Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Intranasal Delivery of Bone Marrow Stromal Cells Preconditioned with Fasudil to Treat a Mouse Model of Parkinson’s Disease
Authors Tang Y, Han L, Bai X, Liang X, Zhao J, Huang F , Wang J
Received 14 November 2019
Accepted for publication 13 January 2020
Published 23 January 2020 Volume 2020:16 Pages 249—262
DOI https://doi.org/10.2147/NDT.S238646
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Yilin Tang,1,* Linlin Han,1,* Xiaochen Bai,1,2 Xiaoniu Liang,1 Jue Zhao,1 Fang Huang,2 Jian Wang1
1Department of Neurology and National Clinical Research Center for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai 200040, People’s Republic of China; 2The State Key Laboratory of Medical Neurobiology, The Institutes of Brain Science and the Collaborative Innovation Center for Brain Science, Shanghai Medical College, Fudan University, Shanghai 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian Wang
Department of Neurology and National Clinical Research Center for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai 200040, People’s Republic of China
Tel +86-133 2193 4789
Fax +86-21-5288 8163
Email [email protected]
Objective: Stem cell transplantation is a promising strategy with great potential to treat Parkinson’s disease (PD). Nevertheless, improving the cell delivery route and optimising implanted cells are necessary to increase the therapeutic effect. Herein, we investigated whether intranasal delivery of bone marrow stromal cells (BMSCs) has beneficial effects in a PD mouse model and whether the therapeutic potential of BMSCs could be enhanced by preconditioning with fasudil.
Methods: A PD mouse model was developed by intraperitoneally administering 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mice were treated intranasally with phosphate buffered saline (PBS), BMSCs, or BMSCs preconditioned with fasudil. One month later, the effects of BMSC treatment were analysed.
Results: Our study showed that fasudil could accelerate the proliferation of BMSCs and promote brain-derived neurotrophic factor (BDNF) secretion in vitro. Intranasally administered BMSCs were capable of surviving and migrating in the brain. Intranasal delivery of BMSCs preconditioned with fasudil significantly improved motor function and reduced dopaminergic neuron loss in substantia nigra; treatment with BMSCs and PBS resulted in similar outcomes. Preconditioning with fasudil inhibited the activation and aggregation of microglia, suppressed immune response, and reinforced BDNF secretion in MPTP-PD mice significantly more than treatment with BMSCs alone.
Conclusion: The present study demonstrates that intranasally administering BMSCs preconditioned with fasudil is a promising cell-based therapy for PD.
Keywords: Parkinson’s disease, intranasal delivery, bone marrow stromal cells, fasudil
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by dopaminergic neuron loss in the nigrostriatal system. In the past few decades, stem cell transplantation has emerged as a treatment strategy with great potential to cure PD.1–4 This beneficial effect might be attributed to the process whereby the damaged cells are replaced with transplanted cells. However, data in the literature suggest that stem cells are more likely to induce repair by stimulating the secretion of growth and differentiation factors.5,6
Presently, the routes by which stem cells reach the brain are either ineffective owing to the presence of the blood-brain barrier (BBB) (as in intravenous injections) or are rather invasive (such as intraventricular injections). Thus, the development of a new approach is necessary for stem cell-based therapy. Intranasal administration allows for transport of cells across the barrier to the central nervous system (CNS).7 Intranasal route of delivery could thus potentially become a key part of the cell therapy method for treating neurological diseases. Intranasal administration is a non-invasive and convenient method that bypasses the BBB and delivers cells directly to the CNS.8–10 However, only a few reports on intranasal administration of cell therapy for PD are currently available.11,12
Stem cell treatment is still mainly experimental. Its limitation lies in the unstable and adverse effects of treatment, which can be attributed to various reasons including insufficient graft survival, cell differentiation and proliferation under both in vivo and in vitro conditions, and formation of teratomas. To enhance the therapeutic potential of stem cells, approaches including genetic modification are being investigated.13 It was proven recently that fasudil, which is a Rho kinase (ROCK) inhibitor, is an ideal candidate for cell optimisation. Rho kinase accumulation is involved in various neural functions such as inhibition of tumour cell proliferation, neuronal differentiation and axonal regeneration.14–16 Recent studies suggest that ROCK inhibitors can help enhance the growth and differentiation of stem cells in vitro, and further improve the engraftment and survival after transplantation.17–19 Li et al demonstrated that combined treatment of neural stem cells (NSCs) and fasudil injected intraperitoneally additionally improved motor capacity of PD mice.12 However, fasudil injection administered every other day for 12 days (6 injections in total) was rather invasive and time-consuming. Whether fasudil pretreatment on stem cells in vitro before intranasal application could improve its therapeutic effect in PD mice remains unexplored. In this study, we investigated whether intranasal delivery of bone marrow stromal cells (BMSCs) has beneficial effects in PD mice. Furthermore, we analysed if the therapeutic potential of BMSCs could be enhanced by preconditioning with fasudil.
Materials and Methods
Animals
Shanghai SLAC Laboratory Animal Company (Shanghai, China) provided 4-week-old and 2-month-old male C57BL/6 mice. Fifteen mice were employed in each group, and this experiment was repeated for another two times. All experiments were conducted in accordance with the guidelines of the National Institutes of Health guide for the care and use of Laboratory animals. All the mice were raised without pathogens, randomly fed with food and water, and fed in a 12/12-h light/dark cycle in a temperature control room (25 ±2°C) for 1 week before the experimental manipulation.
Isolation and Cultivation of BMSCs
BMSCs were extracted from the pooled bone marrow of mononuclear cell fraction from 4-week-old male C57BL/6 mice. After intraperitoneal injection of metomidine hydrochloride under deep anesthesia, femur and tibia were removed aseptically, and by using a sterile syringe, each piece of bone marrow was washed with 10 mL Dulbecco modified Eagle medium (DMEM, Life Technologies Corp.). The bone marrow was spun at ambient temperature for 5 min at 300 × g after being filtrated using a 100 mL cell strainer (BD Falcon, BD Biosciences).
The cells were collected in 75 cm2 culture flasks. These cells underwent the culture with DMEM in a 25 cm2 tissue culture flask containing 100 mg/mL, 100 mg/mL streptomycin, and 10% fetal bovine serum. Subsequently, they underwent the incubation in the environment of 5% CO2 in O2 and 37°C. The culture medium was renewed on the next day for the removal of nonadherent cells. Next, the culture was changed medium two times a week. Fluorescence-activated cell sorting (FACS) analysis was performed with a FACScan instrument (BD Biosciences) using BD CellQuestPro software and the phycoerythrin (PE)-conjugated anti-mouse-CD11b, -CD34, -CD44, -CD105, -CD Sca-1 (BD Biosciences) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse- CD45 (BD Biosciences).
The Effect of Fasudil Pretreatment on BMSCs
The 3rd generation of BMSCs were divided into control group and Fasudil (from Tianjin Chase Sun Pharmaceutical Co., Ltd, China; Supplementary Figure S1) inducing group, which were cultured in BMSC culture medium and BMSC culture medium with fasudil (15μg/mL) for 72 h.
The proliferation of BMSCs was measured using 5-ethynyl-2ʹ-deoxyuridine (EdU) staining. The cells were seeded in 24-well plates using culture medium. After treatment with fasudil for 72 h, the EdU staining was conducted with Click-iT EdU kit (Invitrogen, USA) and 4ʹ,6-diamidino-2-phenylindole (DAPI) solution (Invitrogen, USA). The proliferation capacity was analyzed by measuring the percentage of Edu-positive cells in DAPI-stained cells.
Cell supernatant was collected from a seeding density of 100,000 cells of BMSCs. Extracellular release of BDNF (Promega, USA) was measured using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions.
MPTP-PD Model and Experimental Design
A PD mice model was developed by intraperitoneally administrating MPTP (Sigma, USA, 30 mg/kg/day) for 5 days consecutively. The mice were divided into 3 groups (15 mice in each), namely phosphate buffered saline (PBS) group, BMSC group and Fasudil-BMSC group.
Cell Application
Before cell application, BMSCs were washed using PBS and underwent centrifugation three times for 5 min at 200 g.
On the 3rd and 5th day after modeling, PBS, BMSCs (2.4×106/mice) and BMSCs preconditioned with fasudil (2.4×106/mice) were slowly introduced into the nasal cavity, respectively. After being slightly anesthetized with ether, the mice were placed in a neck brace, with a finger placed on the underside of the neck to restrict fluid flow down the trachea. To increase the barrier permeability of nasopharyngeal mucosa and facilitate the cell entry, all animals were treated with 100 u hyaluronidase (Sigma) dissolved in 24 μL of sterile PBS before vehicle or cell therapy. Mice received two times for each nostril alternate applications (left–right) of 6 μL drops with cell suspension/hyaluronidase or PBS (2 min after the first set, the second right–left application set was conducted). According to the identical procedure, alternating 6 μL portions for every nostril, the MSCs suspension (2.4×106 in 24 μL of sterile PBS) or vehicle (PBS) was applied 1hr after IN pretreatment using hyaluronidase.
Behavioral Assessment
One month after the last cells or PBS administration, motor behaviour was analysed by rotarod test and pole test.
Rotarod Test
All sessions were performed in the range (8 and 12 am). To learn how to keep their balance, mice first received a 300 s training session on the slowly rotating rod (16 rpm). 1hr later, mice were re-placed in the rod, and the time (latency) until a mouse fell off the rod rotating as being continuously accelerated was measured (increased per 30 s, from 16 to 32 rpm in 5 min). The combined measures of the rotarod performance of each mouse were yielded as the area under the mean time curve on the rod against rotation speed (overall rod performance scores, ORP).
Pole Test
For the assessment of bradykinesia in the mice, a pole test was performed. The mouse was placed on top of a vertical rough-surfaced stick (with a diameter of 10 mm, and a height of 58 cm). The time to turn and reach the floor were recorded. Two days before the test, each rat was trained to descend the pole. On the day of the test, the mice could practice five times and then three times for up to 4 min each.
Tissue Fixation and Freeze-Sectioning
One month after the last cells or PBS administration, the mice were killed. Mice underwent the perfusion in the left ventricle with saline solution and then with 4% paraformaldehyde for 15 min at pH 7.4 in 0.1M phosphate buffer. The brains underwent removal and cryoprotection by being soaked in 10%, 20% and 30% sucrose solution per day in phosphate buffer until they sank. The brains were harvested and froze in liquid nitrogen. The parallel series of 10-μm-thick coronal sections were obtained on a cold microtome.
Immunofluorescence
In immunostaining analysis, non-specific binding was blocked with 3% bovine serum (Serotec, UK), Such binding underwent permeabilization for 30mins with 0.1% Triton X-100 in 1% BSA-PBS. The sections underwent the incubation overnight at 4°C with monoclonal rabbit anti-TH (1:500; Millipore), anti-GFP (1:500; Abmart), anti-iNOS (1:500; Proteintech), anti-Arg-1 (1:200; BD), anti-Nestin (1:100; eBioscience). Subsequently, they underwent the incubation with the relevant Alexa Fluor 488-conjugated secondary anti-body (1:1000; Invitrogen) or Alexa Fluor 555-conjugated secondary antibody (1:1000; Invitrogen) for 2hrs at ambient temperature. The nucleus was stained by DAPI (Invitrogen). The control section ran under the same protocol, but the major antibodies were omitted.
Western Blot Analysis
After rapid brain removal, the tissue was separated from the brain and homogenized on ice with a micro-content electric tissue homogenizer (KIMBLEKONTES, USA) in frozen lysis buffer (1×PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS, RIPA) containing protease inhibitors. Lysates underwent the centrifugation at 4°C for 20 min at 10,000 × g, and the authors collected the supernatants. With the use of a Bradford protein assay, the concentrations of protein were ascertained. Protein with equal amounts (30 µg) underwent the separation using the SDS-PAGE and electroblotted on nitrocellulose filter (NC) membrane (Millipore). After non-specific antibody binding was blocked with 5% non-fat dry milk, membranes underwent the incubation at 4°C overnight with monoclonal rabbit anti-TH (1:2000; Millipore), anti-iNOS (1:1000; Proteintech), anti-Arg-1(1:1000; BD). After being washed in TBST, the immunoblots underwent the incubation with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) for 1 h. Using enhanced chemiluminescence (ECL) reagents (Millipore, USA), the immunoblots were developed, and they underwent the measurement using Quantity Software (Bio-Rad, CA). For the comparison of protein loading, antibody directed against GAPDH was employed.
The Measurement of Striatal Biogenic Amines
The striatum samples were weighed and homogenized in 10 volumes of 0.2 M perchloric acid that contains 100 ng/mL isoproterenol and 100 µM EDTA-2Na (the internal standard for measuring the content of 5-HT and catecholamine) for 20 s in a homogenizer at a maximum setting on ice. After 30 min in the mixture of ice and water, the homogenate was centrifuged at 4°C for 20 min at 15,000 × g. For adjusting the pH value to nearly 3.0, 1 M sodium acetate solution was added. After filtration (0.45 µm), the samples were injected in an HPLC system.
Using an HPLC-electrochemical detection system, 5-hydroxytryptamine (5-HT), noradrenaline (NE), homovanillic acid (HVA), dihydroxyphenylacetic acid (DOPAC) and DA were detected. In brief, 20 µL of the homogenate sample was injected to the HPLC-electrochemical detection system using an L-2200 autosampler (HITACHI, Tokyo, Japan) at 4°C, and then the sample underwent the separation at 25°C on a reverse-phase analytical column (EICOMPAK SC-5ODS, 3.0 mm×150 mm; Eircom, Kyoto, Japan), the elution with 0.1 M sodium acetate/citric acid (pH 5.4) that contained 5 mg/L EDTA-2Na, 190 mg/L sodium 1-octanesulfonate, and 17% methanol, and ascertained by an 2465 electrochemical detector (Waters, Milford, MA, USA) at 30°C at a flow rate of 0.5 mL/min. With the use of a computer with Millennium32 Chromatography Manager Software (Waters), the chromatograms were recorded and studied.
Cytokine ELISA Assay
The mentioned culture supernatants yielded here were collected to cytokine concentrations of IL-1β, IL-4, IL-6, IL-10, TNF (Multisciences, China), and the secretion of neurotrophic factor BDNF (Promega, USA) in the brain by a sandwich ELISA kits in line with the instructions of the manufacturer. The determinations were conducted in duplicate. The results are denoted as pg/mL.
Lentivirus Transfection
To explore the possible routes of migration, an emerald green fluorescent protein (eGFP) lentivirus controlled by a cytomegalovirus promoter was employed. The virus was stored at −80°C and the final virus titer was calculated to be 4.35 ×106 infectious units/mL. MSCs were transfected at 80% confluence. The culture medium underwent the aspiration and discard, and the cells were washed two times in PBS. Serum-free medium (5 mL) was mixed with 1 mL virus for every 0.8–1.0 ×106 cells, giving a multiplicity of infection of approximately five. The cells underwent the incubation at 37°C for 4–5 hrs. DMEM containing 20% FBS/1% PS (5 mL) was added and the cells incubated overnight. In the other morning, the cells were washed two times in PBS and replaced the medium.
Results
Characteristics of BMSCs
Cell surface markers of BMSCs were verified (Supplementary Figure S2). According to flow cytometry analysis, these cells expressed CD105 (99.53%), CD44 (99.87%) and Sca-1 (97.81%) surface markers but were CD11b (0.12%), CD34 (0.42%) and CD45 (0.15%) negative, showing the consistency with the typical surface markers of the BMSCs undifferentiated. The lack of expression of CD34 and CD45 revealed that the hematopoietic stem cells’ population was depleted. After 72 hrs of primary cell culture, adherent cells with long fusiform were observed, and about 14–17 days later, cells reached confluence.
The Effect of Fasudil Pretreatment on BMSCs
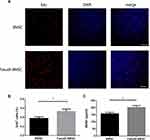
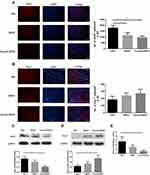
We detected the role of fasudil in the proliferation of BMSCs using EdU staining. The result of EdU staining showed that fasudil obviously promoted the proliferation of BMSCs at 72 h compared with nontreated cells (Figure 1A and B, P < 0.05).
Extracellular release of BDNF was measured using ELISA. After treatment with fasudil for 72 hrs, release of BDNF showed a remarkable increase with respect to control (Figure 1C, P < 0.05).
Behavioral Alterations in MPTP PD Mice
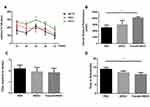
The rotarod test and pole test were performed for examining the motor activity in the 1st month after BMSCs instillation in the mice (Figure 2).
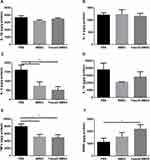
The rotarod performance was measured as 5 consecutive trials for 3 groups. In comparison with the PBS group, the stay time on the rod at 24 rpm was obviously up-regulated in Fasudil-BMSC mice. The ORP score on rotarod test in Fasudil-BMSC group significantly increased (p<0.05) in comparison with the PBS group. In the pole test, a sensitive approach to determine bradykinesia of the MPTP PD mice, time to descend on pole test significantly reduced in Fasudil-BMSC mice (p<0.05). Taken together, BMSCs preconditioned with fasudil obviously promoted motor dysfunction in comparison with the PBS group. However, BMSCs alone did not significantly improve motor dysfunction.
Dopamine Neuron Loss in the Substantia Nigra and the Olfactory Bulb
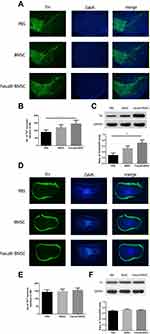
The loss of dopamine neurons in the area of the substantia nigra (SN) is a typical pathological event of PD. BMSCs preconditioned with fasudil protected TH+ DA neurons in the substantia nigra pars compacta (SNpc) when compared with PBS group (Figure 3A and B, P < 0.05). Mice treated with BMSCs and PBS had no obvious difference in the loss of DA neurons. We further ascertained the levels of Nigral TH using Western blot in all mice, and similar results were observed (Figure 3C, P < 0.01).
The OB, containing considerable dopamine neurons, is likely to be covered in the pathogenesis of PD. Accordingly, by immunostaining and Western blot analysis, the expression of TH in the OB was investigated. No difference of dopamine neuron loss in the OB was observed (Figure 3D–F).
Striatal Biogenic Amines in the Brain
To explore whether intranasal BMSCs affect the neurotransmitters, DA levels and its major metabolites (namely NE, HVA, and DOPAC), as well as NE, 5-HT were investigated by HPLC assay at the 1st month after cell application (Figure 4). The concentration of DA detected in Fasudil-BMSC group was significantly higher than in PBS group (p<0.05), and the release of neurotransmitters including DOPAC and 5-HT in the striatum was remarkably promoted in Fasudil-BMSC group compared with PBS group (DOPAC, p<0.01; 5-HT, p<0.01). The levels of DA, DOPAC, HVA, NE and 5-HT showed no obvious difference between BMSC and PBS group.
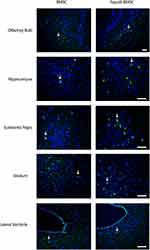
The Distribution of BMSCs in the Brain
One month later, the mice were sacrificed and the brain was removed for immunostaining to detect the distribution of GFP-BMSCs. Intranasal application of GFP-MSCs resulted in the appearance of cells in the hippocampus, olfactory bulb, substantia nigra, striatum and lateral ventricle (Figure 5).
M1 and M2 Microglia in the Brain
M1 and M2 microglial activation are capable of exerting either conducive or detrimental influences on dopamine neuron injury. iNOS and Arg-1 represent M1 and M2 microglia/macrophages, respectively. M1 iNOS expression and M2 Arg-1 expression in brain were measured by immunostaining. Figure 6A and B show that intranasal BMSC or Fasudil-BMSC suppressed iNOS expression in SN (p<0.05). Arg-1 expression was higher in Fasudil-BMSC and BMSCs group compared with PBS group, but no significant difference was found.
Subsequently, M1 iNOS expression and M2 Arg-1 expression in the brain were measured by Western blot. Figure 6C–E show that iNOS expression was decreased (p<0.05), Arg-1 expression was increased (p<0.05), and iNOS/Arg-1 ratio was obviously decreased (p<0.05) in Fasudil-BMSC group compared with PBS group.
Inflammatory Cytokines in the Brain
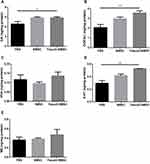
According to neuroinflammation contributes to neuronal degeneration in PD extensively investigated in many studies, the levels of inflammatory cytokines in the brain were compared. The release of IL-6 (p<0.01), TNF-α (p<0.05) was reduced in Fasudil-BMSC group compared with PBS group, and IL-6 (p<0.05), TNF-α (p<0.05) significantly lower in BMSC group in comparison with PBS group (Figure 7A–E).
The Secretion of BDNF in the Brain
Given the important role of BDNF in neuronal cell survival, the effect of BMSCs on inducing such neurotrophic factor was determined. Figure 7F shows that the secretion of BDNF in the brain was significantly enhanced (p<0.05) in Fasudil-BMSC group in comparison with PBS group.
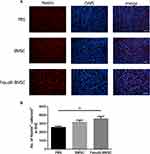
Nestin+ Cells in the Subventricular Zones (SVZ)
To determine whether BMSCs delivery stimulates endogenous neurogenesis after cell transplantation, the expression of Nestin, a neuronal precursor cell marker, was observed. The Nestin+ cells in SVZ were increased in Fasudil-BMSC group compared with PBS group (p<0.01), to promote the mobilization of endogenous stem cells (Figure 8).
Discussion
This study demonstrated that BMSCs when intranasally administered were capable of surviving and migrating to the brain. Fasudil pretreatment was applied to the transplanted cells, which improved the therapeutic capability of BMSCs.
The routes of MSCs delivery into the brain remain a challenge: intravenous injection, where MSCs may be trapped in the organs other than the brain, whereas direct injection into the CNS (such as transcranial or intraventricular injection), is quite invasive. Conversely, intranasal delivery of MSCs effectively bypasses the BBB and rapidly enters the brain, minimizing its distribution. Therefore, intranasal delivery of MSCs may be a non-invasive and safe CNS targeted therapy and a promising option for the treatment of neurological diseases.20 The pathways and mechanisms of intranasal delivery of MSCs from the nasal passages to the brain are not yet understood. In our study, intranasal application of GFP-MSCs was observed in the OB, hippocampus, substantia nigra, striatum and lateral ventricle. Some previous studies also observed that stem cells appeared along the olfactory pathway.11,21,22 Two branches of MSCs migration from the nasal mucosa into the CNS have been demonstrated by some studies:7 The first branch is partly related to the rostral migratory stream (RMS), the known migration path of neural progenitor cells from the SVZ to OB; The stem cells injected into the OB have been demonstrated to migrate bidirectionally using the RMS. The second branch of OR travels along the surface of the brain through cerebrospinal fluid and then into the brain parenchyma. Nevertheless, further research is essential to elucidate the potential migration routes of MSCs after intranasal administration and to determine the fate of cells in the brain after prolonged administration.
BMSCs are ideal candidate cells for cell transplantation therapy, because of their accessibility for amplified culture, immunosuppressive properties, pluripotent potential, and allograft potential thereby eliminating ethical considerations.23,24 An increasing number of evidences show that BMSCs have therapeutic effects on PD.6 However, our study indicates that there is no significant improvement with BMSCs transplantation alone, which is not consistent with the previous findings of Danielyan et al.11 According to Danielyan et al, the behaviour of 6-hydroxydopamine (6-OHDA)-lesioned PD rats improved by intranasal delivery of BMSCs and their TH level increased after 4.5 months. This discrepancy between the two studies was perhaps due to different animal models and the varying duration of treatment in the two research. We chose 1 month as the time point for our study because it has previously been demonstrated that at this time point there is an apparent decrease in striatal dopamine after MPTP administration.25,26 Indeed, we observed that merely intranasal BMSCs had a tendency to attenuate behavioural impairment, protect dopaminergic neurons in MPTP PD mice, and downregulate the levels of proinflammatory cytokines in the brain. A longer evaluation duration and multiple time points are necessary to observe accurate benefits.
BMSCs transplantation by itself might not be adequate for functional recovery and nerve regeneration; combined intervention was required to improve graft survival and efficiency of BMSCs and to increase their therapeutic potential in the targeted organ. Fasudil, a ROCK inhibitor, is getting increasing attention for its ability to promote optimisation of transplanted cells. ROCK is vital for the basic processes of cell proliferation and survival. Blockade of ROCK promotes the mobilisation of adult brain neural stem cells and promotes the survival and proliferation of stem cells in vitro.27 Animal transplantation model showed that suppressing Rho and/or ROCK can prevent acute apoptosis of grafted cells.28 Accordingly, this study aimed to evaluate whether fasudil could enhance the therapeutic effect of BMSCs in MPTP mice by optimising transplanted cells. We found that fasudil accelerated the proliferation of BMSCs and promoted BDNF secretion in vitro. Intranasal delivery of BMSCs preconditioned with fasudil significantly improved the motor function and the expression of TH in the SN. It indicates that fasudil is an ideal candidate that can optimise BMSCs and further enhance the therapeutic capability of BMSCs transplantation in MPTP-PD mice.
Presently, many researchers accept that BMSCs promote regeneration mainly through the secretion of an array of trophic factors and cytokines.13 BDNF, in particular, is an important neurotrophic factor that promotes neurogenesis and angiogenesis. It plays a role in several stages of development of neural circuits including neural stem cell survival and differentiation, axonal growth, and refinement of developing circuits.29 BDNF, which is secreted from MSCs, elicits neurotrophic and neuroprotective effects on DA neurons.30 BDNF can also be secreted by endogenous neural stem cells and glial cells, which are activated by transplantation of MSCs.13 Our study demonstrated that the secretion of BDNF in the brain was significantly enhanced in the Fasudil-BMSC group. Elevated levels of BDNF in the brain appear to be one of the reasons for the protection/survival of existing dopaminergic neurons in the injured areas thereby resulting in functional and motor improvement.
Improved outcome after transplantation of BMSCs has also been attributed to the inhibition of inflammation.31 Many evidences show that microglial cell proliferation and inflammation are related to the pathogenesis of PD.32 Activated microglia are present in the SN and striatum of brains from both PD animal models and PD patients.13 iNOS and Arg-1 are two representatives of M1 and M2 microglia/macrophages. M1-polarized microglia/macrophages are capable of up-regulating the expression of iNOS and secreting inflammatory cytokines. M2-polarized microglia/macrophages are capable of up-regulating the expression of Arg-1 and increasing anti-inflammatory factors.33 In the study, Fasudil-BMSC treatment significantly suppressed the iNOS expression and up-regulated the Arg-1 expression, effectively reversed the ratio of iNOS/Arg-1 in the SN, indicating that inflammatory M1 was transformed to anti-inflammatory M2 microglia. The results might indicate that Fasudil-BMSC might protect against dopamine neurons by reversing inflammatory M1 to anti-inflammatory M2 cells. Moreover, here we show that the levels of IL-6, TNF-a were decreased in Fasudil-BMSC and BMSC group. According to the result, the anti-inflammatory and immunomodulatory effects of BMSCs may be part of the basis for the therapeutical effect in PD animal models.
In conclusion, our findings provide direct evidence that preconditioning with fasudil enhances the therapeutic potential of BMSCs transplantation in MPTP-PD mice when compared with BMSCs independently. We show that intranasal application of BMSCs preconditioned with fasudil can suppress immune response, reverse inflammatory M1 phenotype to anti-inflammatory M2 phenotype microglia, and reinforce neurotrophic factor BDNF secretion. Although further investigation is necessary to analyse the anti-inflammatory effect and neuroprotective effect of Fasudil-BMSC, the positive effects of BMSCs preconditioned with fasudil make it a promising cell-based therapy for PD.
Ethical Approval
The study was approved by the Ethics Committee of Fudan University Ethics Committee and conducted in accordance with the guidelines of the National Institutes of Health guide for the care and use of Laboratory animals.
Acknowledgment
This work was supported by grants (grant numbers: 81371413, 81801260, 81771372, 81571232) from the National Natural Science Foundation of China, and The Project (grant number: 2016YFC1306500) from Ministry of Science and Technology of China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang Y, Chen S, Yang D, Le WD. Stem cell transplantation: a promising therapy for Parkinson’s disease. J Neuroimmune Pharmacol. 2007;2:243–250. doi:10.1007/s11481-007-9074-2
2. Blandini F, Cova L, Armentero MT, et al. Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat. Cell Transplant. 2010;19:203–217. doi:10.3727/096368909X479839
3. Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci Lett. 2001;316:67–70. doi:10.1016/S0304-3940(01)02384-9
4. Hellmann MA, Panet H, Barhum Y, Melamed E, Offen D. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett. 2006;395:124–128. doi:10.1016/j.neulet.2005.10.097
5. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi:10.1002/(ISSN)1097-4644
6. Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871–3882. doi:10.1007/s00018-013-1290-8
7. Jiang Y, Zhu J, Xu G, Liu X. Intranasal delivery of stem cells to the brain. Expert Opin Drug Deliv. 2011;8:623–632. doi:10.1517/17425247.2011.566267
8. Wei N, Yu SP, Gu X, et al. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013;22:977–991. doi:10.3727/096368912X657251
9. Erdo F, Bors LA, Farkas D, Bajza A, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–170. doi:10.1016/j.brainresbull.2018.10.009
10. Bors L, Erdő F. Overcoming the blood–brain barrier. challenges and tricks for CNS drug delivery. Sci Pharm. 2019;87:6. doi:10.3390/scipharm87010006
11. Danielyan L, Schäfer R, von Ameln-mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14:3–16. doi:10.1089/rej.2010.1130
12. Li Y, Yu J, Xi J, et al. Fasudil enhances therapeutic efficacy of neural stem cells in the mouse model of MPTP-induced Parkinson’s disease. Mol Neurobiol. 2017;54:5400–5413. doi:10.1007/s12035-016-0027-8
13. Glavaski-Joksimovic A, Bohn MC. Mesenchymal stem cells and neuroregeneration in Parkinson’s disease. Exp Neurol. 2013;247:25–38. doi:10.1016/j.expneurol.2013.03.016
14. Chen M, Liu A, Ouyang Y, Huang Y, Chao X, Pi R. Fasudil and its analogs: a new powerful weapon in the long war against central nervous system disorders? Expert Opin Investig Drugs. 2013;22:537–550. doi:10.1517/13543784.2013.778242
15. Liu J, Gao HY, Wang XF. The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regen Res. 2015;10:1892–1896. doi:10.4103/1673-5374.170325
16. Compagnucci C, Barresi S, Petrini S, et al. Rho kinase inhibition is essential during in vitro neurogenesis and promotes phenotypic rescue of human induced pluripotent stem cell-derived neurons with oligophrenin-1 loss of function. Stem Cells Transl Med. 2016;5:860–869. doi:10.5966/sctm.2015-0303
17. Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722–732. doi:10.1002/(ISSN)1098-2795
18. Croze RH, Buchholz DE, Radeke MJ, et al. ROCK inhibition extends passage of pluripotent stem cell-derived retinal pigmented epithelium. Stem Cells Transl Med. 2014;3:1066–1078. doi:10.5966/sctm.2014-0079
19. Rizzino A. Stimulating progress in regenerative medicine: improving the cloning and recovery of cryopreserved human pluripotent stem cells with ROCK inhibitors. Regen Med. 2010;5:799–807. doi:10.2217/rme.10.45
20. Li Y, Feng L, Zhang G, Ma C. Intranasal delivery of stem cells as therapy for central nervous system disease. Exp Mol Pathol. 2015;98:145–151. doi:10.1016/j.yexmp.2015.01.016
21. Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(Suppl 1):S123–S139. doi:10.3727/096368914X684970
22. Galeano C, Qiu Z, Mishra A, et al. The Route by Which Intranasally Delivered Stem Cells Enter the Central Nervous System. Cell Transplant. 2018;27:501–514. doi:10.1177/0963689718754561
23. Volarevic V, Markovic BS, Gazdic M, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15:36–45. doi:10.7150/ijms.21666
24. Casiraghi F, Perico N, Cortinovis M, Remuzzi G. Mesenchymal stromal cells in renal transplantation: opportunities and challenges. Nat Rev Nephrol. 2016;12:241–253. doi:10.1038/nrneph.2016.7
25. Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi:10.1016/S0306-4522(96)00545-3
26. Jakowec MW, Nixon K, Hogg E, McNeill T, Petzinger GM. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J Neurosci Res. 2004;76:539–550. doi:10.1002/(ISSN)1097-4547
27. Ding J, Li QY, Yu JZ, et al. Fasudil, a Rho kinase inhibitor, drives mobilization of adult neural stem cells after hypoxia/reoxygenation injury in mice. Mol Cell Neurosci. 2010;43:201–208. doi:10.1016/j.mcn.2009.11.001
28. Koyanagi M, Takahashi J, Arakawa Y, et al. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res. 2008;86:270–280. doi:10.1002/(ISSN)1097-4547
29. Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi:10.1038/nrn3379
30. Singh M, Kakkar A, Sharma R, et al. Synergistic effect of BDNF and FGF2 in efficient generation of functional dopaminergic neurons from human mesenchymal stem cells. Sci Rep. 2017;7:10378.
31. van Velthoven CT, Sheldon RA, Kavelaars A, et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke. 2013;44:1426–1432. doi:10.1161/STROKEAHA.111.000326
32. Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi:10.1016/j.nbd.2009.11.004
33. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi:10.1172/JCI59643
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.