Zunyi 563000, People’s Republic of China; 3Department of Anesthesiology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, People’s Republic of China; 4Department of Physiology, Zunyi Medical University, Zunyi 563000, People’s Republic of China
*These authors contributed equally to this work
Background: TRPM8 channel plays central roles in the sensitization of nociceptive transduction and is thought as one of the potential targets for the treatment of neuropathic pain. However, the specific molecular mechanisms are still less clear.
Methods: Sciatic chronic constriction injury (CCI) rats were intrathecally administered with AMTB (TRPM8-selective antagonist) or PDTC (nuclear factor-kappa B (NF-κB) inhibitor). Cold-, thermal- and mechanical-pain thresholds were examined in CCI and sham-operated rats before and after intrathecal administration of AMTB or PDTC. Protein expression levels of TRPM8 and NF-κB p65, p-PKC/PKC value and p-PKA/PKA value in the CCI ipsilateral L4-6 dorsal root ganglions (DRGs) were analyzed. In addition, the co-expression of TRPM8 and NF-κB was evaluated in DRG.
Results: Intrathecal injection of AMTB decreased the cold hypersensitivity and aggravated the thermal-hyperalgesia in the next 2 weeks after CCI surgery. The protein expression of TRPM8 and NF-κB p65 in the ipsilateral DRGs significantly increased after CCI surgery, which can be reversed by intrathecal administration of AMTB. The PKC, PKA, p-PKC/PKC and p-PKA/PKA values showed significantly increase after CCI surgery, while intrathecal AMTB administration offset the expression increase of PKC, p-PKC and p-PKC/PKC but PKA or p-PKA/PKA in the DRG. NF-κB inhibitor not only efficiently increased the cold-, thermal-pain threshold of CCI rats, but also enhanced AMTB’s anti-cold pain effect although exerted no anti-thermal hyperalgesia effect compared with TRPM8 blockade group. Immunofluorescence results showed co-expression of TRPM8 and NF-κB in DRG neurons.
Conclusion: TRPM8 channels in DRGs participate in the pathogenesis of cold and thermal hyperalgesia (not mechanical allodynia) in rats with neuropathic pain, which could be regulated by PKC (not PKA) and NF-κB signaling. TRPM8 channel, PKC and NF-κB are potential targets for cold hyperalgesia treatment in neuropathic pain patients.
Keywords: TRPM8, neuropathic pain, dorsal root ganglia, PKC, NF-κB
Introduction
More than one-third of pain-related symptoms are accounted for neuropathic pain, which refers to the dysfunction of somatosensory system or the pain caused by diseases.1 The clinical manifestations of neuropathic pain include allodynia, sensory paralyzes and spontaneous pain.2,3 The pathogenesis of neuropathic pain is complex, and the ideal therapeutic method is yet to be found.4 Pathological cold pain is a common symptom in several kinds of neuropathic pain, which are presented as cold allodynia, a pain response to cold temperatures that do not normally provoke pain, and/or cold hyperalgesia, an increased sensitivity to painful cold temperature.5,6
Transient receptor potential melastatin 8 (TRPM8) ion channel belongs to the transient receptor potential protein family, mainly locating in small neurons of the dorsal root ganglion (DRG) and trigeminal ganglia.7 The role of TRPM8 in nerve injury-evoked cold and mechanical allodynia was recently described.8,9 In animals with neuropathic pain, the expression of TRPM8 in sensory neurons significantly increased compared with normal ones, which induces increased sensitivity to cold.10 For example, rats with sciatic nerve chronic constriction injury (CCI) showed elevated expression of TRPM8 in spinal cord and DRG.11,12 TRPM8 channels also play vital roles in the sensitization of nociceptive transduction; therefore, it is thought as one of the potential targets for neuropathic pain treatment.13 However, the specific molecular mechanisms for its roles in neuropathic pain regulation are less clear.
PKC, PKA and their phosphorylation form have been proved to be involved in the regulation of neuropathic pain.14–16 Nuclear factor-kappa B (NF-κB) took part in the transmission of nociceptive sensory information in the DRG17 and spinal cord18,19 in murine with neuropathic pain. PKA and PKC can activate NF-κB in DRG and maintaining persistent inflammatory pain in rat.20 Alleviation of the inflammatory pain can be induced by reducing the formation of NF-κB through antioxidant regulation.21 It is reported that PKC/NF-κB signaling in rat DRG involved in inflammation-induced neuropathic pain.20,22 Recently, Liu et al23 found that cold stimuli induce inflammatory responses in primary airway epithelial cells of asthmatic mice through TRPM8. We hypothesize that TRPM8 blocking can attenuate neuropathic pain via PKC or PKA/NF-κB signaling in the DRG.
To analyze TRPM8’s effect on thermal-, mechanical- and cold-pain in CCI rats and to examine contributions from PKC, PKA and NF-κB signaling, TMPM8 blocker N-(3-aminopropyl)-2-[(3-methylphenyl) methoxy]-N-(2-thienylmethyl)-benzamide (AMTB) and NF-κB blocker pyrrolidinedithiocarbamate ammonium (PDTC) were intrathecally injected, then the NF-κB p65 expression, p-PKC/PKC value, p-PKA/PKA value and the co-expression of TRPM8 and NF-κB were evaluated in the ipsilateral DRG.
Methods
Ethical approval and animal preparation
Ethical approval was given by the Experimental Animal Care and Use Committee of Zunyi Medical University with approval number ZMC2013-0009. Thirty male and 30 female Sprague-Dawley rats (180–200 g, 16–20 weeks old) were purchased from the Animal Experimental Center of Third Military Medical University (Chongqing, China) and housed in groups of three to four under a standard 12-hr light/dark cycle with access to food and water ad libitum for at least 1 week before the beginning of the experiments. Experiment procedures and general handling complied with the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health (8th Edition, 2011).
Intrathecal catheter placement and drug administration
Rats were anesthetized with 10% chloral hydrate (0.4 mL/100 g intraperitoneally). Intrathecal catheter placement surgeries were conducted as reported.24 A skin incision about 2 cm in length was made and muscles were bluntly dissected to expose the spinal dura mater. A syringe needle was used to pierce the spinal dura mater, and a polyethylene catheter was implanted into the lumbar enlargement and fixed. Twenty-four hours later, rats exhibiting neurological deficits were excluded from the experiments. If the catheter was in the subarachnoid space, 20 μL of 2% lidocaine injection through intrathecal catheter will induce paralysis of hind limb within 30 s after lidocaine administration. Penicillin at a dose of 120 mg per day was administered for 3 days after the operation, and the rats were raised separately in cages. TRPM8 antagonist AMTB hydrochloride and NF-κB blocker PDTC were purchased from Sigma-Aldrich (Shanghai, China). From the second day after CCI surgery, in the next two weeks, AMTB and PDTC were given intrathecally twice a day. In the Sham group, 20 μL normal saline was applied intrathecally; 10 μL of 10 μM25 AMTB+10 μL normal saline and 10 μL of 12.2 mM PDTC+10 μL normal were applied intrathecally in the TRPM8 blockade group and NF-κB inhibition group, respectively.
CCI model establishment
CCI operation was performed 5 days after intrathecal catheter placement. The surgical procedure was conducted with reference to Bennett’s work.26 Rats were anesthetized with 10% chloral hydrate (0.4 mL/100 g intraperitoneally), and the right common sciatic nerve was bluntly dissected and exposed behind the femur. Four loose ligatures were made with a 5–0 silk thread at an interval of 1 mm, and the desired degree of constriction was to induce slight tremor of the calf muscle and not to arrest blood flow. For rats in the Sham group, the sciatic nerve only received blunt dissection through the biceps femoris, no ligatures were applied before closing the incisions. Penicillin at a dose of 120 mg per day was administered for 3 days after the operation, and the rats were raised separately in cages.
Pain threshold tests
The pain threshold tests were performed before CCI surgery (baseline pain threshold), and on the 1st, 3rd, 7th, 10th and 14th day after CCI surgery.
The cold hyperalgesia was determined in accordance with Bennett’s method.26 Rats were placed on a stainless plate cooled with ice (4±1°C) under a transparent plastic cover. When rats adapted to the testing environment and stayed still, the total number of right hind paw withdrawals from the cold surface not related to general movement was quantified over the subsequent 20-min period.
Thermal hyperalgesia was measured using a TF2 beam-thermal system (YuYan Instruments, Shanghai, China), and expressed as paw withdrawal latency (PWL, in second) of the right hind paw. Heat was generated from the optical radiation and reached 50°C. Tests were performed after rat adapted to the testing environment and stayed still. Length of time course from the beginning of beam on the right hind paw to the appearance of first paw withdrawal movement were recorded. The maximum time of beam was limited to 30 s in order to prevent tissue injury. Six measurements were taken for each rat, at 10-min intervals, and the mean was regarded as PWL.
Mechanical withdrawal thresholds (MWTs, in gram) were assessed using an electronic von Frey plantar aesthesiometer (IITC, Wood Dale, IL, USA) and served as an index of mechanical allodynia as reported.27 Graded mechanical stimulation was applied vertically by the test probe on the right hind paw, and the test was completed when the rats lifted their hind paw. Six measurements were taken for each rat, at 5-min intervals, and the mean was regarded as MWT.
Western blotting
After behavioral tests, rats in each group were anesthetized and sacrificed on the 7th or 14th day after CCI surgery to perform Western blotting analysis as reported elsewhere.28 The collected ipsilateral L4-6 DRGs (the sciatic nerve is originated from the L4 to S3 segments of the sacral part of the spinal cord) tissue was mechanically homogenized and centrifuged. The supernatant was collected and stored at – 80°C. Protein concentrations of the supernatant were determined using the BCA protein assay kit (Beyotime, China). Equal amounts of total protein (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to membranes for immunoblotting. The primary antibodies used were listed as follows: rabbit monoclonal [EPR4196(2)] to TRPM8 (1:500, Abcam, Shanghai, China); rabbit monoclonal anti phospho-NF-κB p65 (Ser536) (1:1000, Abcam, Shanghai, China); rabbit anti-PKA 2 beta [EP2648] (1:5000, Abcam, Shanghai, China); rabbit monoclonal [E151] to PKA R2 (phospho S99) (1:2500, Abcam, Shanghai, China); rabbit anti-PKC alpha (1:1000, Abcam, Shanghai, China); rabbit anti-PKC alpha (phospho S657+Y658, 1:1000, Abcam, Shanghai, China). Protein expression was visualized with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (1:5000, Abcam, Shanghai, China), and was detected using the Odyssey Infrared Imaging System (LI-COR, USA) and the digital images were analyzed by densitometry using ImageJ software (National Institutes of Health, USA). Protein levels were normalized to β-actin as the loading control. Relative optical density of the protein bands was measured after subtracting the film background. Data are expressed as mean ratio±S.E.M of the target protein/β-actin protein.
Immunocolocalization of TRPM8 and NF-κB-p65 in DRG
The rats were sacrificed and perfused transcardially with PBS followed by 4% paraformaldehyde. L4 DRGs were removed and fixed in 4% paraformaldehyde overnight at 4°C, placed in 20% sucrose solution overnight at 4°C and embedded in O.C.T. compound (Sakura Finetek, Japan). Slices (10 μm thick) were made using a cryostat (Leica, Germany). For immunofluorescence analysis of TRPM8 and NF-κB-p65, the sections were incubated in 1% normal goat serum in PBS with 0.3% Triton X-100 for 1 hr. The L4 DRG sections were incubated with rabbit monoclonal [EPR4196(2)] to TRPM8 antibody (1:300, Abcam, Shanghai, China) and mouse monoclonal anti- NF-κB p65 (F-6) antibody (1:200, Santa Cruz, USA) overnight at 4°C. TRPM8 antibody was labeled with goat-anti-rabbit-DyLight 649 (red); NF-κB-p65 antibody was labeled with goat-anti-mouse-FITC (shown in green) as a secondary antibody. Cellular nuclei were stained with DAPI (shown in blue). The sections were examined under a confocal microscopy (Leica, Germany).
Statistical analysis
Statistics were performed using GraphPad Prism statistical software (Version 7.0, GraphPad Software, USA). All data were expressed as mean±SD. Differences among groups were assessed with one-way ANOVA or two-way ANOVA. P-value <0.05 was considered statistically significant.
Results
TRPM8 blocker reduces cold pain, aggravates thermal pain but has no effect on mechanical pain in CCI rats
For rats in the Sham group, intrathecal injection of normal saline did not induce behaviors of neuropathic pain. For the rats in CCI group, neuropathic pain behaviors such as toe closing, licking and back flexion can be observed on the first day after CCI surgery, but without motor dysfunction and autonomy.
As compared with the Sham group, the paw lift times on cold plate increased, and the thermal withdraw latency and mechanical paw withdrawal threshold decreased significantly in CCI group since the first day after CCI surgery (Figure 1A–C). When blocked TRPM8 with AMTB, compared with the CCI group, both the paw lift times on cold plate (Figure 1A) and the thermal withdraw latency decreased (Figure 1B) on the 3th, 7th, 10th and 14th day after CCI surgery, but rats in CCI and AMTB group showed no difference on mechanical paw withdrawal thresholds at all time points after CCI surgery (Figure 1C).
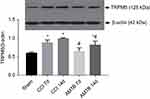
CCI surgery increases TRPM8 protein expression, while TRPM8 blockade reduces CCI induced TRPM8 overexpression in DRGs
The TRPM8 protein expression was evaluated before CCI, on the 7th and 14th day after CCI. Compared with the Sham group, the expression of TRPM8 in the L4-6 DRGs in the CCI group was significantly increased on the 7th and 14th day after CCI surgery. Compared with the CCI group, the expression level of TRPM8 protein in AMTB group was significantly decreased on the 7th and 14th day after CCI surgery (Figure 2).
Intrathecal injection of AMTB decreases PKC overexpression and p-PKC/PKC level in DRGs of CCI rats
Compared with the Sham group, CCI increased the expression of PKC, p-PKC as well as the value of p-PKC/PKC (Figure 3A). In addition, CCI increased the expression of PKA and p-PKA although did not change the p-PKA/PKA value compared with sham-operated rats (Figure 3B).
Compared with CCI group, intrathecal injection of AMTB decreased CCI-induced overexpression of PKC and elevated p-PKC/PKC value (Figure 3A) but showed no effect on PKA, p-PKA and p-PKA/PKA (Figure 3B).
NF-κB participates in the neuropathic pain regulation of TRPM8
To evaluate whether NF-κB participates in the neuropathic pain regulation of TRPM8, we detected NF-κB p65 expression with Western blotting and retested the pain thresholds after blocking NF-κB with its specific inhibitor PDTC.
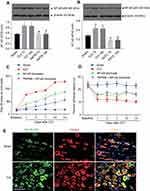
The expression level of NF-κB p65 in the CCI group was significantly increased on the 7th and 14th day after CCI surgery when compared with the Sham group. Its expression in the TRPM8 group was significantly lower than that of the CCI group on the 7th and 14th day after CCI surgery (Figure 4A). In the NF-κB inhibition experiments, NF-κB p65 expression in the CCI group was significantly increased on the 7th and 14th day after CCI surgery when compared with the Sham group. Interestingly, NF-κB inhibition with PDTC significantly decreased NF-κB p65 expression on the 7th and 14th day after CCI surgery compared with that of CCI group at the same time point (Figure 4B).
Pain behavior tests showed that compared with the CCI group, NF-κB blockade with PDTC significantly decreased the paw lift times and reduced thermal hyperalgesia since the first day after CCI surgery (Figure 4C and D). When the TRPM8 and NF-κB were both inhibited, the paw lift times on cold plate decreased more on the 3rd, 7th, 10th and 14th day after CCI surgery compared with that of NF-κB blockade group, but no difference of thermal hyperalgesia was found between NF-κB inhibition group and NF-κB + AMTB blockade group at these time points (Figure 4C and D).
Co-localization of TRPM8 and NF-κB p65 in DRG neurons
To determine the possibility that TRPM8-NF-κB signaling in the DRG neuron regulates pain intensity in neuropathic pain rats, the co-localized expression of TRPM8 and NF-κB p65 was assessed by immunofluorescence detected by confocal microscopy. As shown in Figure 4E, both TRPM8 and NF-κB p65 were localized in the cytoplasm in the Sham and CCI rat DRG. In the same cells, we observed that NF-κB p65 (shown in green) and TRPM8 (shown in red) localized to the same sites (yellow areas).
Discussion
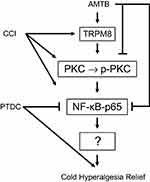
In the present study, intrathecal injection of TRPM8 antagonist AMTB significantly decreased the cold hypersensitivity and increased the thermal hypersensitivity in rats with chronic sciatic nerve ligation. In addition, TRPM8 blockade reduced the nerve injury-induced overexpression of NF-κB p65 and p-PKC/PKC in the L4-6 DRGs, while NF-κB inhibition reduced cold hyperalgesia and enhance the anti-cold hyperalgesia effect of AMTB, which indicates that NF-κB and PKC pathways may play roles in the pathogenesis of TRPM8-mediated cold/thermal hyperalgesia after nerve injury (Figure 5).
Previous studies have shown that TRP channels are closely related to neuropathic pain, especially TRPM8, TRPV1 and TRPA1 are vital in the pathogenesis of peripheral and central neuropathic pain.29–31 These TRP channels have been proved to be involved in the occurrence of pain induced by chemical, temperature and mechanical stimulation. Proudfoot et al11 found that, after sciatic nerve injury, the expression of TRPM8 in the DRGs was significantly increased. Su et al32 observed the same results in rats with chronic neuropathic pain, and concluded that the upregulation of TRPM8 channel plays an important role in the generation and maintenance of neuropathic pain. Our results of TRPM8 expression are in line with these reports. Besides, we found that blocking TRPM8 increased the thermal hypersensitivity in CCI rats. TRPM8 expression may directly influence the thermal pain threshold given that TRPM8 blocker reduced TRPM8 expression in CCI rats (Figure 2). There may be correlations between TRPM8 and other thermo-sensitive receptors, such as Nav1.7,33 TRPA134 and TRPV1,35 which need further research to detect the expression of these receptors when TRPM8 was blocked.
For the decreased expression of TRPM8 in CCI rats received intrathecal injection of AMTB (Figure 2), we speculate that intrathecal administration of AMTB blocks the TRPM8-mediated pain signaling and reduces pain perception, which could be the cause of the decreased TRPM8 overexpression as shown in CCI rats (Figure 2, CCI group).
Consistent with results reported previously,36,37 we found that the CCI injury upregulated the expression of PKC and PKA, it also elevated the value of p-PKC/PKC and p-PKA/PKA in the DRGs of rats. However, TRPM8 blocking can only reduce p-PKC/PKC but not p-PKA/PKA; hence, it is reasonable to consider that PKC (not PKA) signaling is involved in the TRPM8-regulated hyperalgesia induced by nerve injury although PKA involved the neuropathic pain event. Mandadi et al38 also proved that treatment with PKC agonists on the TRPM8 expressing sensory neurons will induce desensitization of TRPM8, which supports our finding on the relationship between PKC and TRPM8-regulated hyperalgesia. Premkumar and colleagues39 suggested that the negative regulation of TRPM8 by PKC occurs indirectly via PKC-mediated activation of phosphatase calcineurin. Therefore, further studies are needed to elucidate the putative sites of PKC phosphorylation, and their mechanisms in pain regulation in DRG neurons expressing TRPM8.
To evaluate whether NF-κB participates in TRPM8’s neuropathic pain regulation effects, we detected NF-κB p65 expression and the pain thresholds after blocking NF-κB. CCI caused NF-κB p65 overexpression while TRPM8 blocking decreased its overexpression. In the NF-κB inhibition experiment, it is interesting to find that PDTC significantly decreased NF-κB p65 expression. In the pain behavior tests, NF-κB blockade significantly decreased the cold- and thermal-hyperalgesia. NF-κB inhibition can facilitate the anti-cold hyperalgesia effect of TRPM8 blocker but NF-κB inhibition cannot enhance the anti-thermal hyperalgesia effect of TRPM8 blocker. These data suggest that NF-κB contributes to TRPM8’s neuropathic pain regulation effect, and intervening NF-κB activity can exert anti-neuropathic pain effect.
To evaluate the possibility that TRPM8-NF-κB signaling in the DRG neurons regulates neuropathic pain intensity, the co-localized expression of TRPM8 and NF-κB was assessed by immunofluorescence. As shown in Figure 4E, both TRPM8 and NF-κB were localized in the cytoplasm of DRG neurons, suggesting that TRPM8-NF-κB signaling in the DRG neurons takes part in the pain regulation of TRPM8.
There are some limitations for this study. AMTB and PDTC given intrathecally act on TRPM8 and NF-κB in the spinal dorsal horn, even the brain, and the pain behavior responses are combined effects from DRG and the central nervous system. Further studies that using the PKC inhibitor and kinase activity assays are warranted to find out the specific link between TRPM8, PKC and NF-κB in the pathogenesis of neuropathic pain.
Conclusion
In summary, the current study proved that TRPM8 in L4-6 DRGs participated in the pathogenesis of cold and thermal hyperalgesia of rats with chronic nerve injury. PKC and NF-κB signaling were involved in the TRPM8-mediated neuropathic pain regulation.
Acknowledgments
The authors thank Prof. Yu Zhang for the edit and revision of this manuscript. This work was supported by a grant from National Natural Science Foundation of China (Grant No.81450054 to Bangyong Qin) and scientific research projects from The Science and Technology Department of Guizhou Province (Grant No.2014-7583 to Bangyong Qin and grant No.LH[2015]7554 to Song Cao). This work is also supported by the Innovative Training Program of Zunyi Medical University (Grant No. [2015]3109).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Baron R. Mechanisms of disease: neuropathic pain–a clinical perspective. Nat Clin Pract Neurol. 2006;2(2):95–106. doi:10.1038/ncpneuro0113
2. Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans–how symptoms help disclose mechanisms. Nat Rev Neurol. 2013;9(10):572–582. doi:10.1038/nrneurol.2013.180
3. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi:10.1038/nrdp.2017.2
4. Meacham K, Shepherd A. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21(6):28.
5. Yin K, Zimmermann K, Vetter I, Lewis RJ. Therapeutic opportunities for targeting cold pain pathways. Biochem Pharmacol. 2015;93(2):125–140. doi:10.1016/j.bcp.2014.09.024
6. Shepherd AJ, Mohapatra DP. Attenuation of unevoked mechanical and cold pain hypersensitivities associated with experimental neuropathy in mice by angiotensin II Type-2 receptor antagonism. Anesth Analg. 2018. doi:10.1213/ANE.0000000000003857
7. Raddatz N, Castillo JP, Gonzalez C, Alvarez O, Latorre R. Temperature and voltage coupling to channel opening in transient receptor potential melastatin 8 (TRPM8). J Biol Chem. 2014;289(51):35438–35454. doi:10.1074/jbc.M114.612713
8. Harrington AM, Hughes PA, Martin CM, et al. A novel role for TRPM8 in visceral afferent function. Pain. 2011;152(7):1459–1468. doi:10.1016/j.pain.2011.01.027
9. Lolignier S, Gkika D, Andersson D. New insight in cold pain: role of ion channels, modulation, and clinical perspectives. J Neurosci. 2016;36(45):11435–11439. doi:10.1523/JNEUROSCI.2327-16.2016
10. Colburn RW, Lubin ML, Stone DJ
11. Proudfoot CJ, Garry EM, Cottrell DF, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16(16):1591–1605. doi:10.1016/j.cub.2006.07.061
12. De Caro C, Russo R. Antinociceptive effect of two novel transient receptor potential melastatin 8 antagonists in acute and chronic pain models in rat. Br J Pharmacol. 2018;175(10):1691–1706. doi:10.1111/bph.14177
13. Basso L, Altier C. Transient receptor potential channels in neuropathic pain. Curr Opin Pharmacol. 2017;32:9–15. doi:10.1016/j.coph.2016.10.002
14. Xie JD, Chen SR, Chen H, Pan HL. Bortezomib induces neuropathic pain through protein kinase C-mediated activation of presynaptic NMDA receptors in the spinal cord. Neuropharmacology. 2017;123:477–487. doi:10.1016/j.neuropharm.2017.06.027
15. Hohmann SW, Angioni C, Tunaru S, Lee S. The G2A receptor (GPR132) contributes to oxaliplatin-induced mechanical pain hypersensitivity. Sci rep. 2017;7(1):446.
16. Wang C, Gu L, Ruan Y, et al. Facilitation of MrgprD by TRP-A1 promotes neuropathic pain. FASEB J. 2018;33(1):fj201800615RR.
17. Wen J, Jones M, Tanaka M, et al. WWL70 protects against chronic constriction injury-induced neuropathic pain in mice by cannabinoid receptor-independent mechanisms. J Neuroinflammation. 2018;15(1):9. doi:10.1186/s12974-018-1220-7
18. Lee KM, Jeon SM, Cho HJ. Tumor necrosis factor receptor 1 induces interleukin-6 upregulation through NF-kappaB in a rat neuropathic pain model. Eur J Pain. 2009;13(8):794–806. doi:10.1016/j.ejpain.2008.09.009
19. Lim H, Lee H, Noh K, Lee SJ. IKK/NF-kappaB-dependent satellite glia activation induces spinal cord microglia activation and neuropathic pain after nerve injury. Pain. 2017;158(9):1666–1677. doi:10.1097/j.pain.0000000000000959
20. Souza GR, Cunha TM, Silva RL, et al. Involvement of nuclear factor kappa B in the maintenance of persistent inflammatory hypernociception. Pharmacol Biochem Behav. 2015;134:49–56. doi:10.1016/j.pbb.2015.04.005
21. Pinho-Ribeiro FA, Zarpelon AC, Mizokami SS, et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-kappaB activation. J Nutr Biochem. 2016;33:8–14. doi:10.1016/j.jnutbio.2016.03.013
22. Zhao R, Pei GX, Cong R, Zhang H, Zang CW, Tian T. PKC-NF-kappaB are involved in CCL2-induced Nav1.8 expression and channel function in dorsal root ganglion neurons. Biosci Rep. 2014;34(3). doi:10.1042/BSR20140005
23. Liu H, Hua L, Liu Q, Pan J, Bao Y. Cold stimuli facilitate inflammatory responses through transient receptor potential melastatin 8 (TRPM8) in primary airway epithelial cells of asthmatic mice. Inflammation. 2018;41(4):1266–1275. doi:10.1007/s10753-018-0774-y
24. Zhou R, Xu T, Liu X, et al. Activation of spinal dorsal horn P2Y13 receptors can promote the expression of IL-1beta and IL-6 in rats with diabetic neuropathic pain. J Pain Res. 2018;11:615–628. doi:10.2147/JPR.S154437
25. Mueller-Tribbensee SM, Karna M, Khalil M, Neurath MF, Reeh PW, Engel MA. Differential contribution of TRPA1, TRPV4 and TRPM8 to colonic nociception in mice. PLoS One. 2015;10(7):e0128242. doi:10.1371/journal.pone.0128242
26. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107.
27. Cao S, Deng W, Li Y, et al. Chronic constriction injury of sciatic nerve changes circular RNA expression in rat spinal dorsal horn. J Pain Res. 2017;10:1687–1696. doi:10.2147/JPR.S139592
28. Cao S, Liu Y, Wang H, et al. Ischemic postconditioning influences electron transport chain protein turnover in Langendorff-perfused rat hearts. PeerJ. 2016;4:e1706. doi:10.7717/peerj.1706
29. Chen K, Zhang ZF, Liao MF, Yao WL, Wang J, Wang XR. Blocking PAR2 attenuates oxaliplatin-induced neuropathic pain via TRPV1 and releases of substance P and CGRP in superficial dorsal horn of spinal cord. J Neurol Sci. 2015;352(1–2):62–67. doi:10.1016/j.jns.2015.03.029
30. Pinheiro Fde V, Villarinho JG, Silva CR, et al. The involvement of the TRPA1 receptor in a mouse model of sympathetically maintained neuropathic pain. Eur J Pharmacol. 2015;747:105–113. doi:10.1016/j.ejphar.2014.11.039
31. Moran MM, Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol. 2018;175(12):2185–2203. doi:10.1111/bph.14044
32. Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12:120. doi:10.1186/1471-2202-12-120
33. Yang L, Dong F, Yang Q, et al. FGF13 Selectively regulates heat nociception by interacting with Nav1.7. Neuron. 2017;93(4):806–821.e809. doi:10.1016/j.neuron.2017.01.009
34. de David Antoniazzi CT, De Pra SD, Ferro PR, et al. Topical treatment with a transient receptor potential ankyrin 1 (TRPA1) antagonist reduced nociception and inflammation in a thermal lesion model in rats. Eur J Pharm Sci. 2018;125:28–38. doi:10.1016/j.ejps.2018.09.012
35. Barrett KT, Roy A, Rivard KB, Wilson RJA, Scantlebury MH. Vagal TRPV1 activation exacerbates thermal hyperpnea and increases susceptibility to experimental febrile seizures in immature rats. Neurobiol Dis. 2018;119:172–189. doi:10.1016/j.nbd.2018.08.004
36. Ma W, St-Jacques B. Signalling transduction events involved in agonist-induced PGE2/EP4 receptor externalization in cultured rat dorsal root ganglion neurons. Eur J Pain. 2018;22(5):845–861. doi:10.1002/ejp.1172
37. Yang J, Hsieh CL. Role of transient receptor potential vanilloid 1 in electroacupuncture analgesia on chronic inflammatory pain in mice. Biomed Res Int. 2017;2017:5068347.
38. Mandadi S, Armati PJ, Roufogalis BD. Protein kinase C modulation of thermo-sensitive transient receptor potential channels: implications for pain signaling. J Nat Sci Biol Med. 2011;2(1):13–25. doi:10.4103/0976-9668.82311
39. Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci. 2005;25(49):11322–11329. doi:10.1523/JNEUROSCI.3006-05.2005
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.