Back to Journals » Journal of Pain Research » Volume 12
Intramuscular electrical stimulus potentiates the motor cortex modulation effects on pain and descending inhibitory systems in knee osteoarthritis: a randomized, factorial, sham-controlled study
Authors da Graca-Tarragó M , Lech M, Dal Moro Angoleri L, Santos DS , Deitos A, Brietzke AP , Torres ILS , Fregni F, Caumo W
Received 21 July 2018
Accepted for publication 20 September 2018
Published 3 January 2019 Volume 2019:12 Pages 209—221
DOI https://doi.org/10.2147/JPR.S181019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Maria da Graca-Tarragó,1,2 Mateus Lech,2 Letícia Dal Moro Angoleri,2 Daniela Silva Santos,2 Alícia Deitos,1,2 Aline Patrícia Brietzke,1,2 Iraci LS Torres,1,3 Felipe Fregni,4 Wolnei Caumo1,2,5,6
1Post-Graduate Program in Medical Sciences, School of Medicine, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; 2Laboratory of Pain and Neuromodulation, HCPA, Porto Alegre, Brazil; 3Pharmacology Department, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; 4Department of Physical Medicine and Rehabilitation, Harvard Medical School, Boston, MA, USA; 5Surgery Department, School of Medicine, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; 6Pain and Palliative Care Service, HCPA, Porto Alegre, Brazil
Background: Neuroplastic changes in nociceptive pathways contribute to severity of symptoms in knee osteoarthritis (KOA). A new look at neuroplastic changes management includes modulation of the primary motor cortex by transcranial direct current stimulation (tDCS).
Objectives: We investigated whether tDCS combined with intramuscular electrical stimulation (EIMS) would be more efficacious than a sham (s) intervention (s-tDCS/s-EIMS) or a single active(a)-tDCS/s-EIMS intervention and/or s-tDCS/a-EIMS in the following domains: pain measures (visual analog scale [VAS] score and descending pain modulatory system [DPMS], and outcomes, and analgesic use, disability, and pain pressure threshold (PPT) for secondary outcomes.
Registration: The trial is registered in Clinical trials.gov: NCT01747070.
Methods: Sixty women with KOA, aged 50–75 years old, randomly received five sessions of one of the four interventions (a-tDCS/a-EIMS, s-tDCS/s-EIMS, a-tDCS/s-EIMS, and s-tDCS/a-EIMS). tDCS was applied over the primary motor cortex (M1), for 30 minutes at 2 mA and the EIMS paraspinal of L1–S2.
Results: A generalized estimating equation model revealed the main effect of the a-tDCS/a-EIMS in the VAS pain scores at end treatment compared with the other three groups (P<0.0001). There existed a significant effect of time and a significant interaction between group and time (P<0.01 for both). The delta-(Δ) pain score on VAS in the a-tDCS/a-EIMS group was –3.59, 95% CI: –4.10 to –2.63. The (Δ) pain scores on VAS in the other three groups were: a-tDCS/s-EIMS=−2.13, 95% CI: −2.48 to –1.64; s-tDCS/a-EIMS=−2.25, 95% CI: −2.59 to –1.68; s-tDCS/s-EIMS MR =–1.77, 95% CI: –2.08 to –1.38. The a-tDCS/a-EIMS led to better effect in DPMS, PPT, analgesic use, and disability related to pain.
Conclusion: This study provides additional evidence regarding additive clinical effects to improve pain measures and descending pain inhibitory controls when the neuromodulation of the primary motor cortex with tDCS is combined with a bottom-up modulation with EIMS in KOA. Also, it improved the ability to walk due to reduced pain and reduced analgesic use.
Keywords: osteoarthritis, electroacupuncture, pain pressure threshold, conditioned pain modulation, CPM, transcranial direct current stimulation, tDCS
Introduction
Knee Osteoarthritis (OA) is one of the world’s leading causes of disability in older patients.1 Also, a peripheral persistent inflammatory process and anatomic lesions can induce significant atrophy of gray matter in OA compared with controls.2,3 Along with pain severity, in the OA, there is also a reorganization in the somatotopy within the somatosensory and motor cortex.4 Likewise, there is a neuroplasticity state adjustment linked to an increased level of serum brain-derived neurotrophic factor (BDNF). This neurotrophin plays a critical role in activity-dependent modulation at cortical synapses, and it is also associated with the disinhibition of motor cortex excitability.5 Also, it is a marker of neuroplasticity state related to transcranial direct current stimulation (tDCS) effect.6 Hence, tDCS is a promising technique because it modulates the thalamocortical synapses in a top-down manner within pain pathways7 and it might change in thalamic inhibitory pathways, cingulate cortex, and periaqueductal gray matter induced by tDCS.8–10Also, it is supported by its efficacy to treat acute postoperative pain6 and chronic pain (eg, trigeminal neuralgia, phantom pain, and fibromyalgia).11
Likewise, meta-analyses have demonstrated the efficacy of needling (ie, acupuncture and electroacupuncture) to relieve pain in knee osteoarthritis (KOA).12,13 Also, intramuscular electrical stimulation (EIMS), which differs from acupuncture in its theoretical basis. It is a technique that uses the insertion of needles with electrical stimulation into the motor points, local and paravertebral muscles.14 Several studies have reported that EIMS can be an effective modality for musculoskeletal pain (ie, myofascial pain syndrome, chronic tensional headache, upper trapezius muscle, and lower back pain).15,16 Additionally, recently we found that in severe KOA, one session of EIMS led changes in the motor cortex excitability, such as reduced intracortical facilitation and increased the cortical silent period.17
Thus, the most promising areas of research give theoretical and experimental support to ongoing testing of a combined therapy to provide best protocols to maximize the clinical effect of tDCS and EIMS. Extant literature provides evidence that the tDCS modulates the thalamocortical synapses by top-down effects through of neuronal and synaptic activity, as well as in neuronal network dynamics, with an impact on deep brain structures in ways that modify cortical excitability;18 while the EIMS modulates pain processing by bottom-up effects, activating inhibitory systems by stimulating afferent Ab and Ad fibers fibers19 as well through the enkephalinergic, serotonergic, and noradrenergic inhibitory systems.20 Notwithstanding, it persists a gap to investigate if the tDCS effects can be optimized when combined with peripheral electrical neuromodulatory approaches.
Thus, we tested the hypothesis that the combined therapy (active (a) -tDCS/a-EIMS) would be more efficacious than sham (s) interventions (s-tDCS/s-EIMS) or single interventions (a-tDCS/s-EIMS and/or s-tDCS/a-EIMS) in the following domains: pain measures (visual analog scale [VAS] score and descending pain modulatory system [DPMS]) (primary outcomes), analgesic use, disability, and pain pressure threshold (PPT) (secondary outcomes). Also, we examined whether the effect of EIMS and tDCS on PPT could be associated with the serum level of the BDNF.
Patients and methods
Design overview, setting, and participants
The Research Ethics Committee at the Hospital de Clínicas de Porto Alegre approved the protocol according to the Declaration of Helsinki. All patients provided oral and written informed consent before participating in this randomized, double blind, factorial design, four-groups, parallel clinical trial. De-identified data relating to intervention and primary outcomes will be made available on request to Caumo W ([email protected]) with no time restriction.
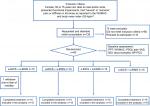
Our patients were recruited from the general population through the referrals from chronic pain specialists and from physiatrists of our institution, as well as by free posters in different hospital services and mass-dissemination newspapers. Inclusion criteria: women 50–75 years old, right-handed to minimize potential bias related to laterality, since we applied the tDCS on the motor cortex in the brain hemisphere contralateral to treated knee. In addition, they needed to report moderate-to-severe pain and stiffness of the knee, and functional impairment without significant relief with medication. The symptoms should have been present for at least 6 months. At baseline, a physiatrist with over 10 years of experience in treating OA evaluated the radiographs of knees and the degree of OA by the Kellgren–Lawrence (K–L) grading scale.21 This scale is validated and has high reproducibility grading KOA.22 Grading of 3–4 K–L has been included. The interview at baseline consisted of the Western Ontario and McMaster Universities (WOMAC) Index. Patients were eligible if they graded pain as moderate, severe, or extreme in at least one of five issues: pain when lying down, sitting, standing, walking, or climbing stairs.23 They should present stiffness in the morning or late in the day and report moderate, severe, or extreme difficulty in at least 1 of 17 questions. Exclusion criteria: unable to read and patients with a body mass index >35 kg/m2, since excessive weight could interfere with the capacity to walk and, consequently, the outcomes measures. Also, subjects using corticosteroids, with other concomitant rheumatology, orthopedic or neurological diseases, or with other uncompensated chronic diseases (ie, ischemic heart disease, kidney or hepatic insufficiency), were excluded. Additionally, we excluded patients with previous surgery on the affected area in the last 6 months, previous experience with acupuncture, or underwent to physical therapy in the last 30 days. The flowchart of the study is presented in Figure 1.
Interventions
Patients were randomly assigned to receive four intervention groups: a-tDCS/a-EIMS (n=15), a-tDCS/s-EIMS (n=15), s-tDCS/a-EIMS (n=15), and s-tDCS/s-EIMS (n=15). They received five treatment sessions over consecutive days. Immediately after they received one session of a-tDCS or s-tDCS, all patients received one session of a-EIMS or s-EIMS.
a-tDCS
The a-tDCS anodal was applied in the primary motor cortex (M1) contralateral to the treated knee and the cathode in the contralateral supraorbital region. Five sessions were held, lasting 30 minutes, with an intensity of 2 mA, ramp-up and ramp-down duration of 30 seconds, with a current density of 0.057 mA/cm2.24 The rubber electrodes used were attached to a sponge soaked in 0.9% saline and had a size of 35 cm2 (5 cm vs 7 cm). The distance maintained between the electrodes was 7–8 cm. The sponges were changed for every patient to minimize the adverse effect.
s-tDCS
Sham stimulation was performed the same way as the active stimulation, but the tDCS device was prepared to turn off after 30 seconds of ramp-up, mimicking the feel of active stimulation to the patient, as a longitudinal clinical trial had previously validated.25
Active electrical (a-EIMS)
This study uses acupuncture needles with guide tubes (DongBang Acupuncture, Inc., Chungnam, Korea), 40 mm in length and 0.25 mm in diameter. The EIMS was applied to the needles with an electroacupuncture device (Sikuro, São Paulo, Brazil) with a constant current at a frequency of 2 Hz and intensity adjusted to the patient’s tolerability during 30 minutes. The location of the EIMS is based on sensitized roots by chronic pain in KOA and the affected muscles.26 Twelve needles were deeply inserted along the spinous processes maintaining a distance from the spinous process line of 2 cm at L1–S2 (nerve roots involved in the knee) and to the corresponding dermatomes at the following correspondent’s anatomic sites: vast medial, rectus femoris, vast lateral, anterior tibialis muscles, and the pes anserine bursae.17
Sham (s-EIMS)
Patients also received five sessions of 30 minutes. The electroacupuncture device (Sikuro) was prepared so that no electrical stimulation passed to the patient, but it remained on with the diode blinking, promoting visible and audible electrical stimulation. Rubber electrodes were placed on the sites where the needles were placed.
Instruments and assessments
Outcomes
The primary clinical outcome was the VAS pain score and the main quantitative sensory testing outcome was the DPMS function assessed by the changes in the numerical pain scale (NPS) (0–10) during the conditioned pain modulation (CPM) task. The secondary outcomes were the WOMAC index, PPT, and analgesic use.
Primary outcomes
i) VAS was used to measure pain intensity, ranging from 0 mm (no pain) to 100 mm (worst pain, the worst pain possible).
ii) CPM task was a conditioning stimulus. It consists of immersing the nondominant hand in cold water (0°C–1°C) for 1 minute. In the 30 final seconds, we measured the initial PPT1 in the dominant arm (test stimulus), considering the sensation of pain as 6 of 10 by the patient. Before the CPM task, we measured PPT0, obtaining the value of referred pain as 6 of 10 by the patient. The CPM task activates the diffuse noxious inhibitory-control-like effect. The efficiency of DPMS was evaluated by subtracting the PPT1 from the PPT0, where negative values indicate inhibitory of the DPMS.27 We chose the right arm for test stimulus, because it is consistent with the paradigm studied in most of the existing literature. Additionally, a previous study showed that the arrangement of the test stimulus and conditioned stimulus do not influence the CPM28
Secondary outcomes
iii) PPT: We used an electronic algometer (J-Tech Medical Industries, Midvale, UT, USA) to perform the tests. Before completing the trial, patients were advised to differentiate the sensations of pressure and pain. Patients were instructed to alert when experiencing pain onset verbally. We carried out three successive measurements at 3–5-minute intervals. According to a previous study, a lower PPT in the patellar tendon was a predictor of pain intensity and disability scores in KOA. We measured the PPT in the patellar tendon of the leg that we applied the EIMS, that is the sclerotomal hyperalgesia.26
iv) WOMAC index was used to assess the functional capacity related to pain, stiffness, and difficulties during daily activities. The answers refer to the symptoms of the last 48 hours. The score is obtained as follows: pain (0–20), stiffness (0–8), and physical function (0–68). The global score adds the scores of the three subscales; the maximum score is 96.29
v) They registered the analgesic use during the treatment period in a diary. To run the analysis we classified the analgesic intake in two categories: if they used at least three times a week or if they used painkillers more than three times a week.
Other instruments and assessment control for potential confounding factors
All questionnaires applied were validated for the Brazilian population. We used the Pittsburgh Sleep Quality Index to assess the sleep quality, Beck Depression Inventory to evaluate depressive symptoms and the Brazilian Portuguese Catastrophizing Scale to measure the catastrophizing of pain.30–32 Demographic data and medical comorbidities were determined using a standardized questionnaire. At the baseline and the end treatment, blood samples were collected to measure serum BDNF. These blood samples were centrifuged at 4,500× g in plastic tubes for 10 minutes at 4°C and stored at –80°C for hormone assay. An ELISA using a ChemiKine BDNF Sandwich ELISA Kit, CYT306 (Chemicon/Millipore, Billerica, MA, USA) was used to determine the serum BDNF. The lower detection limit of the kit is 7.8 pg/mL for BDNF.
Use of analgesics at baseline was computed from the average weekly intake in the last 3 months. In data analysis, we considered the analgesic intake as a dichotomous variable, according to two categories: analgesic use at least three times a week or analgesic use more than three times a week. This approach was adopted because patients with chronic pain take rescue analgesics erratically, and the frequency of use varies weekly depending on the pain level.
Sample size
We defined the sample size by a difference with clinical relevance between interventions based on the VAS pain score according to previous study.33 To reject the null hypothesis, we accepted an error type I of 5%. Thus, for an error type II of 10%, we needed 52 patients divided into four balanced groups (n=13) in a 1:1:1:1 ratio to test 1.25 points in the mean difference of VAS (0–100 mm) between groups. The effect size (f) for this difference was 0.25, as determined by ANOVA for repeated measures of five measures (post-randomization period) (1.25/5) and a 0.5 variation coefficient. Taking into account multiple outcomes and possible losses, the final sample size was 60 patients (15 per group).34
Randomization and allocation concealment
Randomization was generated in blocks of four. Before the recruitment phase, randomization was created using a computer system by researchers who did not administer the intervention. They set the sequence in separately sealed envelopes. The envelope was opened only by the physician who would apply the intervention and only at the time of administering it for one of the four groups: 1) a-tDCS/a-EIMS, 2) a-tDCS/s-EIMS, 3) s-tDCS/a-EIMS and 4) s-tDCS/s-EIMS.
Blinding
The authors took the following measures to avoid bias: The same physician applied all the interventions, and the participants were instructed to clarify their doubts regarding interventions only with the doctor who applied the interventions; participants had no opportunity to meet each other. Two evaluators blind to randomization administered the scales. Furthermore, to assess whether blinding was adequate, we asked participants at the treatment end to guess whether they had received a-tDCS/a-EIMS, a-tDCS/s-EIMS, s-tDCS/a-EIMS, and s-tDCS/s-EIMS and to rate their confidence in the treatment on a Likert scale with five categories (no confidence to completely confident).
Statistical analysis
The mean differences between the interventions groups at baseline were assessed using ANOVA, and categorical variables were examined using chi-square or Fisher’s exact and Kruskal–Wallis tests. The values are presented as the mean (SD) or frequency. Continuous variables were tested for normality using the Shapiro–Wilks test.
Generalized estimating equations (GEEs) were conducted with an exchangeable working correlation structure to account for correlation between the sessions from a single participant to examine the changes in the outcome measures (VAS pain score, change on NPS [1–10] during CPM task, PPT, and WOMAC) between four experimental groups.35 The factors were the interventions groups (ie, a-tDCS/a-EIMS, a-tDCS/s-EIMS, s-tDCS/a-EIMS, and s-tDCS/s-EIMS) and the time. We used a GEE model to analyze the treatment effect according to the group (ie, a-tDCS/a-EIMS, a-tDCS/s-EIMS, s-tDCS/a-EIMS, and s-tDCS/s-EIMS) on the VAS pain score during the 5 days of the treatment period. In this GEE model, the treatment group was the factor, the pain scores on VAS the dependent variable, and the analgesic use the covariate. The magnitude of the effect of treatment group on the number of analgesics used throughout the 5 days of treatment was presented by the number needed to treat (NNT). The Bonferroni’s test was used to adjust the differences for multiple comparisons. We lost four daily assessments in one patient (1.33%), which was not a significant value to influence the analysis of the dataset. Spearman’s correlation analysis was used to analyze the correlation between BDNF and PPT. All analyses were performed with two-tailed tests at the 5% significance level. The data were analyzed using SPSS version 24.0 (SPSS, Chicago, IL, USA).
Results
Patients’ characteristics
Patients’ demographic and clinical characteristics are shown in Table 1. Fifty-nine patients completed the study; one patient in the a-tDCS/a-EIMS group withdrew because of fear of needles. There was no significant difference between groups. There were no significant side effects, and minor side effects due to tDCS were reported by <12% of patients, which was similar between groups.
The rates of patients that assumed to receive active interventions were the following: A total of 87.5% in the a-tDCS/a-EIMS, 78.6% in the a-tDCS/s-EIMS, 86.7% in the s-tDCS/a-EIMS, and 73.3% in the s-tDCS/s-EIMS. Whereas, 2 (20%) patients in the a-tDCS/s-EIMS and 2 (20%) in the s-tDCS/s-EIMS reported that they did not know their treatment (χ2= 3.81; P=0.70). Regarding confidence rates in the treatment classified as confident or completely confident were the following: A total of 86.7% in the a-tDCS/a-EIMS, 73.3% in the a-tDCS/s-EIMS, 83.3% in the s-tDCS/a-EIMS, and 73.3% in the s-tDCS/s-EIMS, (χ2= 12.19; P=0.43).
Analysis of the treatment effect on the primary outcomes
Pain on VAS
A GEE model revealed the main effect for treatment group for the pain scores in the VAS. The a-tDCS/a-EIMS produced a higher reduction in the pain scores in the VAS compared with all three groups across the overtime (Wald χ2= 172.49, Df =3, P<0.0001) (see Figure 2). In addition, there was a significant main effect for time (Wald χ2=100.93, Df =4, P<0.001) and a significant time × group interaction (Wald χ2= 24.17, Df =12, P<0.01).
The within-subject test according to the treatment group analyzed by the GEE is presented in Table 2. We can see the main effect for treatment group in the pain scores in all groups (Wald χ2=8.69, Df =3, P<0.03) and a main effect for time (Wald χ2=13.41, Df =4, P<0.009), as well the time× group interaction (Wald χ2=37.90, Df =12, P<0.001). This analysis indicates that all groups showed significantly decreased the pain scores over time. However, the group that received two active interventions (a-tDCS/a-EIMS) showed the larger reduction in the VAS score (see Table 2). The effect size (ES) within the group-treatment measured by the VAS score was larger for the a-tDCS/a-EIMS group (d=1.86). The a-tDCS/a-EIMS group showed an increment in the ES of 34.4% compared with s-tDCS/a-EIMS group. The analysis revealed a main effect for treatment group in the analgesic doses used (Wald χ2=6.19, Df =1, P<0.01) and an interaction between the analgesic doses used× treatment group (Wald χ2=10.10, Df =3, P<0.01) (Table 2 shows the risk for the analgesic use and the NNT according to treatment group).
Effect on the descending modulatory system on CPM
A GEE model revealed the main effect for treatment group for the change in the NPS (0–10) during CPM task (Wald χ2=8.78, Df =3, P=0.03), and the main effect for time (Wald χ2=18.35, Df =1, P=0.00), and a significant time× group interaction (Wald χ2=20.01, Df =3, P=0.00). The combined active groups (a-tDCS/a-EIMS) showed a statistically significant increase in the function of DPMS compared with sham. The effect of a single active intervention used in the DPMS was not statistically different to sham. The change on the NPS (0–10) during the CPM task by the treatment groups is presented in Figure 3.
Analysis of the treatment effect on the secondary outcomes: PPT, WOMAC score, and analgesic consumption
Analgesic use during the week treatment
The analgesic use at least three times during the week treatment occurred in 74% of patients in the s-tDCS/s-EIMS and 45% of patients in the a-tDCS/a-EIMS. The relative risk (RR) for using analgesics was 0.60 (95% CI: 0.46 to 0.76), and the NNT was 3.33 (2.33–5.86). The analgesic use occurred in 69.2% in a-tDCS/s-EIMS. The RR for using analgesics at least three times during the week compared with a-tDCS/ a-EIMS was 0.65 (95% CI: 0.50 to 0.84), and the NNT was 4.16 (95% CI: 2.68 to 9.36). The analgesic use occurred in 49.3% in the s-tDCS/a-EIMS and the RR for using analgesic at least three times during the week compared with a-tDCS/ a-EIMS was 0.92 (95% CI: 0.68 to 1.23), and the NNT was 25 (95% CI: α to 5.61). It was possible to see that the groups that received a-EIMS had a higher reduction in the analgesic use, independent of being combined with a-tDCS or s-tDCS.
Treatment effect on PPT and WOMAC score
A GEE model revealed the main effect for treatment group for the PPT (Wald χ2=8.99, Df =3, P=0.03), and the main effect for time (Wald χ2=51.48, Df =1, P=0.00), and a significant time × group interaction (Wald χ2=2.72, Df =3, P=0.01). All three groups with an active treatment showed a statistically significant increase in the PPT (a-tDCS/a-EIMS, s-tDCS/a-EIMS, a-tDCS/s-EIMS) compared to s-tDCS/s-EIMS.
A GEE model showed a main effect for treatment group for the WOMAC scores (Wald χ2=8.48, Df =3, P=0.03), the main effect for time (Wald χ2=86.80, Df =1, P<0.001), and a significant time × group interaction (Wald χ2=27.02, Df =3, P<0.001). A considerable improvement in the WOMAC scores was observed in the group with combined active groups (a-tDCS/a-EIMS), with a difference statically significant than groups with single active treatment (a-tDCS/s-EIMS, s-tDCS/a-EIMS) or (s-tDCS/s-EIMS) (Table 3). The WOMAC scores decreased significantly in the groups that received single active intervention compared with s-tDCS/s-EIMS.
The relationship between serum BDNF and PPT at the end of treatment
The comparisons between groups are presented in Table 4. No significant differences in serum BDNF were observed between the intervention groups neither at baseline nor at the treatment end.
A higher level of serum BDNF at baseline was correlated negatively with the PPT at the end of treatment, independent of the intervention group. The scatterplot of the negative correlation between PPT and BDNF is presented in Figure 4. The Spearman correlation coefficient was r2=−0.37, and the 95% CI was –0.57 to –0.13, P<0.001. That is, patients with higher PPT presented a lower level of serum BDNF at the baseline or vice versa.
  | Figure 4 Scatterplot of the correlation between BDNF and pain pressure threshold in the intervention group (n=60). Abbreviations: BDNF, brain-derived neurotrophic factor. |
Discussion
Our findings provide evidence that our novel approach to applying a combined active interventions tDCS and EIMS over the M1 improved pain scores and the DPMS function. Also, it improved the disability and reduced analgesic use. Even though, when we evaluated the effect of each active treatment combined with their respective sham, both reached a better result compared with s-tDCS/s-EIMS in the pain scores and the disability due to pain. However, the improvement in the DPMS function during CPM task produced by each treatment separately did not reach statistical significance compared with the s-tDCS/s-EIMS group. Together, these data suggest that the combined active intervention (a-tDCS/a-EIMS) is more efficient to improve symptoms of KOA. Finally, we found that BDNF levels at baseline predicted a higher PPT at treatment end independent of the intervention group.
Our results extend data that the combination of two active interventions (a–tDCS with a–EIMS) was more effective to improve pain than each one used alone in KOA. It is important to stress that we observed this effect in OA, which has a pathophysiological mechanism distinct of the other chronic pain pathologies that applied to the tDCS combined with some other therapy, such as low back pain, fibromyalgia, and myofascial pain. In addition, this study was the first to investigate the effect of the tDCS combined with EIMS to treat KOA in a factorial design. These effects might be clinically relevant since they produced an improved pain and disability in a short term compared with each one individually. Accordingly, they emerge as a novel therapeutic possibility to treat KOA, and confirm our hypothesis that the combined therapy could be more effective because tDCS modulates in a top-down manner, whereas the EIMS modulates in a bottom-up. Also, they are aligned to results of previous studies, which found a better effect when the tDCS was combined with another therapy (ie, tDCS/trigger point injection in myofascial pain and tDCS/aerobic exercises in fibromyalgia).36–38 In the same way, in low back pain, it was found that the tDCS combined with the peripheral electrical stimulation resulted in 25% reduction for an immediate effect on recurrent back pain;38,39 whereas, in chronic low back pain, 12 sessions of tDCS simultaneously with transcutaneous electrical nerve stimulation (TENS) on nonconsecutive days produced a pain relief that lasted up to 3 months.40
In the present study, the tDCS was combined with EIMS, a technique based on the insertion of needles, that promotes effects for 72 hours given its effects on A delta fibers, thereby promoting activation of enkephalinergic , serotonergic, and noradrenergic systems;23 whereas in previous studies, the tDCS was used combined with the TENS,36–40 which activates cutaneous afferent fibers, and its effect on inflammation is possibly time-dependent.41 In the other way, EIMS activates the K+/Na+ pumps and can lead to increments of BDNF secretion.6,42 Its action promotes a reduction of N-methyl-D-aspartate receptor function and glutamate excitotoxicity.43 In the spinal cord, the BDNF modulates fast excitatory (glutamatergic) and inhibitory (GABAergic/glycinergic) signals, as well as slow peptidergic neurotransmission. It also regulates the expression of neuronal genes involved in the central sensitization process through transcription factors, such as c-fos and c-Jun.44
According to our findings, our combined approach (bottom-up with EIMS and top-down with tDCS) induced a synergistic effect strengthening the descending inhibitory pain system. Also, the result that a-tDCS combined with a-EIMS was better than every single active intervention may be explained by the occurrence of a priming effect, when one intervention favors a response to another.38,45 According to the literature, a-tDCS promotes long-term potential at the cortical level and increases the excitability based on default effects, whereas the EIMS induces long-term depression (decreased excitability) at the spinal level.45,46 Thus, the possible hypothesis is that these results occur due to the principles of homeostatic metaplasticity, where long-term potentiation comes from low neural activity, which is a mechanism of the tDCS effect. Additionally, the modulation of the synaptic plasticity threshold with a-tDCS/a-EIMS optimizes the neurophysiological effects of these two techniques toward increased neuronal excitability at M1 and greater analgesic efficacy.47 Few potential mechanisms can be discussed to explain this synergistic effect, such as, 1) one of the primary mechanisms would be the restoration of inhibitory/excitatory balance by promoting excitation in both peripheral and central areas. In fact, it has been shown that chronic pain syndromes are associated with a lack of inhibitory activity that can be reestablished by increased excitation that leads to secondary inhibition; 2) the changes in cortical excitability promoted by these two interventions may promote the release of endogenous opioids.9,48 Thus, the increase in the effect of a-EIMS in the sequence of a-tDCS on the pain neural networks can interfere with functional connectivity, synchronization, and oscillatory activities in cortical and subcortical networks.49 This result suggests that both interventions, in combination, led to stronger activation in the systems involved in pain processing (ie, opioidergic, glutamatergic, noradrenergic, GABAergic, etc.); however, how the combined therapy affects these systems in the long term needs to be investigated by future research.
We observed that the BDNF levels at baseline were correlated negatively with the PPT at the end of treatment, independent of the intervention group. Although the explanation for this finding is not clear, the BDNF levels are higher in situations of inflammation and persistent pain, where it might play a pro-nociceptive role.50,51 According to a previous study, serum levels of BDNF were higher in patients with KOA than in healthy patients of the same age.52 In other chronic pain conditions, such as fibromyalgia, our group already had demonstrated a negative correlation between BDNF and PPT.53 We hypothesize that the more dysfunctional patients have higher levels of BDNF, a more disinhibited descending inhibitory system, a lower PPT, and pain with less responsiveness to treatment.
Several issues concerning the design of our study should be addressed: first, when they were asked about the tDCS use, <12% of patients guessed the intervention correctly. Thus, it is improbable that unblinding could change the directions of our conclusions. Second, although we found immediate pain relief for these patients, we believed that more sessions would lead to better and long-lasting effects.54 However, future studies with more sessions and long-term follow-up will still be needed to allow us to assess the long-term clinical impact of these treatment possibilities in KOA. Third, we included only women because KOA’s prevalence is higher in females. Hence, we cannot extrapolate our findings for men. Fourth, the formal assessment of awareness of group allocation (either active or sham) and the high level of confidence in the treatment demonstrated that the sham method seemed to have blinded patients’ effectively.
Conclusion
This study provides additional evidence regarding additive clinical effects to improve pain measures and descending pain inhibitory controls when the neuromodulation of the primary motor cortex with tDCS is combined with a bottom-up modulation with EIMS in KOA. Also, it improved the ability to walk due to reduced pain and reduced analgesic use.
Acknowledgments
This research was supported by grants and material support from the following Brazilian agencies: Committee for the Development of Higher Education Personnel – CAPES - PNPD/CAPES (Coordenação de Aperfeicoamento de Pessoal de Nível Superior - Programa Nacional de Pós Doutorado [National Post Doctorate Program]; grants to Alicia Deitos and Aline Patrícia Brietzke and material support); the National Council for Scientific and Technological Development - CNPq (grants to Iraci LS Torres and Wolnei Caumo); the Postgraduate Program in Medical Sciences at the School of Medicine of the Federal University of Rio Grande do Sul (material support); the International Cooperation Program – CAPES (023/11) (to Wolnei Caumo and Felipe Fregni); the Postgraduate Research Group at the Hospital de Clínicas de Porto Alegre FIPE-HCPA (Fundo de Incentivo a Pesquisa e Eventos/Hospital de Clínicas de Porto Alegre; material support); the Foundation for Support of Research at Rio Grande do Sul (FAPERGS [Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul]; material support); and the Brazilian Innovation Agency (FINEP [Financiadora de Estudos e Projetos]); process number 1245/13 to Iraci LS Torres and Wolnei Caumo).
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Issa SN, Sharma L. Epidemiology of osteoarthritis: an update. Curr Rheumatol Rep. 2006;8(1):7–15. | ||
Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–4007. | ||
Schmidt-Wilcke T, Gänssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28(1):1–4. | ||
Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. | ||
Dussán-Sarria JA, da Silva NRJ, Deitos A, et al. Higher cortical facilitation and serum BDNF are associated with increased sensitivity to heat pain and reduced endogenous pain inhibition in healthy males. Pain Med. 2018;19(8):1578–1586. | ||
Ribeiro H, Sesterhenn RB, Souza A, et al. Preoperative transcranial direct current stimulation: exploration of a novel strategy to enhance neuroplasticity before surgery to control postoperative pain. A randomized sham-controlled study. PLoS One. 2017;12(11):e0187013. | ||
Stagg CJ, O’Shea J, Kincses ZT, Woolrich M, Matthews PM, Johansen-Berg H. Modulation of movement-associated cortical activation by transcranial direct current stimulation. Eur J Neurosci. 2009;30(7):1412–1423. | ||
García-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83(2):259–273. | ||
Maarrawi J, Peyron R, Mertens P, et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 2007;69(9):827–834. | ||
Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain. 2012;153(12):2359–2369. | ||
David M, Moraes AA, Costa MLD, Franco CIF. Transcranial direct current stimulation in the modulation of neuropathic pain: a systematic review. Neurol Res. 2018;40(7):557–565. | ||
Zhang Q, Yue J, Golianu B, Sun Z, Lu Y. Updated systematic review and meta-analysis of acupuncture for chronic knee pain. Acupunct Med. 2017;35(6):392–403. | ||
Chen N, Wang J, Mucelli A, Zhang X, Wang C. Electro-acupuncture is beneficial for knee osteoarthritis: the evidence from meta-analysis of randomized controlled trials. Am J Chin Med. 2017;45(5):965–985. | ||
Kim TH, Lee CR, Choi TY, Lee MS. Intramuscular stimulation therapy for healthcare: a systematic review of randomised controlled trials. Acupunct Med. 2012;30(4):286–290. | ||
Couto C, de Souza IC, Torres IL, Fregni F, Caumo W. Paraspinal stimulation combined with trigger point needling and needle rotation for the treatment of myofascial pain: a randomized sham-controlled clinical trial. Clin J Pain. 2014;30(3):214–223. | ||
Chassot M, Dussan-Sarria JA, Sehn FC, et al. Electroacupuncture analgesia is associated with increased serum brain-derived neurotrophic factor in chronic tension-type headache: a randomized, sham controlled, crossover trial. BMC Complement Altern Med. 2015;15:144. | ||
Tarragó MG, Deitos A, Brietzke AP, et al. Descending control of nociceptive processing in knee osteoarthritis is associated with intracortical disinhibition: an exploratory study. Medicine. 2016;95(17):e3353. | ||
Lang N, Siebner HR, Ward NS, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22(2):495–504. | ||
Baldry P. Management of myofascial trigger point pain. Acupunct Med. 2002;20(1):2–10. | ||
Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. | ||
Ravaud P, Giraudeau B, Auleley GR, et al. Radiographic assessment of knee osteoarthritis: reproducibility and sensitivity to change. J Rheumatol. 1996;23(10):1756–1764. | ||
Ravaud P, Auleley G, Amor B, Dougados M. Radiographic assessment of progression in knee osteoarthritis. Rheumatol Eur. 1995;24:129–132. | ||
Nunes G, de Castro LV, Wageck B, Kume V, Chiesa GS, de Noronha M. Translation into portuguese of questionnaires to assess knee injuries. Acta Ortop Bras. 2013;21(5):288–294. | ||
Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56–92. | ||
Brunoni AR, Vanderhasselt MA, Boggio PS, et al. Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology. 2013;38(1):58–66. | ||
Imamura M, Imamura ST, Kaziyama HH, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum. 2008;59(10):1424–1431. | ||
Willer JC, de Broucker T, Le Bars D. Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. J Neurophysiol. 1989;62(5):1028–1038. | ||
Klyne DM, Schmid AB, Moseley GL, Sterling M, Hodges PW. Effect of types and anatomic arrangement of painful stimuli on conditioned pain modulation. J Pain. 2015;16(2):176–185. | ||
Berrada K, Abouqal R, Allali F. FRIO133-normative values for the Western Ontario and MCMaster Universities in the general population compared with knee osteoarthritis patients. Ann Rheumatol Disord. 2011;70(3):88. | ||
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. | ||
Warmenhoven F, van Rijswijk E, Engels Y, et al. The Beck Depression Inventory (BDI-II) and a single screening question as screening tools for depressive disorder in Dutch advanced cancer patients. Support Care Cancer. 2012;20(2):319–324. | ||
Sehn F, Chachamovich E, Vidor LP, et al. Cross-cultural adaptation and validation of the Brazilian Portuguese version of the pain catastrophizing scale. Pain Med. 2012;13(11):1425–1435. | ||
da Graca-Tarragó M, Deitos A, Patrícia Brietzke A, et al. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med. 2016;17(5):877–891. | ||
Morgan TM, Case LD. Conservative sample size determination for repeated measures analysis of covariance. Ann Biom Biostat. 2013;1(1):1–6. | ||
Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. | ||
Choi YH, Jung SJ, Lee CH, Lee SU. Additional effects of transcranial direct-current stimulation and trigger-point injection for treatment of myofascial pain syndrome: a pilot study with randomized, single-blinded trial. J Altern Complement Med. 2014;20(9):698–704. | ||
Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci. 2016;10:68. | ||
Schabrun SM, Jones E, Elgueta Cancino EL, Hodges PW. Targeting chronic recurrent low back pain from the top-down and the bottom-up: a combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul. 2014;7(3):451–459. | ||
Boggio PS, Amancio EJ, Correa CF, et al. Transcranial DC stimulation coupled with TENS for the treatment of chronic pain: a preliminary study. Clin J Pain. 2009;25(8):691–695. | ||
Hazime FA, Baptista AF, de Freitas DG, et al. Treating low back pain with combined cerebral and peripheral electrical stimulation: A randomized, double-blind, factorial clinical trial. Eur J Pain. 2017;21(7):1132–1143. | ||
Desantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10(6):492–499. | ||
Muro M, Nagata A, Sakuma C, Moritani T, Yona M, Sakamoto K. Observation of high and low frequency muscle fatigue by means of phosphorus-31 NMR. Ann Physiol Anthropol. 1986;5(2):89–96. | ||
Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord. 2015;174:432–440. | ||
West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3(12):921–931. | ||
Giordano J, Bikson M, Kappenman ES, et al. Mechanisms and effects of transcranial direct current stimulation. Dose Response. 2017;15(1):155932581668546. | ||
Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120(2):482–503. | ||
Müller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neuroscientist. 2015;21(2):185–202. | ||
Dossantos MF, Ferreira N, Toback RL, Carvalho AC, Dasilva AF. Potential mechanisms supporting the value of motor cortex stimulation to treat chronic pain syndromes. Front Neurosci. 2016;10:18. | ||
Shafi MM, Westover MB, Fox MD, Pascual-Leone A. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur J Neurosci. 2012;35(6):805–825. | ||
Merighi A, Salio C, Ghirri A, et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85(3):297–317. | ||
Klein K, Aeschlimann A, Jordan S, Gay R, Gay S, Sprott H. ATP induced brain-derived neurotrophic factor expression and release from osteoarthritis synovial fibroblasts is mediated by purinergic receptor P2X4. PLoS One. 2012;7(5):e36693. | ||
Simão AP, Mendonça VA, de Oliveira Almeida TM, et al. Involvement of BDNF in knee osteoarthritis: the relationship with inflammation and clinical parameters. Rheumatol Int. 2014;34(8):1153–1157. | ||
Zanette SA, Dussan-Sarria JA, Souza A, Deitos A, Torres IL, Caumo W. Higher serum S100B and BDNF levels are correlated with a lower pressure-pain threshold in fibromyalgia. Mol Pain. 2014;10(1):46. | ||
Castillo-Saavedra L, Gebodh N, Bikson M, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain. 2016;17(1):14–26. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.