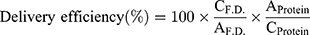Back to Journals » International Journal of Nanomedicine » Volume 15
Intracellular Distribution of Lipids and Encapsulated Model Drugs from Cationic Liposomes with Different Uptake Pathways
Authors Takikawa M , Fujisawa M , Yoshino K, Takeoka S
Received 17 June 2020
Accepted for publication 31 August 2020
Published 29 October 2020 Volume 2020:15 Pages 8401—8409
DOI https://doi.org/10.2147/IJN.S267638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Masato Takikawa,1 Mizuki Fujisawa,2 Kazuma Yoshino,2 Shinji Takeoka2,3
1Department of Advanced Science and Engineering, Graduate School of Advanced Science and Engineering, Waseda University, Tokyo 169-8555, Japan; 2Department of Life Science and Medical Bioscience, Graduate Schoolof Advanced Science and Engineering, Waseda University (TWIns), Tokyo 162-8480, Japan; 3Institute for Advanced Research of Biosystem Dynamics, Waseda Research Institute for Science and Engineering, Waseda University, Tokyo 169-8555, Japan
Correspondence: Shinji Takeoka
Department of Life Science and Medical Bioscience, Graduate School of Advanced Science and Engineering, Waseda University (TWIns), Shinjuku-ku, Tokyo 162-8480, Japan
Tel +81-3-5369-7324
Fax +81-5369-7324
Email [email protected]
Aim: The uptake pathway of liposomes into cells is mainly via endocytosis or membrane fusion; however, the relationship between the uptake pathway and the intracellular pharmacokinetics of the liposome components remains unclear. This study aimed at revealing the relationship by using cationic liposomes having similar physical properties and different uptake pathways.
Materials and Methods: We prepared cationic liposomes composed of amino acid-type lipids, K3C14 and K3C16, which have different uptake pathways by a hydration method, and fluorescently modified them by encapsulating FITC-dextran and surface conjugation with Alexa Fluor® 488 (AF488). Then, we investigated their intracellular distribution in HeLa cells over time.
Results: The liposomes had similar physical properties and did not cause significant cell mortality after treatment for 180 min. The delivery rate and efficiency of encapsulated FITC-dextran with the fusogenic K3C16 liposomes were 3 and 1.6 times higher, respectively, than with the endocytic K3C14 liposomes. FITC-dextran molecules delivered with K3C16 liposomes were observed throughout the cytosolic space after 10 min, while those delivered with K3C14 liposomes were mainly observed as foci and took 60 min to diffuse into the cytosolic space. K3C14 lipids modified with AF488 were distributed mostly in the cytosolic space. In contrast, fluorescently labeled K3C16 lipids were colocalized with the plasma membrane of 50% of the HeLa cells after 10 min and were gradually internalized intracellularly.
Conclusion: Fusogenic K3C16 liposomes internalized into HeLa cells faster than endocytic K3C14 liposomes, and their components differently distributed in the cells.
Keywords: cationic liposome, amino lipid, intracellular delivery, endocytosis, membrane fusion, intracellular pharmacokinetics
Introduction
There are various types of bioactive macromolecules, such as peptides, proteins, nucleic acids, and polysaccharides, in living cell systems. Recent studies have demonstrated that new therapeutics using biomacromolecules can regulate cellular functions, leading to disease treatments with high efficacy.1,2 The in vitro or ex vivo delivery of biomacromolecules are well-known investigative approaches toward developing treatments for various types of diseases,3 for instance, in immunotherapy and regenerative medicine.4–6 The performance of such therapeutics, including drug delivery systems, is usually related mainly to their bioavailability and the efficacy at the target sites, and both these factors are strongly related to the pharmacokinetics.7,8
Liposomes, vesicles composed of lipid bilayers, can be used as drug delivery systems, which encapsulate and embed hydrophilic or hydrophobic drugs.9,10 Liposomes are usually taken up intracellularly via endocytosis, where liposomes pass through endosomes and lysosomes,11,12 and need membrane fusion or lipid mixing with the endosomal or lysosomal membranes to intracellularly release encapsulated compounds. Recently, several studies have reported liposomes that can deliver encapsulated compounds directly into the cytosol via membrane fusion rather than via endocytic pathways.13–15 Some studies have revealed a relationship between the uptake pathways and the intracellular pharmacokinetics of the liposomal compositions, and there are rare combinations of liposomes having similar lipid compositions but different uptake pathways.
In previous work, we have developed cationic liposomes composed of various types of cationic lipids bearing an amino acid as the head group.16,17 We have also demonstrated that some of these cationic liposomes could efficiently deliver bioactive compounds into various types of cell lines, with limited cytotoxicity, leading to the exhibition of bioactivity in the living cells.18,19 In particular, cationic liposomes composed of a lysine head group, a propyl spacer, and ditetradecyl tail chains (K3C14) exhibited prominent cellular internalization and lysosome rupture, which is important for intracellular delivery, while retaining the function of the encapsulated compounds.20 In contrast, cationic liposomes composed of K3C16, which has tail chains that are just two carbons longer than K3C14, displayed cellular internalization via membrane fusion.21
Herein, we report the effect of the uptake pathways of cationic liposomes composed of amino acid-type lipids on the intracellular pharmacokinetics of the liposomal compounds, including the lipids and the encapsulated compounds. Cationic liposomes, composed of K3C14 or K3C16, with different uptake pathways were fluorescently labeled on the liposome surface, or by encapsulation of a fluorescent compound, and their intracellular distributions were analyzed over time using confocal laser scanning microscopy.
Materials and Methods
Materials
The synthesis of the cationic lipids, 1,5-ditetradecyl-N-lysil-N-trityl-L-glutamate (K3C14) and 1,5-dihexadecyl-N-lysil-N-trityl-L-glutamate (K3C16), was performed as previously reported.17 HeLa cells were purchased from the Japanese Collection of Research Bioresource Cell Bank (Osaka, Japan). Alexa Fluor® 488 (AF488) NHS ester (succinimidyl ester) and LysoTracker® Red DND-99 were purchased from Invitrogen (Carlsbad, CA, USA), and DAPI (4,6-diamidino-2-phenylindole) was purchased from PromoCell (Heidelberg, Germany). FITC-dextran (MW 40 kDa) was from Sigma-Aldrich (St Louis, MO, USA).
Preparation of Cationic Liposomes
Cationic liposomes were prepared by a hydration method, followed by size control with an extruder (LIPEX® Extruder, Transferra, Burnaby, Canada). For the study of the delivery efficiency and localization of encapsulated compounds, cationic liposomes encapsulating FITC-dextran were prepared. Cationic lipids (K3C14, K3C16: 5 mg/mL) were hydrated with FITC-dextran solution [5 mg/mL in HEPES buffer (20 mM, pH 7.4)] overnight at room temperature (rt), followed by the size control with the extruder at 50°C (final membrane pore size: 0.10 μm). Unencapsulated FITC-dextran was removed with ultracentrifugation (33,000 rpm, 30 min, 4°C; 2×) (Optima™ LE-80K Ultracentrifuge, Beckman Coulter; Brea, CA, USA), and finally, the liposome pellets were resuspended gently in HEPES buffer by pipetting several times. For the study of the localization of cationic lipids after being taken up by cells, cationic liposomes modified with fluorescent probes on their surface were prepared according to the protocol previously reported.21 Briefly, cationic lipids were hydrated with HEPES buffer (20 mM, pH 7.4) overnight at rt, followed by size control with the extruder at 50°C (final membrane pore size: 0.10 μm). The lipids were stirred with a 5% lipid molar concentration of AF488 NHS ester dissolved in DMSO, for conjugation with the primary amines of the cationic lipids, at rt in the dark for 1.5 h. The mixed solution was then filtrated with an Amicon Ultra centrifugal filter (MWCO: 100 kDa), and the resulting pellets were suspended in HEPES buffer by pipetting.
Characterization of Cationic Liposomes
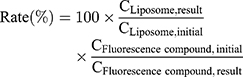
The liposomes were characterized in terms of their size, polydispersity index (PDI), and zeta potential using a Zetasizer, Nano-ZS90 (Malvern, UK). The liposome concentrations were determined using fluorescamine, which reacts with the first amine on the head group of cationic lipids, leading to fluorescence emission at 495 nm. The amount of encapsulated FITC-dextran was evaluated by measuring the fluorescent intensity at 520 nm with a fluorometer (RF-5300PC, Shimadzu Corporation, Japan), and the amount of AF488 conjugated with the cationic liposomes was evaluated by measuring the fluorescent intensities (em: 480 ± 20 nm, ex: 528 ± 20 nm) with a microplate reader (Powerscan HT, BioTek Instruments; Winooski, VT, USA). The rate of the FITC-dextran encapsulation and the modification with AF488 were calculated using the following formula;
where 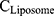 and
and 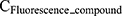 are the concentrations of the cationic liposome (μg/mL) and the fluorescent compound (FITC-dextran, AF488) (μg/mL), respectively. All samples were stored at 4°C until used, and they were used within 3 days after preparation.
are the concentrations of the cationic liposome (μg/mL) and the fluorescent compound (FITC-dextran, AF488) (μg/mL), respectively. All samples were stored at 4°C until used, and they were used within 3 days after preparation.
Cationic liposomes were observed using transmission electron microscopy (TEM) (JEM-1230, JEOL, Japan) to investigate their shape and morphology following a previously reported protocol.19 Briefly, liposome samples were dropped onto copper grids (COL-C15, Okenshoji, Japan) and were stained with samarium acetate (Sigma-Aldrich; St Louis, MO, USA). Imaging was conducted after the complete evaporation of the buffer.
For the stability testing of the cationic liposomes, all samples were stored at 4°C for 5 days after preparation. The liposomes were ultracentrifuged and resuspended again after the preparation or the storage, and their physical properties were determined in terms of size, PDI, zeta potential, and the leakage of FITC-dextran.
Cytotoxicity Testing
The cytotoxicity of the cationic liposomes was evaluated as previously described.19 Briefly, 5×103 HeLa cells were seeded into a 96-well cell culture plate and incubated with DMEM containing 10% fetal bovine serum (FBS) overnight at 37°C in an atmosphere of 5% CO2. Then, the cells were treated with cationic liposomes (fc 100 to 500 μM) for 180 min and were washed with heparan sulfate (20 U/mL) containing phosphate-buffered saline (PBS) three times. Considering that the uptake of liposomal compounds via membrane fusion occurs quite soon after the treatment, the treatment time was set at a maximum of 180 min, and the cytotoxicity was evaluated at this time. The cytotoxicity was evaluated using the WST-1 assay (Takara Bio, Shiga, Japan) following the manufacturer’s protocol. Statistical difference was determined by one-way analysis of variance (ANOVA), followed by a Tukey–Kramer post hoc test.
Intracellular Delivery of Liposome-Encapsulated FITC-Dextran to HeLa Cells
For the evaluation of the delivery efficiency of encapsulated FITC-dextran, 1×105 HeLa cells were seeded on cell culture dishes (φ 35 mm) and were incubated overnight. After the HeLa cells were treated with the cationic liposomes encapsulating FITC-dextran (fc, FITC-dextran: 50 μg/mL) for 10, 20, 30, 60, 120, and 180 min, the cells were washed with cold PBS containing heparan sulfate (20 U/mL), followed by lysing with 0.5% Triton X-100 dissolved in PBS. In this experiment, the concentration of the FITC-dextran was fixed; however, the concentration of each lipid was different (K3C14: 94.8 μM, K3C16: 73.5 μΜ) because the encapsulation efficiencies of the liposomes were slightly different. The amount of FITC-dextran taken up by the HeLa cells was calculated by measuring the fluorescent intensity in the solution containing the lysed cells using a microplate reader as described above, and the concentration of the cellular protein was also determined using a Pierce 660 nm protein assay following the manufacturer’s protocol. The calibration curves for the concentrations of FITC-dextran and protein were prepared using the FITC-dextran used in the liposome preparation and BSA contained in the protein measurement kit, respectively. The delivery efficiency was calculated from the following equation:
where 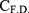 and
and 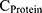 are the concentrations of FITC-dextran (μg/mL) and the protein (μg/mL) after liposome treatment, and
are the concentrations of FITC-dextran (μg/mL) and the protein (μg/mL) after liposome treatment, and 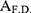 and
and 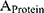 are the amount of FITC-dextran and protein per 1.0x105 cells, respectively.
are the amount of FITC-dextran and protein per 1.0x105 cells, respectively.
Statistical analysis was performed with a two-way ANOVA, followed by a Tukey–Kramer post hoc test. Data with P < 0.05 were considered to be statistically significant.
Intracellular Distribution of FITC-Dextran with Cationic Liposomes
For the evaluation of the intracellular delivery of FITC-dextran, 5×104 HeLa cells were seeded onto glass-bottom dishes (φ 35 mm) and were incubated overnight. The medium was exchanged with DMEM containing the cationic liposomes encapsulating FITC-dextran (fc, FITC-dextran: 50 μg/mL, K3C14: 94.8 μM, K3C16: 73.5 μΜ), and the cells were incubated for 10, 20, 30, 60, 120, and 180 min. At each time point, the medium was removed, and the cells were washed three times with cold PBS containing heparan sulfate. After fixation with 4% paraformaldehyde and staining the nuclei with DAPI, the cells were observed with confocal laser scanning microscopy (CLSM).
To observe the localization of FITC-dextran in the cytosolic space, HeLa cells were treated with cationic liposomes at the concentrations given above for 150 min. LysoTracker Red DND-99 (f.c. 50 μM) was then added to the cells and they were incubated for another 30 min, followed by fixation and observation with CLSM.
Intracellular Distribution of the Cationic Lipids After Surface Modification with AF488
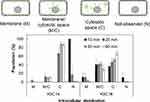
HeLa cells (1×105) were seeded onto glass-bottom dishes (φ 35 mm) and incubated overnight. The medium was exchanged with DMEM containing AF488-conjugated cationic liposomes (fc cationic liposomes: 100 μM), and the cells were incubated for 10, 20, 30, and 60 min. Then, the medium was removed, and the cells were washed three times with cold PBS containing heparan sulfate. After fixation with 4% paraformaldehyde, the cells were observed with CLSM. For the analysis of the cellular entry route, the localization of AF488-labeled lipids was evaluated using four categories: colocalization with the plasma membrane (M); colocalization with the plasma membrane and localization in the cytosolic space (M/C); localization in the cytosolic space (C); and not observed (N). Each analysis was conducted for approximately 120 cells per dish, and experiments were repeated in triplicate.
Results
Characterization of Cationic Liposomes
The size and zeta potential of the cationic liposomes containing K3C14 and K3C16 were 121 ± 35 nm and 43.2 ± 2.9 mV, and 139 ± 47 nm and 40.5 ± 1.1 mV, respectively (Table 1), and the PDI values of less than 0.20 indicated the liposomes did not aggregate in the dispersion. The encapsulation of FITC-dextran did not affect the physical properties of the liposomes; however, modification with AF488 NHS increased the size of the K3C14 liposomes from 139 to 193 nm because hydrophobic interactions of the AF488 moieties caused aggregation. Such a small change in liposome size with AF488 modification should not affect the cell internalization of K3C14 liposomes. The encapsulated amounts of FITC-dextran in the K3C14 and K3C16 liposomes were 4.2 and 3.5 mg/mL, respectively, when the lipid concentration was set at 5.0 mg/mL (Table 1). The encapsulation efficiencies of FITC-dextran were calculated as 26.1% and 25.3% for the K3C14 and K3C16 liposomes, respectively, and these values were not significantly different. The surface modification efficiencies of the K3C14 and K3C16 liposomes were 60.9% and 71.4%, respectively, and 3.05 and 3.57 mol% of the lipids were modified with AF488 NHS in the K3C14 and K3C16 liposomes, respectively. The shape and morphology of cationic liposomes composed of K3C14 and K3C16 were analyzed using TEM, and they were observed as round shape particles without aggregations (Figure S1). None of the samples showed precipitation or aggregation during storage at 4°C for at least 5 days, and there was no change in the physical properties nor was there leakage of encapsulated FITC-dextran (Table S1).
 |
Table 1 Characterization of Cationic Liposomes Encapsulating FITC-Dextran or Surface Modified with AF488 (n = 3) |
Cytotoxicity Testing
A WST-1 assay was performed to investigate the cytotoxicity of cationic liposomes composed of K3C14 and K3C16. No significant cell mortality was observed after 180 min treatment of these liposomes over the concentration range (100–500 μM), and there were no significant differences between their cytotoxicity at any doses (Figure 1).
Intracellular Distribution of FITC-Dextran Delivered by Cationic Liposomes
HeLa cells were treated with cationic liposomes encapsulating FITC-dextran, and the intracellular distribution of the FITC-dextran was observed under CLSM (Figure 2). In each case, the fluorescent intensity increased depending on the treatment time. When the HeLa cells were treated with K3C14 liposomes encapsulating FITC-dextran, the FITC-dextran was observed as fluorescent foci adjacent to the plasma membranes after 10 min. After 20 min, FITC-dextran was observed to be dispersed in the cytosol, especially around the plasma membranes, and after 30 min, the FITC-dextran was also observed near the nucleus. When the HeLa cells were treated with the K3C16 liposomes, the green fluorescent signals of FITC-dextran were observed throughout the cytosol after 10 min, and the positions of the nuclei were distinctly observed as holes. The fluorescent intensity increased uniformly throughout the cytosol with increasing treatment time up to 30 min, and the green fluorescent foci were partially observed when the treatment was continued for over 60 min.
In addition, the colocalization of FITC-dextran with LysoTracker Red DND-99 was observed after treatment for 180 min with the liposome samples. At this time, the LysoTracker DND-99 was observed strongly around the nucleus, and the yellow foci indicating the colocalization of FITC-dextran and LysoTracker DND-99 were observed, particularly in the HeLa cells treated with K3C14 rather than K3C16 liposomes.
Efficiency of the Intracellular Delivery of FITC-Dextran Using Cationic Liposomes
HeLa cells were treated with cationic liposomes encapsulating FITC-dextran, and the efficiency of the delivery of FITC-dextran using the cationic liposomes was evaluated at different time points (Figure 3). The delivery efficiency of both liposome samples increased with increasing treatment time. For the K3C14 liposomes, the delivery efficiency reached 4.5% after 60 min. For the K3C16 liposomes, the delivery efficiency reached 6.1% after 60 min. The delivery efficiencies of the liposomes were significantly different after 20 min (P < 0.05), at 20 min the K3C16 liposomes showed a delivery efficiency of 4.7%, which was almost equal to the efficiency at 60 min for the K3C14 liposomes. Hence, the K3C16 liposomes exhibited a higher delivery efficiency and higher rate of delivery than the K3C14 liposomes.
Intracellular Distribution of Cationic Lipids Modified with AF488
HeLa cells were treated with cationic liposomes modified at the surface with AF488, and the uptake behavior of the liposomes was analyzed by categorizing the lipid distribution in the cells (Figures 4 and S2). The intracellular fluorescent intensity was increased with increasing treatment time, as was observed with the liposomes encapsulating FITC-dextran.
When the HeLa cells were treated with K3C14 liposomes for 10 min, there were no fluorescent-positive cells. The percentage of HeLa cells that had K3C14 distributed only in the cytosolic space (C) increased with increasing treatment time until the total reached 90%, while the percentage of K3C14 distributed in the plasma membrane and in the cytosolic space (M/C) remained relatively constant at approximately 10%. With the K3C16 liposomes, 90% of the cells were fluorescent positive after 10 min, and K3C16 was observed to be colocalized in the plasma membranes of 50% of the cells (M, M/C). The percentage of M and M/C gradually decreased as the treatment time increased, and finally, there were almost no cells graded as M. Conversely, the percentage of cells graded as C continuously increased until 70% was reached.
Discussion
Cationic liposomes composed of K3C14 or K3C16 were evaluated in terms of the size, zeta potential, encapsulation efficiency for FITC-dextran, and the modification efficiency for AF488 NHS. The differences in the lipid type did not affect the physical properties of the liposomes, the encapsulation efficiency for FITC-dextran, or the modification efficiency with AF488 NHS. The encapsulation of FITC-dextran did not affect the physical properties of the liposomes. Surface modification of the K3C16 liposomes with AF488 NHS slightly increased the size and PDI, and decreased the zeta potential, of the liposomes. However, because only 3.57 mol% of the K3C16 lipids in the liposome membrane were modified with AF488, the surface modification did not change the liposome properties or cause severe aggregation. In contrast, although the modification ratio of the K3C14 liposomes (3.05 mol%) was similar to that of the K3C16 liposomes, the modification of the surface of the K3C14 liposomes with AF488 NHS increased the size and the PDI of the liposomes, but the size distribution did not change from a single peak. These results indicated that the K3C14 liposomes became slightly aggregated to create larger aggregates after the surface modification. We hypothesized that the surface modification of liposomes would possibly affect the liposome properties depending on the structure of lipid. The uptake pathway of nanoparticles has been reported to be severely affected by the size of the nanoparticles, and nanoparticles that have sizes in the order of several hundreds of nm are usually taken up via caveolae- or clathrin-dependent endocytosis.22,23 In the case of the K3C14 liposomes, the size difference before and after the surface modification with AF488 NHS was less than 100 nm, and we judged that such a difference would not alter the main uptake pathway of the K3C14 liposomes.
To analyze the intracellular delivery of FITC-dextran with K3C14 and K3C16 liposomes, HeLa cells were washed with PBS containing heparan sulfate to remove free cationic liposomes encapsulating FITC-dextran, or those modified with AF488, from the plasma membrane.24 Thus, the fluorescence observed in the peripheral region of the cells indicated that the fluorescent molecules were present inside the cell or in the plasma membrane. In addition, because the K3C14 and K3C16 liposomes were prepared with similar sizes, zeta potentials, and PDI values, the delivery efficiency and the intracellular distribution of the liposomal compounds were considered to depend only on the uptake pathway of the liposomes.
In the study, to investigate the delivery behavior of cationic liposomes, although the concentration of FITC-dextran treated to the cells were aligned, the concentration of K3C14 liposomes was 1.3 times higher than that of K3C16 liposomes because the liposomal encapsulation efficiency was quite affected by the lipid composition. However, the delivery rate and efficiency of K3C16 liposomes were 3 and 1.6 times higher, respectively, than those of K3C14 liposomes. Thus, we judged that the comparison of the delivery rates and efficiencies between these cationic liposomes is not overestimated and valid enough.
The delivery efficiency of the K3C14 liposomes reached a plateau after 60 min, although that of the K3C16 liposomes continued to increase even after 120 min. This difference is likely to be because the K3C14 liposomes were intracellularly taken up mainly via active transport (ie, endocytosis), while the K3C16 liposomes were taken up via both active and passive transport (ie, membrane fusion). HeLa cells treated with K3C14 liposomes showed green fluorescence near the plasma membrane after 10 min, and the intracellular distribution of FITC-dextran proceeded toward the perinuclear region as the treatment time increased. The green fluorescence up until 30 min was mainly observed as green foci, which is similar to previous studies using endocytic delivery systems.19,25 This occurs because the FITC-dextran delivered via endocytosis has not yet escaped from the endosomes at this time. After treatment for more than 60 min, the green fluorescence was detected throughout the cytosol, suggesting that the FITC-dextran had been gradually diffused in the cytosol.
With the K3C16 liposomes, the FITC-dextran was observed throughout the cytosol even after treatment for only 10 min, and the fluorescent intensity increased with increasing treatment time. This result indicated that the FITC-dextran molecules delivered by K3C16 liposomes via membrane fusion were directly released into the cytosolic space and dispersed without being trapped in the endosomes.
A previous study revealed that K3C14 liposomes had a higher potency than K3C16 liposomes in the ability to rupture endosomes and lysosomes during internalization in THP-1 cells.21 However, in this study, when HeLa cells were treated with LysoTracker Red DND-99, after treatment for 180 min with liposomes encapsulating FITC-dextran, the colocalization of LysoTracker Red DND-99 with FITC-dextran was observed more in the cells treated with K3C14 liposomes than with K3C16 liposomes. These results indicate that the colocalization with LysoTracker Red DND-99 observed after the treatment with K3C14 liposomes was with FITC-dextran trapped in endosomes or lysosomes. Furthermore, the colocalization was not strongly observed in the cells treated with K3C16 liposomes because the FITC-dextran was delivered mainly via membrane fusion, and was not delivered via endocytic pathways.
To evaluate the intracellular distribution of the liposomes composed of cationic lipids, the surface lipids of the cationic liposomes were modified with AF488, and the free AF488 NHS was removed by centrifugation. Thus, the green fluorescence observed in the cells treated with the AF488-modified liposomes was from the cationic lipids modified with AF488. When HeLa cells were treated with K3C14 liposomes, almost no cells fused with K3C14 lipids on the plasma membrane (M) were observed, and it took 20 min for the K3C14 lipids to be detected intracellularly. In contrast, 90% of cells showed intracellular localization of K3C16 lipids after 10 min (M, M/C, C), and 50% of the cells showed membrane fusion (M, M/C), indicating that the internalization of K3C16 liposomes occurred mainly via rapid membrane fusion. When the cells were treated with K3C14 liposomes, the percentage of cells categorized as N and C, with increasing time, decreased and increased, respectively, while the percentage categorized as M/C remained constant. This result indicated that the K3C14 liposomes were taken up mainly via endocytic pathways, and their fusogenic potential was quite low. Interestingly, when the cells were treated with K3C16 liposomes, the percentage of cells graded as M continuously decreased with time, and the percentage of cells graded as M/C was almost constant until a final decrease was observed. The percentage of cells graded as C kept increasing until a plateau was reached. Thus, the gradual variation in the cells in the different categories after treatment with the two types of liposomes can be summarized as follows: K3C14: I) N to M/C or C, II) M/C to C; K3C16: I) N to M or M/C or C, II) M to M/C, III) M/C to C. This sequence can be explained by the intracellular internalization of the cationic lipids fused with the plasma membrane. The fused lipids in the plasma membrane were internalized intracellularly during the endocytosis of other liposomes and the membrane recycling of the intracellular vesicles into the plasma membrane.26,27
Furthermore, in order to confirm the uptake pathways of cationic liposomes composed of K3C14 and K3C16, HeLa cells were treated with several types of endocytosis inhibitors, and the uptake behaviors of cationic liposomes were investigated. Interestingly, in some cases, the fusogenic potentials of K3C14 and K3C16 liposomes varied from the result without endocytosis inhibitors, and the fusogenic potential of K3C14 liposomes sometimes surpassed that of K3C16 liposomes (Figure S3). Considering some preliminary tests including above, we hypothesized that the fusogenic potential of our liposomes would be affected by i) liposomal membrane fluidity,28–30 ii) liposomal elasticity,31 iii) hydrophilic/hydrophobic balance of lipids composing liposomes,14,32 and iv) the membrane composition or fluidity of liposome-treated cells.33–35 The relationship between the lipid chemical structure and their liposomal fusogenic potential will be reported in the future reports.
Conclusion
Cationic liposomes with different uptake pathways were fluorescently labeled on the liposome surface, or by encapsulation of a fluorescent compound, and the intercellular pharmacokinetics of the liposomes were investigated in relation to their uptake pathways. The delivery rate and efficiency of fusogenic K3C16 liposomes were 3 and 1.6 times higher, respectively, than those of endocytic K3C14 liposomes. In addition, the fusogenic cationic lipids internalized into the cytosolic space after membrane fusion. This study provides useful information for intracellular pharmacokinetic studies using liposomal drug delivery systems.
Acknowledgments
This project was partially supported by the Leading Graduate Program in Science and Engineering, Waseda University from MEXT, Japan. We thank to Dr. Ueda (RIKEN Cluster for Pioneering Research) and the support by Materials Characterization Support Unit, RIKEN CEMS for TEM observation and the discussion. We also thank Victoria Muir, PhD, from Edanz Group for editing a draft of this manuscript.
Disclosure
S.T. is an inventor of the cationic lipid and reports a patent WO-A1-2008/062911 issued. The other authors declare no conflicts of interest in this work.
References
1. Dong L, Fang Y, Ning G. The smart drug delivery system and its clinical potential. Theranostics. 2016;6:1306–1323.
2. Xing H, Wang H, Wu B, Zhang Z. Lipid nanoparticles for the delivery of active natural medicines. Curr Pharm Des. 2017;23:1–9.
3. Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538:183–192. doi:10.1038/nature19764
4. Thurber GM, Yang KS, Reiner T, et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat Commun. 2013;4:1504.
5. Elgart V, Lin J-R, Loscalzo J. Determinants of drug-target interactions at the single cell level. PLoS Comput Biol. 2018;14:e1006601. doi:10.1371/journal.pcbi.1006601
6. Lee H, Fonge H, Hoang B, Reilly RM, Allen C. The effects of particle size and molecular targeting on the intratumoral and subcellular distribution of polymeric nanoparticles. Mol Pharm. 2010;7:1195–1208.
7. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi:10.1038/nrc1893
8. Leucuta SE. Subcellular drug targeting, pharmacokinetics and bioavailability. J Drug Target. 2014;22:95–115.
9. Kim J-S. Liposomal drug delivery system. J Pharm Investig. 2016;46:387–392.
10. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi:10.2147/IJN.S68861
11. Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145.
12. Straubinger RM, Hong K, Friend DS, et al. Endocytosis of liposomes and intracellular fate of encapsulated molecules: encounter with a low pH compartment after internalization in coated vesicles. Cell. 1983;32:1069–1079. doi:10.1016/0092-8674(83)90291-X
13. Sun L, Gao Y, Wang Y, et al. Guiding protein delivery into live cells using DNA-programmed membrane fusion. Chem Sci. 2018;9:5967. doi:10.1039/C8SC00367J
14. Kolašinac R, Kleusch C, Braun T, Merkel R, Csiszár A. Deciphering the functional composition of fusogenic liposomes. Int J Mol Sci. 2018;19:346.
15. Yang J, Bahreman A, Daudey G, et al. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent Sci. 2016;2:621–630.
16. Obata Y, Suzuki D, Takeoka S. Evaluation of cationic assemblies constructed with amino acid based lipids for plasmid DNA delivery. Bioconjugate Chem. 2008;19:1055–1063. doi:10.1021/bc700416u
17. Sarker SR, Arai S, Murate M, et al. Evaluation of the influence of ionization states and spacers in the thermotropic phase behaviour of amino acid-based cationic lipids and the transfection efficiency of their assemblies. Int J Pharm. 2012;422:364–373.
18. Sarker SR, Aoshima Y, Hokama R, Inoue T, Sou K, Takeoka S. Arginine-based cationic liposomes for efficient in vitro plasmid DNA delivery with low cytotoxicity. Int J Nanomed. 2013;8:1361.
19. Sarker SR, Takikawa M, Takeoka S. In vitro delivery of cell impermeable phallotoxin using cationic liposomes composed of lipids bearing lysine head group. ACS Appl Bio Mater. 2020;3:2048–2057. doi:10.1021/acsabm.9b01167
20. Li T, He J, Horvath G, Próchnicki T, Latz E, Takeoka S. Lysine-containing cationic liposomes activate the NLRP3 inflammasome: effect of a spacer between the head group and the hydrophobic moieties of the lipids. Nanomedicine. 2018;14:279–288. doi:10.1016/j.nano.2017.10.011
21. He J, Li T, Próchnicki T, Latz E, Takeoka S. Membrane fusogenic lysine type lipid assemblies possess enhanced NLRP3 inflammasome activation potency. Biochem Biophys Rep. 2019;18:100623.
22. Zhu J, Liao L, Zhu L, et al. Size-dependent cellular uptake efficiency, mechanism, and cytotoxicity of silica nanoparticles toward HeLa cells. Talanta. 2013;107:408–415. doi:10.1016/j.talanta.2013.01.037
23. Ha KD, Bidlingmaier SM, Liu B. Macropinocytosis exploitation by cancers and cancer therapeutics. Front Physiol. 2016;7:381.
24. McNaughton BR, Cronican JJ, Thompson DB, Liu D. Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins. Proc Natl Acad Sci. 2009;106:6111–6116.
25. Sarker SR, Hokama R, Takeoka S. Intracellular delivery of universal proteins using a lysine headgroup containing cationic liposomes: deciphering the uptake mechanism. Mol Pharm. 2014;11:164–174.
26. Sahay G, Querhes W, Alabi C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31:653–658.
27. Hsu VW, Prekeris R. Transport at the recycling endosome. Curr Opin Cell Biol. 2010;22:528–534.
28. Felgner PL, Kumar R, Sridhar CN, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J.Biol.Chem. 1994;269:2550–2561.
29. Xu Y, Szoka FC
30. Tokudome Y, Saito Y, Saito F, Kikuchi M, Hinokitani T, Goto K. Preparation and characterization of ceramide-based liposomes with high fusion activity and high membrane fluidity. Colloids Surf B Biointerfaces. 2009;73:92–96.
31. Guo P, Liu D, Subramanyam K, et al. Nanoparticle elasticity directs tumor uptake. Nat Commun. 2018;9:130. doi:10.1038/s41467-017-02588-9
32. Pogodin S, Werner M, Sommer JU, et al. Nanoparticle-induced permeability of lipid membranes. ACS Nano. 2012;6:10555–10561.
33. Lu M, Zhao X, Xing H, et al. Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int J Pharm. 2018;550:100–113. doi:10.1016/j.ijpharm.2018.08.040
34. Bompard J, Rosso A, Brizuela L, et al. Membrane fluidity as a new means to selectively target cancer cells with fusogenic lipid carriers. Langmuir. 2020;36:5134–5144.
35. Carradori D, Dos Santos A, Masquelier J, et al. The origin of neural stem cells impacts their interactions with targeted-lipid nanocapsules: potential role of plasma membrane lipid composition and fluidity. J Control Release. 2018;292:248–255.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.