Back to Journals » Clinical Ophthalmology » Volume 8
Intracameral phenylephrine and ketorolac injection (OMS302) for maintenance of intraoperative pupil diameter and reduction of postoperative pain in intraocular lens replacement with phacoemulsification
Authors Lindstrom R, Loden J, Walters T , Dunn S, Whitaker J, Kim T, Demopulos G, Tjia K
Received 20 June 2014
Accepted for publication 29 July 2014
Published 5 September 2014 Volume 2014:8 Pages 1735—1744
DOI https://doi.org/10.2147/OPTH.S69710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Richard L Lindstrom,1 James C Loden,2 Thomas R Walters,3 Steven H Dunn,4 J Steven Whitaker,5 Terry Kim,6 Gregory A Demopulos,5 Khiun Tjia7
1Minnesota Eye Consultants, Minneapolis, MN, USA; 2Loden Vision Centers, Goodlettsville, TN, USA; 3Texan Eye Care, Austin, TX, USA; 4Houston Eye Associates, Houston, TX, USA; 5Omeros Corporation, Seattle, WA, USA; 6Duke University Eye Center, Durham, NC, USA; 7Isala Clinics, Zwolle, the Netherlands
Background: The purpose of this study was to evaluate the effect of OMS302 on intraoperative pupil diameter and early postoperative ocular pain when administered during intraocular lens replacement surgery.
Methods: Four hundred and six patients (406 study eyes; 202 in the OMS302 group and 204 in the placebo group) were entered into this randomized, double-masked, placebo-controlled, multicenter Phase III study, which was conducted at 15 centers in the USA and the Netherlands. The patients received OMS302 (60.75 mM phenylephrine HCl and 11.25 mM ketorolac tromethamine) or placebo in irrigation solution during intraocular lens replacement. No other changes in procedure were required. Coprimary endpoints were change in pupil diameter over time from surgical baseline to end of procedure and patient-reported ocular pain during the first 12 hours postoperatively. Secondary endpoints included additional measures of pupil diameter and postoperative pain.
Results: OMS302 was superior to placebo in maintaining intraoperative mydriasis, preventing miosis, and reducing postoperative pain. The weighted mean (standard error) difference (OMS302 – placebo) in change in the area under the curve from baseline for pupil diameter was 0.590 ([0.049]; 95% confidence interval 0.494 to 0.686; P<0.0001). For ocular pain scores, the weighted mean (standard error) difference was –4.580 ([1.192]; 95% confidence interval –6.917 to 2.244; P=0.0002). All secondary efficacy results favored OMS302. Specifically, analyses supporting prevention of miosis (patients with ≥6 mm pupil diameter at completion of cortical clean-up and those with <6 mm diameter at any time during surgery) were significant for OMS302 (95.9% versus 77.0% and 9.2% versus 38.0%, respectively; P<0.0001 for each endpoint). OMS302 was well tolerated and not associated with any unexpected adverse events.
Conclusion: OMS302 maintained mydriasis, prevented miosis, and reduced early postoperative pain when administered in irrigation solution during intraocular lens replacement, with a safety profile similar to that of placebo. OMS302 is preservative-free and bisulfite-free, and its administration does not require any modification to the surgical procedure.
Keywords: intraocular lens replacement surgery, cataract, mydriasis, miosis, postoperative pain, OMS302
Corrigendum for this paper has been published
Introduction
Intraoperative lens replacement (ILR) for cataract extraction and lens replacement and for clear or refractive lens exchange is the most common surgical procedure performed in the USA.1 A leading cause of visual impairment worldwide, the prevalence of cataract increases with age,2 and surgical removal of the lens remains the only effective treatment for cataracts impairing visual acuity.3 Coupled with increasing life expectancy and population growth, the demand for ILR procedures is expected to increase dramatically in the next 20 years.
Sufficient mydriasis during ILR is critical to a successful surgical outcome by providing visualization of the surgical field, a good red reflex, and adequate room for intraocular manipulation of surgical instruments.4,5 However, because pupil dilation is typically achieved by application of topical mydriatic agents preoperatively,6,7 the initial mydriatic effect achieved may not be sustained throughout the procedure, particularly in cases where the surgical trauma itself induces intraoperative miosis through prostaglandin-related stimulation of the iris.7–9 Failure to maintain an adequate pupil diameter further increases the difficulty and risks (both intraoperative and postoperative) associated with the procedure.10–12 Although several alternative methods to maintain pupil dilation may be used, their implementation requires additional pharmacological measures and/or pupil expansion devices that may add complexity and time to the surgical procedure.13–15
Intraocular lens replacement may also be associated with postoperative pain. Results of a prospective study performed specifically to evaluate postoperative pain following cataract surgery reported that approximately one third of patients undergoing cataract surgery report ocular pain during the first 4 postoperative hours, with a quarter of these patients experiencing moderate to severe pain.16 The study also reported that over half of patients experienced postoperative eye symptoms, such as foreign body sensation, pruritus, and irritation, which are often interpreted as ocular findings rather than pain by ophthalmologists.17 As such, given the variable differences associated with each individual’s personal definition of pain and pain severity, the incidence of pain following cataract surgery may be higher than currently reported. Postoperative pain has been associated with clinical outcomes and patient well-being in many surgical settings, and is a primary concern for many surgeons.18
OMS302 is a bisulfite-free and preservative-free investigational drug developed for use during ILR to manage intraoperative pupil diameter and reduce postoperative ocular pain. OMS302 is added to the standard balanced saline irrigation solution bottle for intracameral irrigation in the anterior chamber during ocular surgery and requires no change in surgical procedure. OMS302 contains phenylephrine hydrochloride (PE), an α1-adrenergic receptor agonist, and ketorolac tromethamine (KE), a nonselective cyclo-oxygenase 1 and 2 inhibitor. These two agents are administered topically and used independently in ILR for pupil dilation and management of ocular pain, respectively.19,20 Pharmacologically, the α1-selective agonist activity of PE mediates contraction of the radial iris dilator muscles, causing dilation of the pupil with little or no cycloplegia. PE is not known to have any effect on pain. KE, by inhibiting both proinflammatory cyclo-oxygenase enzymes, inhibits prostaglandin synthesis resulting from surgical trauma, thereby reducing both postoperative pain and the occurrence of intraoperative miosis.21 Intracameral irrigation of OMS302 exposes target tissues to consistent concentrations of PE and KE throughout the entire procedure. In addition, unlike preoperatively administered topical drugs, which are washed out of the eye by the irrigation solution used during ILR, OMS302 continuously bathes the intraocular structures as part of the irrigation solution. This provides an opportunity to maintain the pharmacological effects of PE and KE. Intracameral PE is expected to maintain intraoperative mydriasis, while intracameral KE is expected to reduce postoperative pain and to contribute to prevention of intraoperative miosis.
Here we present the results of a Phase III clinical trial evaluating the effect of OMS302 compared with placebo on intraoperative pupil diameter and pain during the early postoperative period when administered in irrigation solution during phacoemulsification and ILR.
Materials and methods
This was a multicenter, randomized, parallel-group, double-masked, placebo-controlled Phase III study conducted at 15 ophthalmology clinics in the USA and the Netherlands. All sites received approval from an institutional review board or ethics committee. The study was performed in accordance with the ethical principles described in the Declaration of Helsinki, the Code of Federal Regulations, and the International Conference on Harmonisation, including maintenance of patient confidentiality and compliance with the United States Health Insurance Portability and Accountability Act. Informed consent was obtained from all participating patients. This study is registered at ClinicalTrials.gov as NCT01193127.
Study design
This study was designed to evaluate the effect of OMS302 on intraoperative pupil diameter and on early postoperative ocular pain in patients undergoing ILR. Eligible patients were randomly assigned to OMS302 or placebo (1:1) within one business day prior to surgery using a centralized interactive web response system. Randomization was stratified by Lens Opacities Classification II nuclear grade category and site using a permuted block design such that each block contained an equal number of patients per treatment group. Four milliliters of OMS302 (60.75 mM PE and 11.25 mM KE formulated in 20 mm sodium citrate buffer) or placebo (20 mm sodium citrate buffer) were added to 500 mL of the standard balanced saline irrigation solution used during the surgical procedure.
Study procedures were performed at screening, at baseline prior to surgery, on the day of surgery intraoperatively, and postoperatively at 2 hours, 4 hours, 6 hours, 8 hours, 10–12 hours, 24 hours, 48 hours, 7 days, 14 days, and 90 days. All patients received the following standardized preoperative topical treatments: antibiotic (Vigamox® four times daily for 3 days), mydriatic (one drop of PE 2.5% and one drop of tropicamide 1% at approximately 30 minutes, 15 minutes, and 5 minutes prior to surgery), and anesthetic (one drop of lidocaine or tetracaine delivered with the mydriatic agents). Viscoat® (sodium chondroitin sulfate and sodium hyaluronate) viscoelastic was used for all patients during surgery. Postoperatively, all patients continued the Vigamox regimen and received topical KE (beginning the day following surgery, after completion of coprimary postoperative pain endpoint collection) for at least 7 days. All patients were discharged with acetaminophen (paracetamol).
The recording of each operation by video photography through the operating microscope was standardized to start preincision and continue until wound closure. Each video included patient-specific scales of measurement obtained prior to the start of the procedure for use by a single, central reader who measured intraoperative pupil sizes at the time of first incision and every minute thereafter until wound closure. Treatment assignment was masked to all patients, investigators, the central reader of pupil diameters, and other study-related personnel.
Patients
Male and female patients 18 years of age or older scheduled to undergo unilateral primary cataract extraction and lens replacement or refractive lens exchange with a coaxial phacoemulsification device were eligible for this study. Patients were also required to have a best-corrected visual acuity of 20/400 or better in the non-study eye and intraocular pressure between 5 and 22 mmHg, inclusive, in the study eye. Key exclusion criteria included: presence of any connective tissue disorder; use of nonsteroidal anti-inflammatory drugs, cyclosporin, and/or ocular mast cell stabilizers within 7 days of surgery; use of monoamine oxidase inhibitors within 21 days of surgery; repeated use of pilocarpine in the study eye within 6 months of surgery; and history of use of an α1-adrenergic antagonist (eg, tamsulosin). Patients were also excluded if they had narrow-angle or unstable glaucoma, glaucoma being treated with prostaglandins or prostaglandin analogs, pseudocapsular exfoliation, uncontrolled chronic ocular diseases that could affect pupil dilation, active corneal pathology or scarring, extraocular/intraocular inflammation, and/or presence of active bacterial or viral infection in either eye; history of iritis or any ocular trauma with iris damage; or intraocular nonlaser surgery in the study eye within 3 months or intraocular laser surgery in the study eye within 30 days of surgery.
Endpoints and assessments
Two coprimary efficacy endpoints were evaluated: change in pupil diameter over time from surgical baseline (just prior to initial incision) to end of the surgical procedure (immediately following incision closure) and early postoperative pain as assessed by the patient at 2, 4, 6, 8, and 10–12 hours after surgery. Pupil diameter was measured by the central reader using still images of pupil diameter at one-minute intervals obtained from video recordings of each patient’s procedure. Ocular pain was measured postoperatively by a visual analog scale (VAS) using a 100 mm scale, where 0 represents no pain and 100 represents worst pain possible.22
Secondary efficacy endpoints included the categorical endpoints of number and percentage of patients with pupil diameter ≥6 mm at completion of cortical clean-up, pupil diameter <6 mm at any time during surgery, moderate-to-severe pain (VAS ≥40) at any time during the first 12 hours postoperatively, and who reported being pain-free (VAS =0) in the operated eye at all time points during the first 12 hours postoperatively. Safety evaluations included adverse event reports (solicited and voluntary), ophthalmological examinations (iris/pupil, lens status, eyelid erythema and edema, conjunctival erythema and edema, corneal staining and edema, intraocular pressure, and fundus findings), and vital signs.
Statistical analysis
All efficacy and safety analyses were conducted on the full analysis set, which comprised all randomized patients who received the study medication. A sample size of 200 patients in each treatment group provided 99% and 96% power, respectively, to demonstrate the superiority of OMS302 compared with placebo for each of the coprimary endpoints of pupil diameter and ocular pain. Mean (standard deviation [SD]) differences of 0.6 mm (0.7 mm) in pupil diameter and 5.0 mm (13.3 mm) in ocular pain scores between the OMS302 and placebo groups were assumed. The primary analyses of change in pupil diameter and ocular VAS pain scores were based on mean area under the curve (AUC) treatment differences. For the change in pupil diameter, mean AUC was determined by calculation of the pupil diameter AUC from surgical baseline to wound closure using the trapezoidal rule, then determining the mean AUC by dividing the pupil diameter AUC by total surgery time, and subtracting the baseline pupil diameter. The mean AUC of ocular VAS pain scores was determined by first calculating the AUC by the trapezoidal rule using actual VAS pain score collection times and then dividing by the number of hours with VAS results during the first 12 hours postoperatively. Summary statistics of the mean AUC change from baseline in pupil diameter and mean AUC of ocular VAS pain scores were provided by stratum and treatment group. A generalized Cochran–Mantel–Haenszel test stratified by randomization strata was used to compare the two treatment groups.
Additional statistical analyses comparing the OMS302 and placebo groups on the categorical endpoints were performed by chi-square test. Treatment comparisons for ocular pain the day after surgery were performed using a generalized Cochran–Mantel–Haenszel test. A stepdown approach was used to evaluate the statistical significance of the selected secondary endpoints, each tested sequentially at the 0.05 level. All statistical analyses were performed on the intent-to-treat study population using SAS version 9.2 software (SAS Institute, Inc, Cary, NC, USA).
Results
Patient characteristics and disposition
Of 451 patients screened for this study, 416 were randomized at 15 study sites in the USA and the Netherlands. Ten randomized patients were not treated with study medication, so were excluded from all analyses (Figure 1). All efficacy and safety analyses were performed for the full analysis population set, which consisted of 406 patients (204 placebo, 202 OMS302) who received study medication.
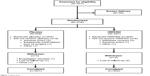  | Figure 1 Patient flow. |
All but five patients completed the study. Three patients (one placebo, two OMS302) were lost to follow-up, one placebo patient refused to return for the final visit, and one placebo patient was withdrawn by the investigator due to persistent anterior chamber inflammation and poor compliance with anti-inflammatory treatment. Demographic and baseline characteristics were similar, with no clinically meaningful differences between the OMS302 and placebo groups (Table 1). The majority of patients were over the age of 65 years, female, and white.
  | Table 1 Demographic and baseline characteristics (full analysis population set) |
Efficacy
Pupil diameter during surgery
OMS302 was superior to placebo in maintaining mydriasis during the surgical procedure (Table 2). The mean AUC pupil diameter change from baseline was +0.1 mm for the OMS302 group compared with −0.5 mm for the placebo group; the Cochran–Mantel–Haenszel-weighted mean (standard error) difference (OMS302 – placebo) was 0.590 (0.049) mm (95% confidence interval 0.494 to 0.686; P<0.0001). As indicated by this result and depicted in Figure 2, patients in the placebo group experienced steady pupil constriction after initiation of the procedure whereas OMS302 patients did not. One placebo patient required insertion of a Malyugin Ring® during surgery. Overall mean surgery time in this study was approximately 10 minutes, and substantial variability in mean pupil diameter in both treatment groups was observed approximately 21 minutes following the start of surgery due to the limited number of patients undergoing surgery for this duration.
All secondary efficacy analyses of intraoperative pupil diameter were supportive of the coprimary efficacy analysis (Table 2). Significantly more OMS302-treated patients had a pupil diameter ≥6 mm (95.9%) at the completion of cortical clean-up and significantly fewer OMS302-treated patients experienced a decrease in pupil diameter to <6 mm at any time during the procedure (9.2%) compared with placebo patients (77.0% and 38.0%, respectively; P<0.0001 for both endpoints). In a prespecified secondary efficacy analysis, only 1.0% of OMS302 patients experienced substantial intraoperative miosis (minimum decrease in pupil diameter of 2.5 mm) compared with 26.5% of placebo patients (P<0.0001).
Early postoperative ocular pain
Analyses of early postoperative ocular pain VAS scores are presented in Table 3 and depicted graphically in Figure 3. OMS302 was superior to placebo in reducing early postoperative pain (P=0.0002); the weighted mean (standard error) difference (OMS302 – placebo) in AUC of ocular pain scores during the first 12 hours postoperatively was −4.580 (1.192) mm (95% confidence interval −6.917 to −2.244). The mean AUC of ocular pain VAS for the OMS302 group was less than half that for the placebo group (4.3 mm versus 8.9 mm, respectively). Secondary analyses of ocular pain were also supportive of the coprimary endpoint analysis (Table 3). A greater proportion of OMS302-treated patients compared with placebo-treated patients reported no ocular pain and a greater proportion of placebo-treated patients reported moderate-to-severe pain during the initial 12 hours postoperatively; differences between treatment groups, however, were not statistically significant (P=0.08 for each of these two endpoints).
  | Figure 3 Mean (± SE) VAS ocular pain scores during the early postoperative period (full analysis population set). |
Safety
Overall, OM302 was well tolerated by patients undergoing ILR. Treatment-emergent adverse events (TEAEs) were reported more frequently by patients treated with placebo (70%) than patients treated with OMS302 (58%). All TEAEs were mild or moderate in severity, with the exception of six severe events experienced by four placebo patients and one OMS302 patient. Of these severe events, only eye inflammation and conjunctival hyperemia, both of which occurred in patients treated with placebo, were considered treatment-related by the investigators.
No deaths occurred during the study and no patients prematurely discontinued the study due to an adverse event. Four patients (two in each treatment group) experienced serious adverse events. Among the OMS302-treated patients, one experienced a myocardial infarction and a second experienced dehydration. Among the placebo-treated patients, one experienced respiratory arrest and pericardial effusion and a second was diagnosed with a malignant lung neoplasm. None of the serious adverse events were considered by the investigators to be treatment-related.
The most frequently observed TEAEs overall were eye pain, headache, posterior capsule opacification, anterior chamber inflammation, ocular discomfort, blurred vision, conjunctival hyperemia, photophobia, and increased intraocular pressure, all of which are anticipated events following ILR surgery (Table 4). TEAEs reported among a greater proportion of placebo patients compared with OMS302 patients included eye pain (37.3% versus 16.8%), blurred vision (7.8% versus 2.5%), and photophobia (6.4% versus 2.0%). Increased intraocular pressure was reported in a higher proportion of patients treated with OMS302 (5.9%) compared with placebo (2.0%). The majority of eye pain, blurred vision, and photophobia reported was experienced within 1–2 days of the surgery and mild in severity, irrespective of treatment received. All instances of increased intraocular pressure were mild or moderate in severity, resolved with or without treatment by the end of the study, and were considered unrelated to study treatment by the investigators.
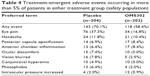  | Table 4 Treatment-emergent adverse events occurring in more than 5% of patients in either treatment group (safety population) |
Ophthalmological examinations were performed for all patients at multiple time points throughout the study. The majority of patients had normal preoperative retinas. Although findings such as macular drusen, retinal pigment epithelium changes, and background diabetic retinopathy were noted for some patients, such findings were present at baseline and did not significantly change during postoperative assessments. Baseline mean (SD) intraocular pressure was 15.4 (2.5) mmHg among placebo subjects and 15.6 (2.7) mmHg among OMS302 subjects. Consistent with reported TEAEs, mean (SD) intraocular pressure post-surgery on day 1 was higher for the OMS302 treatment group (16.9 [5.5] mmHg) compared with placebo (15.1 [4.0] mmHg); by day 2 mean (SD) intraocular pressure had resolved to baseline levels (15.3 [3.6] mmHg for placebo versus 15.7 [3.5] mmHg for OMS302). No notable treatment-related changes in vital signs were observed.
Discussion
In this large, well-controlled, randomized study in which all patients received standard-of-care preoperative topical mydriatic and anesthetic agents, direct application of OMS302 to intraocular tissues during ILR was superior to placebo in maintaining pupil diameter, preventing pupil constriction, and reducing acute postoperative ocular pain. With the exception of the addition of OMS302 or placebo in balanced saline solution used for irrigation, no changes to the ILR procedure were required and all preoperative and postoperative treatments were standard.
During ILR, PE provides a constant mydriatic effect to the iris dilator muscle. This mydriatic effect is complemented by the ability of KE to prevent miosis by inhibiting surgically induced prostaglandin release and its attendant activation of the iris sphincter muscle, a pharmacological activity that is unachievable by an α1-adrenergic receptor agonist (eg, phenylephrine or epinephrine).13 By both maintaining mydriasis and inhibiting miosis, OMS302 can decrease the risk of operative complications related to small pupils. A 6 mm pupil diameter is the recognized threshold associated with complications during ILR;23 small pupil size during these procedures is associated with a greater incidence of intraoperative and postoperative complications, such as posterior capsule breaks, vitreous loss, and posterior capsule opacification resulting from incomplete removal of lens epithelial cells during cortical clean-up.24–26 In this study, all patients received standard-of-care preoperative mydriatic topical drops; however, only about 4% of patients treated with OMS302 had a pupil diameter <6 mm at completion of cortical clean-up compared with 23% of patients treated with placebo (P≤0.0001). This may be a meaningful treatment benefit because OMS302 can decrease surgical risk as well as facilitate cortical clean-up during this important phase of ILR. These beneficial effects on pupil management (ie, maintenance of mydriasis and prevention of miosis) in the OMS302 group versus the control group in this study were present throughout the procedure.
Postoperative pain is recognized as an important factor in both patient and surgeon satisfaction,18 and the reduction in acute postoperative pain following ILR demonstrated with OMS302 represents a significant improvement over current standard postoperative care. OMS302 was associated with a greater than 50% reduction in acute postoperative pain. While the magnitude of the mean treatment effect was modest, even mild ocular pain can be troublesome and inconvenient for patients. Also, approximately 35% more OMS302-treated patients were pain-free postoperatively on the day of surgery, while almost twice as many placebo-treated patients (13.4% for placebo versus 7.9% for OMS302) experienced moderate-to-severe postoperative pain on the day of surgery. Although these secondary endpoint analyses did not reach statistical significance (P=0.08 for each), the study was not powered to demonstrate statistical significance with regard to the secondary endpoints.
No new or unexpected safety findings beyond those commonly observed with ILR surgery were observed in the study and OMS302 was well tolerated. Safety results were consistent with those reported in the Phase II study of OMS302.27 Adverse events of eye pain were reported by approximately 50% more patients receiving placebo than OMS302. Slightly more OMS302-treated patients experienced increased intraocular pressure; however, the number of patients experiencing this event overall was small, and the majority of cases resolved within a few days of onset. None of the increased intraocular pressure experienced during the study was considered related to treatment.
OMS302 is an innovative addition to the available therapies used during ILR. While the separate use of topical PE and KE has been well established in ophthalmological settings,19,20 OMS302 provides a novel method of delivering these agents to intraocular tissues throughout the surgical procedure, thereby maintaining mydriasis while pre-emptively blocking production of prostaglandin, preventing miosis, and reducing postoperative ocular pain. No change in standard operating procedure is required to use OMS302.
Disclosure
This study was sponsored by Omeros Corporation. Statistical support (Edmund Ng, Pacific Northwest Statistical Consulting) for the study data and medical writing services (12 Point LLC) for preparation of this manuscript were funded by Omeros Corporation. JSW and GAD are employees of Omeros Corporation and own stock or stock options in Omeros. RLL and TK are consultants for Omeros Corporation. SHD, TRW, JCL, and KT were principal investigators for this study.
References
Cullen KA, Hall MJ, Golosinskiy A. Ambulatory Surgery in the United States, 2006. National Health Statistics Reports; no 11. Revised. Hyattsville, MD, USA: National Center for Health Statistics; 2009. Available from: http://www.cdc.gov/nchs/data/nhsr/nhsr011.pdf. Accessed August 2, 2014. | ||
Brian G, Taylor H. Cataract blindness – challenges for the 21st century. Bull World Health Organ. 2001;79:249–256. | ||
Fattore G, Torbica A. Cost and reimbursement of cataract surgery in Europe: a cross-country comparison. Health Econ. 2008;17(Suppl 1): S71–S82. | ||
Steinert RF. The intumescent cataract. In: Steinert RF, editor. Cataract Surgery. 3rd ed. Irvine, CA, USA: Saunders Elsevier; 2010. | ||
Ho T, Fan R, Hong WW, Khian KB. Maximal mydriasis evaluation in cataract surgery. J Cataract Refract Surg. 1992;18:375–379. | ||
Levine L. Mydriatic effectiveness of dilute combinations of phenylephrine and tropicamide. Am J Optom Physiol Opt. 1982;59:580–594. | ||
Eyeson-Annan ML, Hirst LW, Battistutta D, Green A. Comparative pupil dilation using phenylephrine alone or in combination with tropicamide. Ophthalmology. 1998;105:726–732. | ||
Waitzman MB. Prostaglandins and the eye: rapid transition from basic to applied and implications for expanded clinical consideration. Metab Pediatr Syst Ophthalmol. 1982;6:17–26. | ||
Srinivasan R, Madhavaranga. Topical ketorolac tromethamine 0.5% versus diclofenac sodium 0.1% to inhibit miosis during cataract surgery. J Cataract Refract Surg. 2002;28:517–520. | ||
Drolsom L, Davanger M, Haaskjold E. Risk factors for an inflammatory response after extracapsular cataract extraction and posterior chamber IOL. Acta Ophthalmol (Copenh). 1994;72:21–26. | ||
Goodman DF, Stark WJ, Gottsch JD. Complication of cataract extraction with intraocular lens implantation. Ophthalmic Surg. 1989;20: 132–140. | ||
Fine IH, Packer M, Hoffman RS. Phacoemulsification in the presence of a small pupil. In: Steinert RF, editor. Cataract Surgery. Philadelphia, PA, USA: Saunders; 2010. | ||
Antcliff RJ, Trew DR. The maintenance of per-operative mydriasis in phacoemulsification with topical diclofenac sodium. Eye (Lond). 1997;11 Pt 3:389–391. | ||
Duffin RM, Pettit TH, Straatsma BR. 2.5% v 10% phenylephrine in maintaining mydriasis during cataract surgery. Arch Ophthalmol. 1983; 101:1903–1906. | ||
Liou SW, Yang CY. The effect of intracameral adrenaline infusion on pupil size, pulse rate, and blood pressure during phacoemulsification. J Ocul Pharmacol Ther. 1998;14:357–361. | ||
Porela-Tiihonen S, Kaarniranta K, Kokki M, Purhonen S, Kokki H. A prospective study on postoperative pain after cataract surgery. Clin Ophthalmol. 2013;7:1429–1435. | ||
Dell SJ, Hovanesian JA, Raizman MB, et al. Ocular Bandage Study Group. Randomized comparison of postoperative use of hydrogel ocular bandage and collagen corneal shield for wound protection and patient tolerability after cataract surgery. J Cataract Refract Surg. 2011;37: 113–121. | ||
Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale J Biol Med. 2010;83:11–25. | ||
Narváez J, Kronberg BP, Park H, Zumwalt JR, et al. Pupil dilation using a standard cataract surgery regimen alone or with atropine 1.0% pretreatment: prospective comparative evaluation. J Cataract Refract Surg. 2010;36:563–567. | ||
Reddy R, Kim SJ. Critical appraisal of ophthalmic ketorolac in treatment of pain and inflammation following cataract surgery. Clin Ophthalmol. 2011;5:751–758. | ||
Arshinoff SA, Opalinski YAV. The pharmacotherapy of cataract surgery. In: Yanoff M, Duker JS, editors. Ophthalmology: Expert Consult. 3rd ed. Edinburgh, UK: Mosby Elsevier; 2009. | ||
Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40: 381–395. | ||
Zanetti FR, Fulco EA, Chaves FR, da Costa Pinto AP, Arieta CE, Lira RP. Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: a randomized trial. Indian J Ophthalmol. 2012;60:277–281. | ||
Guzek JP, Holm M, Cotter JB, et al. Risk factors for intraoperative complications in 1,000 extracapsular cataract cases. Ophthalmology. 1987;94:461–466. | ||
Narendan N, Jaycock P, Johnston RL, et al. The cataract national dataset electronic multicenter audit of 55,567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye (Lond). 2009;23: 31–37. | ||
Apple DJ, Escobar-Gomez M, Zaugg B, Kleinmann G, Borkenstein AF. Modern cataract surgery: unfinished business and unanswered questions. Surv Ophthalmol. 2011;56(6 Suppl):S3–S53. | ||
Crandall A, Dunn S, Whitaker JS, Ng E, Demopulos G, Rosenblatt M. OMS302 maintains mydriasis and decreases postoperative pain in cataract surgery. Poster presented at the American Academy of Ophthalmology Annual Meeting, October 24–25, 2011, Orlando, FL, USA. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



