Back to Journals » Cancer Management and Research » Volume 11
Intra-rectal use of epinephrine in radiotherapy of prostate cancer
Authors Qin SB, Gao XS , Li HZ, Liu CX, Hou DL , Nian WD, Li XY, Wang D
Received 10 September 2018
Accepted for publication 10 April 2019
Published 27 May 2019 Volume 2019:11 Pages 4847—4854
DOI https://doi.org/10.2147/CMAR.S187049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Antonella D'Anneo
Shang-Bin Qin,1 Xian-Shu Gao,1 Hong-Zhen Li,1 Chao-Xing Liu,2 Dong-Liang Hou,3 Wei-Dong Nian,4 Xue-Ying Li,5 Dian Wang6
1Department of Radiation Oncology, Peking University First Hospital, Beijing, People’s Republic of China; 2Department of Radiation Oncology, Shijiazhuang City First Hospital, Shijiazhuang, People’s Republic of China; 3Department of Radiation Oncology, Beijing Shijitan Hospital, Beijing, People’s Republic of China; 4Department of General Surgery, Peking University First Hospital, Beijing, People’s Republic of China; 5Department of Medical Statistics, Peking University First Hospital, Beijing, People’s Republic of China; 6Department of Radiation Oncology, Rush University Medical Center, Chicago, IL, USA
Purpose: The aim of the study was to evaluate the feasibility and toxicity of intra-rectal epinephrine during prostatic radiotherapy.
Materials and methods: A total of 34 patients with prostate cancer were randomized to receive daily intra-rectal epinephrine (4 mg in 40 mL, n=16) or placebo (40 mL normal saline, n=18) 5 min before daily radiotherapy. Physical examination including systolic blood pressure (SBP) and heart rate (HR) was performed before, 5 min after, and 20 min after intra-rectal use. Toxicities were graded using the Radiation Therapy Oncology Group standard. A two-sided Fisher’s exact test was used to compare proportions between groups. A mixed-effects model was used to analyze multiple measurements of SBP and HR. Survival curves were calculated using the Kaplan–Meier method and compared between groups using the log-rank test.
Results: All patients completed the protocol treatment and reported no cardiovascular symptoms after intra-rectal administration. There were no differences in SBP and HR between these two groups at any time point (before, 5 min after, and 20 min after epinephrine). At 5 weeks after the start of radiotherapy, the incidence of rectal toxicity≥grade 2 was 27.8% (5/18) for the control group versus 12.5% (2/16) for the epinephrine group, but was not statistically significant (p=0.4). There was no rectal toxicity≥grade 2 in these two groups beyond 2-year follow-up. The 5-year biochemical relapse-free survival was 75.0% and 72.2% for the epinephrine and control group, respectively.
Conclusion: Results of this pilot randomized trial have demonstrated that intra-rectal administration of epinephrine is feasible and safe in prostatic radiotherapy. Its radio-protective effect warrants further investigation.
Keywords: radiation proctitis, epinephrine, radiotherapy
Introduction
Multiple lines of evidence have demonstrated that radiation dose escalation is associated with improvement in clinical outcomes of patients with prostate cancer receiving radiotherapy.1–3 Due to the close proximity of the prostate to the anterior rectal wall, rectal acute toxicity≥grade 2 is 20–61%4–6 and late toxicity≥grade 2 is 9–37.6% depending on follow-up length and the toxicity criteria1,2,7,8 even in the modern era of image-guided radiotherapy (IGRT). Therefore, radiation proctitis is a limiting factor for dose escalation. In recent years, several radio-protectors including sucralfate retention, misoprostol rectal suppositories, and oral mesalazine, etc, have been investigated in many types of human malignancy including prostate cancer.9–11 However, few of these radio-protectors have been proven effective in improving radiation-induced proctitis. Amifostine has been shown to effectively prevent proctitis in some clinical investigations.12–16 However, its use was not commonly used in clinics due to many reasons including side effects, low level of present evidence, cost–benefit assessment, and noncompliance issues. An implanted rectal spacer can create rectal sparing but was mainly used in early-stage prostate cancer without capsular penetration.17
Epinephrine is one of the most commonly used vasoconstrictors that can prevent peptic ulcer bleeding and nose mucosa bleeding, and can also extend the effect of local anesthetics when its mixture is administered to slow down absorption. Epinephrine was also investigated as a potential radio-protector in many preclinical studies. For instance, Prewitt and Musacchia18 found that intraperitoneal infusion of epinephrine decreases radiation-induced death in hamsters when compared with infusion of saline. The authors believed that epinephrine might reduce blood flow in the spinal cord and improve the anoxic environment in the spinal cord, which relieves radiation damage.18 Steckel et al.19 observed that intra-arterial infusion of epinephrine prevents radiation-induced nephritis in an animal model. Gao et al20 reported that topical use of epinephrine on the skin and oral mucosa significantly reduces radiation-induced damage in a mice model.Altogether, the results of the above preclinical studies have suggested that epinephrine might be an effective radio-protective agent.
We hypothesized that intra-rectal use of epinephrine immediately prior to radiation treatment might relieve radiation proctitis. We therefore launched this pilot randomized study to assess the safety and effectiveness of intra-rectal epinephrine on radiation proctitis in prostate cancer patients receiving radiotherapy since intra-arterial infusion of epinephrine can increase blood pressure and cause other cardio-cerebrovascular events, which can be very dangerous in clinical practice.
Methods and materials
Eligibility and accrual
All patients had a diagnosis of prostate cancer, and elected to receive definitive radiotherapy or adjuvant/salvage radiotherapy after prostatectomy. All patients underwent history and physical examination, as well as normal blood work, including complete blood count, aspartate aminotransferase, alanine transaminase, serum creatinine, and blood urea nitrogen. Definitive versus postoperative radiation and pelvic versus prostate radiation were stratified between the epinephrine group and the saline control group. Patients with evidence of metastatic disease were excluded from this study. Hormonal therapy was permitted. All eligible patients signed informed consent about this study prior to the protocol treatment. This protocol was approved by our Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
Application of epinephrine suspension
Prior to this pilot randomized study, we recruited healthy volunteers for use of the epinephrine dose, in order to decide the clinical safety of intra-rectal infusion of epinephrine. At first, we recruited five volunteers for topical use of epinephrine on nasal mucosa. Epinephrine suspension at a dose concentration of 1:10,000 (0.1 mg/mL) or 1:20,000 (0.05 mg/mL) was dropped on nasal mucosa. The turbinal mucosa was found to turn white and shrank after the use of epinephrine. The onset time and duration of these two dose concentrations were 16.4±5.5 s versus 37±13.9 s and 21±4.2 min versus 14±2.2 min for 0.1 mg/mL versus 0.05 mg/mL, respectively.
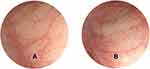
Based on the above results, we chose epinephrine at a concentration of 1:10,000 for intra-rectal use of epinephrine. We then recruited four additional volunteers to receive intra-rectal administration of epinephrine suspension (40 mL, 1:10,000). Rectal mucosa was continuously observed through endoscopy. The onset time to constrict rectal mucosa vessels was 5±0.8 min and the duration of the constriction was 22.5±2.9 min (Figure 1). All healthy volunteers signed informed consent about this study.
Based on the above results in healthy volunteers, we chose intra-rectal use of epinephrine at a concentration of 1:10,000 (0.1 mg/mL) in this randomized pilot study. Patients were randomized to receive either intra-rectal infusion of epinephrine (4 mg/40 mL) (experimental group) or normal saline of equivalent volume (control group) via 50-cm3 syringe in the prone position before each radiation treatment. Patients were asked to remain in the prone position for 5 min after administration, and then asked to become supine for prostatic radiotherapy. Patients were asked to have a bowel movement soon after treatment. All patients were blind to receiving intra-rectal epinephrine or normal saline before and after radiotherapy was initiated.
Radiation therapy
Patients underwent intensity-modulated radiotherapy (IMRT) or three-dimensional radiotherapy to the pelvis, seminal vesicles, and prostate with daily IGRT. Radiotherapy treatment planning was computed tomography based. Before simulation, normal saline of 40 mL was intra-rectally instilled. The prostate, rectum, and bladder were contoured using treatment-planning software after computed tomography-magnetic resonance imaging fusion. The rectum was contoured from the anus (at the level of ischial tuberosities) to a length of 15 cm or until the recto-sigmoid flexure could be identified. For definitive radiotherapy, we prescribed either pelvic radiotherapy (pelvic lymph nodes plus prostate and seminal vesicle) or prostate radiotherapy (prostate and seminal vesicle). The decision to treat pelvic lymph nodes was based on the risk estimate for nodal involvement (greater than 15% from the Roach equations using Gleason scores and pretreatment prostate specific antigen (PSA)). The planning target volume (PTV) was defined as the clinical target volume plus a 0.5 cm margin (0.3 cm to the anterior rectal wall). For adjuvant/salvage radiotherapy, we treated the prostate fossa only. The prostate fossa was contoured based on the Radiation Therapy Oncology Group (RTOG) atlas. A standard fractionation regimen (1.8–2.0 Gy/fraction, 5 fractions/week) was used to treat all cases. Median doses delivered to the pelvis, fossa, and prostate were respectively 46 Gy, 72 Gy, and 76 Gy within 7.2–8.0 weeks. Dose constraints for the rectum were V70≤10%, V60≤20%, and V50≤30%. The prescribed dose must cover at least 95% of the PTV.
On treatment and follow-up evaluations
To evaluate safety, each patient was assessed for systolic blood pressure (SBP) and heart rate (HR) before, 5 min after, and 20 min after intra-rectal infusion of epinephrine or saline. In addition, we saw patients weekly during the course of radiotherapy, at 1 month, 3 months, 6 months, and then every 6 months until 3 years, and then annually after completion of radiation treatment. Toxicities were assessed at the above time points using RTOG acute and late toxicity scoring criteria.21
Statistics
This is the first pilot randomized study to assess the feasibility and acute cardiovascular effects after intra-rectal infusion of epinephrine (primary endpoint). Given no clinical data, we estimate 30 participants (15 for each group) to examine this primary endpoint. Considering 10% of patients might not be compliant or might be lost for follow-up, we therefore planned to recruit 34 participants (a sample size) to this randomized study. Summary statistics such as sample proportions, means, and median values were used to describe the patient characteristics. A two-sided Fisher's exact test was used for comparing proportions across groups. We used a mixed-effects model, an appropriated statistical method for repeated measurements on SBP and HR. Survival curves were calculated with the Kaplan–Meier method and compared with the log-rank test. All analyses were performed using SPSS14.0 software.
Results
Patients and treatment characteristics
Between January 2011 and February 2012, 34 patients with prostate cancer were accrued to this randomized study, 18 to the saline group and 16 to the epinephrine group. Baseline demographics and characteristics of the patients are summarized in Table 1. Both arms of the study were balanced with respect to age, radiation dose, hormonal therapy, and NCCN risk groups (as shown in Table S1.).
 | Table 1 Patient demographics |
Assessment of acute cardiovascular effects after intra-rectal infusion of epinephrine (primary endpoint)
All patients tolerated daily intra-rectal infusion of saline versus epinephrine and radiotherapy well, with a 100% compliance rate. No patient reported cardiovascular symptom side effects after the intra-rectal use of epinephrine or saline. Abnormal increase in blood pressure and/or arrhythmia was not observed after intra-rectal infusion of epinephrine compared with saline control. The differences in SBP (Figure 2) or HR (Figure 3) either before or 5 min or 20 min after epinephrine were not statistically significant (p=0.792 and p=0.542).
 | Figure 2 Systolic blood pressure (mean ± SD) was recorded before, 5 min after, and 20 min after epinephrine. |
 | Figure 3 Heart rate (mean ± SD) was recorded before, 5 min after, and 20 min after epinephrine. |
Assessment of radiation proctitis (secondary endpoint)
All patients completed the protocol treatment without any break related to side effects. At 5 weeks after the start of radiotherapy, the incidence of rectal toxicity≥grade 2 was 27.8% (5/18) for the control group versus 12.5% (2/16) for the epinephrine group, but was not statistically significant (p=0.4). After 2 years, no patients reported rectal toxicity≥grade 2 in either group. Rectal toxicity is summarized in Figure 4. More toxicity details are supplied in Table S2.
 | Table 2 Mean±SD irradiated volume of the rectum (cm3) |
 | Figure 4 Incidence of Radiation Therapy Oncology Group grade 2 toxicity by group in all patients during treatment and follow-up.Abbreviations: m, months; wk, weeks. |
The irradiated volume of rectum was compared between these two groups. The irradiated volumes in the epinephrine group (V55Gy, V65Gy, and V70Gy) were not significantly different from the control, except for V60Gy, which is statistically significantly larger in the epinephrine group compared with the control group (p=0.027) (Table 2).
Biochemical relapse-free survival
All patients were followed at a median 62 months in the range of 57–68 months. The 5-year biochemical relapse-free survival (bRFS) for the epinephrine and control groups was 75.0% and 72.2%, respectively (Figure 5). No patients died at the most recent follow-up.
 | Figure 5 Biochemical relapse-free survival (bRFS) for the epinephrine and control groups. |
Discussion
Results of this pilot randomized study have demonstrated that intra-rectal infusion of epinephrine (1:10,000; 40 mL) does not have any significant effect on HR and SBP, as well as acute rectal toxicity, compared with the control group. Furthermore, the initial follow-up results did show less incidence of chronic rectal toxicity (≥grade 2) than the control group at 24 months after the completion of radiotherapy. However, this type of toxicity difference was not observed simply because no incidence of radiation proctitis≥grade 2 was reported in either group beyond 2 years after all patients completed prostatic radiotherapy. Reasons for this difference were unknown. This might be due to the small sample size including a mixed population receiving definitive radiotherapy (median dose of 76 Gy in 38 fractions) versus postoperative radiotherapy (median dose of 72 Gy in 36 fractions) or receiving pelvic radiotherapy versus prostatic radiotherapy alone, or receiving three-dimensional radiotherapy versus IMRT. Nonetheless, given 100% compliance and no difference in acute cardiovascular toxicity, we believe use of epinephrine in patients with prostate cancer receiving high-dose radiotherapy is worth further investigation.
In addition, another advantage of intrarectal administration of epinephrine prior to radiotherapy of prostate cancer, in comparison with other types of administration such as intravenous administration, is that we are not concerned about epinephrine protecting prostate cancers from radiotherapy. This is because epinephrine is inside the rectum and will not be absorbed into the prostate due to the constriction of mucosal vessels. Also, 5-year bRFS for the epinephrine group is not significantly different compared with the control group (75% versus 72.2%). However, we have to admit that daily intra-rectal infusion of epinephrine prior to radiotherapy via syringe indeed brought inconvenience to our patients as well as a workload burden to our healthcare providers (doctors, nurses, and therapists). We have indeed spent enormous time and effort to keep patient compliance and protocol treatment compliance for this pilot study. Therefore, investigation of epinephrine suspension as a radio-protective agent might not be feasible in a large study on prostatic radiotherapy, but results of this pilot randomized study are very promising, and we would recommend development of a new form of epinephrine such as a suppository for future investigation.
Conclusion
Intra-rectal infusion of epinephrine (1:10,000; 40 mL) suspension prior to prostatic radiotherapy is feasible and safe. Its radio-protective effect needs to be further investigated in a large study on high-dose radiotherapy of prostate cancer with a well-controlled dose and dose delivery.
Ethics approval and consent to participate
The study was approved by the local Institutional Review Board (Peking University First Hospital Biomedical Research Ethics Committee).
Availability of data and materials
Please contact the corresponding author (Dr Xian-Shu Gao) for data requests.
Abbreviation list
SBP, systolic blood pressure; HR, heart rate; RTOG, Radiation Therapy Oncology Group; bRFS, biochemical relapse-free survival; IGRT, image-guided radiotherapy; IMRT, intensity-modulated radiotherapy; PTV, planning target volume; PSA, prostate specific antigen.
Acknowledgments
The abstract of this paper was presented at the 2016 and 2017 ASTRO annual meetings as a poster presentation. The poster’s abstract was published in “Poster Abstracts” in International Journal of Radiation & Oncology & Biology & Physics:
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105.
2. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–1239. doi:10.1001/jama.294.10.1233
3. Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi:10.1016/S1470-2045(14)70040-3
4. Al-Mamgani A, Heemsbergen WD, Peeters ST, Lebesque JV. Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:685–691. doi:10.1016/j.ijrobp.2008.04.063
5. Peeters ST, Heemsbergen WD, van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2005;61:1019–1034. doi:10.1016/j.ijrobp.2004.07.715
6. Wortel RC, Incrocci L, Pos FJ, et al. Acute toxicity after image-guided intensity modulated radiation therapy compared to 3D conformal radiation therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2015;91:737–744. doi:10.1016/j.ijrobp.2014.12.017
7. Wortel RC, Incrocci L, Pos FJ, et al. Late side effects after image guided intensity modulated radiation therapy compared to 3D-conformal radiation therapy for prostate cancer: results from 2 prospective cohorts. Int J Radiat Oncol Biol Phys. 2016;95:680–689. doi:10.1016/j.ijrobp.2016.01.031
8. Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:980–988. doi:10.1016/j.ijrobp.2008.02.073
9. O‘Brien PC, Franklin CI, Dear KB, et al. A phase III double-blind randomised study of rectal sucralfate suspension in the prevention of acute radiation proctitis. Radiother Oncol. 1997;45:117–123.
10. Hille A, Schmidberger H, Hermann RM, et al. A phase III randomized, placebo-controlled, double-blind study of misoprostol rectal suppositories to prevent acute radiation proctitis in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1488–1493. doi:10.1016/j.ijrobp.2005.05.063
11. Resbeut M, Marteau P, Cowen D, et al. A randomized double blind placebo controlled multicenter study of mesalazine for the prevention of acute radiation enteritis. Radiother Oncol. 1997;44:59–63.
12. Athanassiou H, Antonadou D, Coliarakis N, et al. Protective effect of amifostine during fractionated radiotherapy in patients with pelvic carcinomas: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2003;56:1154–1160.
13. Kouloulias VE, Kouvaris JR, Pissakas G, et al. Phase II multicenter randomized study of amifostine for prevention of acute radiation rectal toxicity: topical intrarectal versus subcutaneous application. Int J Radiat Oncol Biol Phys. 2005;62:486–493. doi:10.1016/j.ijrobp.2004.10.043
14. Ben-Josef E, Han S, Tobi M, et al. A pilot study of topical intrarectal application of amifostine for prevention of late radiation rectal injury. Int J Radiat Oncol Biol Phys. 2002;53:1160–1164.
15. Singh AK, Ménard C, Guion P, et al. Intrarectal amifostine suspension may protect against acute proctitis during radiation therapy for prostate cancer: A pilot study. Int J Radiat Oncol Biol Phys. 2006;65:1008–1013. doi:10.1016/j.ijrobp.2006.02.030
16. Simone NL, Menard C, Soule BP, et al. Intrarectal amifostine during external beam radiation therapy for prostate cancer produces significant improvements in quality of life measured by EPIC score. Int J Radiat Oncol Biol Phys. 2008;70:90–95. doi:10.1016/j.ijrobp.2007.05.057
17. Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:971–977. doi:10.1016/j.ijrobp.2015.04.030
18. Prewitt RL, Musacchia XJ. Mechanisms of radio-protection by catecholamines in the hamster (Mesocricetus auratus). Int J Radiat Biol Relat Stud Phys Chem Med. 1975;27:181–191.
19. Steckel RJ, Tobin PL, Stein JJ, Bennett RL. Intra-arterial epinephrine protection against radiation nephritis. A Progress Report. Radiology. 1969;92:1341–1345.
20. Gao X, Nakagawa T, Yamamoto M, et al. Radioprotective effect of epinephrine as a vasoconstrictor in mouse oral mucosa and scalp. Okayama Igakkai zasshi. 1996;108:139–144. doi:10.4044/joma1947.108.3-6_139
21. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi:10.1016/0360-3016(95)00060-C
Supplementary materials
 | Table S1 Acute and late proctitis in the epinephrine and control groups |
 | Table S2 Risk in the epinephrine and control groups |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.