Back to Journals » Infection and Drug Resistance » Volume 12
Interprofessional Antimicrobial Stewardship Influencing Clostridioides difficile Infection: An 8-Year Study Using Antimicrobial Use Density
Authors Yoshida J , Kikuchi T, Ueno T, Mataga A, Asano I, Otani K, Tamura T, Tanaka M
Received 13 May 2019
Accepted for publication 18 October 2019
Published 4 November 2019 Volume 2019:12 Pages 3409—3414
DOI https://doi.org/10.2147/IDR.S184050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Junichi Yoshida, Tetsuya Kikuchi, Takako Ueno, Akiko Mataga, Ikuyo Asano, Kazuhiro Otani, Tetsuro Tamura, Masao Tanaka
Division of Infection Control, Shimonoseki City Hospital, Shimonoseki, Japan
Correspondence: Junichi Yoshida
Division of Infection Control, Shimonoseki City Hospital, 1-13-1, Shimonoseki 750-8520, Japan
Tel +81 83 231 4111
Fax +81 83 224 3838
Email [email protected]
Purpose: To reduce Clostridioides difficile infection (CDI), we implemented interprofessional antimicrobial, infection control, and diagnostic stewardship (ipAS) conducted by physicians/pharmacists, infection control nurses, and medical technologists, respectively. As a numerical indicator for ipAS, we used antimicrobial use density (AUD) in an 8-year study to validate its efficacy in CDI reduction.
Patients and methods: This was an observational study. CDI was defined as stool samples or C. difficile isolates containing toxin A and/or B from a patient with diarrhea occurring three or more times per day. From 2011–2018 at a 10-ward single site the subjects were in-patients with CDI, and the following data were collected: AUDs for 23 antibiotics, and antimicrobial test results. By 2015, we had established ipAS, consisting of culture submission before the administration of broad-spectrum antimicrobials, the promotion of point-of-care testing for diagnosis-based antimicrobials, perioperative prophylactic antibiotics, intervention at positive diagnosis of blood culture, team round for diarrhea, and inspection on contact precautions and disinfection in CDI cases. The study outcomes included annual numbers of CDI patients and blood culture sets. We compared annual AUDs between former (2011–14) and latter (2015–18) periods using Kruskal–Wallis tests and examined the correlation between AUDs and CDI numbers.
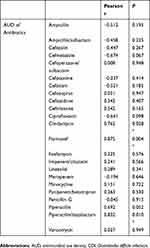
Results: Of a total 50,970 patients, 1,750 patients underwent C. difficile toxin tests, of whom 171 patients (9.8%) were positive for CDI. Between the former and latter periods, AUDs for flomoxef (11.96 to 2.71 by medians), panipenem/betamipron (0.30 to 0.00), and clindamycin (3.87 to 2.19) significantly decreased (P<0.05) as did numbers of CDIs (26.5 to 10) (P=0.043). The correlation analysis revealed a significant correlation between AUD for flomoxef and CDIs (P=0.004) and the AUD for piperacillin/tazobactam and CDIs (P=0.010) with a positive Pearson r.
Conclusion: The integrated antimicrobial, diagnostic, and infection control approach used in ipAS may reduce CDIs.
Keywords: broad-spectrum antibiotics, infection control, diagnosis, multidisciplinary, clindamycin
Introduction
Bui et al reported that antimicrobial stewardship focusing only on high-cost antibiotics results in missed opportunities to reduce Clostridioides difficile infection (CDI), while the use of low-cost antibiotics, such as clindamycin (CLDM), was out of control.1 In addition, Patton et al reported constant CDI in surgery wards despite antibiotic stewardship efforts, demonstrating that the real-world impact of stewardship interventions remains limited.2
To address the complex task of containing CDIs, Yanke et al attempted an interprofessional approach, leading to the detection of barriers to the successful implementation of a CDI prevention bundle, such as a lack of sinks and C. difficile polymerase chain reaction testing.3 Likewise, our community hospital in Japan implemented interprofessional antimicrobial, infection control, and diagnostic stewardship (ipAS) in a similar multifaceted approach, but with cycles of Plan-Do-Check-Act (PDCA). We conducted a retrospective observational study to verify its efficacy in reducing CDIs.
Materials And Methods
Ethics
The institutional ethics board for research (Rinri Kenkyu Iinkai, in Japanese) permitted this study to be published and approved the study protocol (serial number 2018SCHEC-026).
Materials
In a single institute with a total of 10 wards, we collected a consecutive series of C. difficile toxin tests (CDTs) from 2011 to 2018. For the CDTs, we initially employed the TOX A/B QUIK CHEK® test (Nissui Co. Tokyo, Japan). By 2012, we employed the C. DIFF QUIK CHEK COMPLETE® (Alere Medical Co., Ltd, Tokyo, Japan), by 2013, the CD Immunochromato-CD A/B GE test (Nissui Co. Tokyo, Japan), by 2014, the C. DIFF QUIK CHEK COMPLETE (Alere Medical Co., Ltd, Tokyo, Japan), and by 2016 and thereafter, the GE Test Immunochromato-CD GDH/TOX® test (Nissui Co., Tokyo, Japan).
For antimicrobial use density (AUD), we collected data on annual patient-days and the total dose of 23 agents: ampicillin, ampicillin/sulbactam, cefazolin (CEZ), cefmetazole (CMZ), cefoperazone/sulbactam, cefotaxime (CTX), cefotiam, cefozopran, ceftazidime, ceftriaxone (CTRX), ciprofloxacin (CPFX), CLDM, flomoxef (FMOX), fosfomycin, imipenem/cilastatin (IPM/CS), linezolid, meropenem (MEPM), minocycline, panipenem/betamipron (PAPM/BP), penicillin G, piperacillin (PIPC), piperacillin/tazobactam (PIPC/TAZ), and vancomycin.
Methods
Definition
A positive CDT was defined as being positive for toxin A and/or B in feces or culture isolates, regardless of glutamate dehydrogenase (GDH) results, because historically GDH was not available in the initial years of the study. To increase the test sensitivity for C. difficile toxin, as ordered by the attending doctors, we isolated C. difficile strains using cycloserine-cefoxitin-mannitol agar plates, including the Nissui Plate CCMA EX (Nissui Pharmaceutical Co., Ltd, Tokyo, Japan) since 2011, and Vital Media CCMA Medium (Kyokuto Pharmaceutical Industrial Co., Ltd, Tokyo, Japan) since 2015. CDI was defined as a positive CDT in the presence of three or more episodes of diarrhea per day. CDTs were performed in all of the patients who showed diarrhea preceded by antibiotic administration.
Intervention
Previously, physicians had administered antimicrobials to patients at their discretion. Since 2013, however, we restricted the use of carbapenem antibiotics and by 2015, we established a protocol to control the use of broad-spectrum antibiotics, such as MEPM, IPM/CS, cefepime, and PIPC/TAZ based on the following concepts. (1) Antibiotic use must meet the indications and preceding culture sampling as stated by the sepsis guideline4 or (2) must be performed in the presence of microbes producing an extended spectrum beta-lactamase inhibitor. In addition, perioperative patients received prophylactic antibiotics in accordance with the Japanese guidelines for the prevention of surgical site infection,5 a modification of that released by the Centers for Disease Control and Prevention in 1999.6 To assist compliance, we used a clinical pathway in perioperative patients, and administered CEZ as a prophylactic antimicrobial.7 During consultations on suitable agents for anaerobic microbes, CLDM usage was discouraged for CDIs, and the use of metronidazole was encouraged to reduce the risk posed by anaerobes. For PDCA cycles to reduce broad-spectrum antibiotic use, the interprofessional team shared AUD data on the intranet and in monthly audits.
For infection control, patients with CDIs underwent isolation, contact precaution, and sodium hypochlorite disinfection of their environment in accordance with British guidelines.8 Interprofessional team rounds were performed to detect unreported diarrhea cases, which frequently occurred and were amended in a PDCA cycle.
In terms of diagnostic stewardship, blood or other cultures were mandated before the use of broad-spectrum antibiotics. Additionally, we encouraged point-of-care testing such as a urinary antigen test for Streptococcus pneumoniae and Legionella pneumophila (QuickChaser; Mizuho Medie Co., Saga, Japan) for targeted antibiotics. On receipt of positive culture results, microbiological technicians issued intranet alert electronic mails to attending doctors for de-escalation or, on isolation of drug-resistant microbes, for a change to more effective drugs.
Analysis
AUD was calculated as
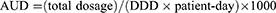
where DDD stands for defined daily dose, as defined by the World Health Organization.9
The primary endpoint was the time shift in the AUDs of 23 antibiotics and numbers of CDIs. For statistical analysis, we used the Kruskal–Wallis test to compare the former (2011–14) and latter (2015–18) periods. Another primary endpoint was the risk of AUDs for CDIs. To assess the correlation between the AUDs of 23 antibiotics and CDI numbers, the Pearson correlation coefficient was analyzed between annual AUDs and CDI numbers.
Secondary endpoints included the time shift in the numbers of blood culture sets, and the number of point-of-care tests performed. For statistical analysis, we used the Kruskal–Wallis test to compare the former and latter periods. We used SPSS Version 24 (IBM Inc., Armonk, NY) for these analyses.
Results
Overview
Of a total 50,970 patients, 1,750 patients underwent CDTs, of whom 171 patients (9.8%) were positive for CDIs. Among the 171 patients, with a median age of 81 (range, 45–105 years), 91 (53.2%) were male and 80 (46.8%) were female.
Primary Endpoint
Comparing the former and latter periods, the AUDs of FMOX, PAPM/BP, and CLDM decreased whereas those of CEZ, CMZ, CTX, and CTRX significantly increased (P<0.05) (Table 1). The numbers of CDI patients decreased significantly (P=0.043). Annually, the number of CDI patients peaked at 35 in 2013 and decreased thereafter (Figure 1).
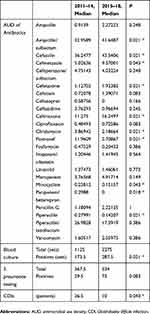 |
Table 1 Comparison Of AUDs For 23 Antibiotics And Numbers Of Blood Culture Sets And Point-Of-Care Tests For Streptococcus pneumoniae, And CDI Cases, As Assessed Using Kruskal-Wallis Tests |
 |
Figure 1 Annual trend of AUDs and number of CDI patients before and after intervention (black bar). FMOX and PIPC/TAZ are significantly correlated with number of CDI patients (Table 2). Time course of AUDs for PIPC/TAZ coincided with that for CDIs. Vertical axis shows AUD and number of CDI patients. Abbreviations: AUD, antimicrobial use density; CDI, Clostridioides difficile infection; FMOX, flomoxef; PIPC/TAZ, piperacillin/tazobactam. |
The correlation analysis revealed a significant correlation (P<0.05) between the AUD of FMOX and CDIs as well as between the AUD of PIPC/TAZ and CDIs, with positive Pearson r values for both (Table 2).
Secondary Endpoints
During the former period, numbers of total blood culture sets and positive blood culture results remained consistently low. After the implementation of ipAS, both numbers significantly increased (both P=0.021 by Kruskal–Wallis test) (Table 2). Likewise, levels of point-of-care testing for S. pneumoniae showed an increased tendency in the latter period (P=0.083).
Discussion
Herein, we report that ipAS decreased CDIs in hospitalized patients, which was associated with increased AUDs for broad-spectrum antibiotics and CLDM, as previously reported in the literature. Likewise, a correlation analysis showed a significant positive relationship between the AUD of PIPC/TAZ and CDI cases. The numbers of blood culture tests and point-of-care tests for S. pneumoniae also increased in the latter period, supporting the role of diagnostic stewardship.
Relevant guidelines, such as those published by the German Society of Infectious Diseases, state that antimicrobial stewardship helps to control CDIs.10 Huttner et al addressed the “4C” (cephalosporins, CPFX and other fluoroquinolones, co-amoxiclav, and CLDM) approach to reduce CDIs.11 We also observed decreased use of CLDM in the current study.
In terms of clinical pathways and antimicrobial stewardship, the combined medical associations of Germany reported that antimicrobial stewardship for surgical site infection (SSI) reduced CDIs.12 Our clinical pathways for perioperative care routinely designated CEZ for prophylaxis against SSI and a duration of treatment of 2 days in accordance with the Japanese guidelines. Recently, a “Global Declaration on Appropriate Use of Antimicrobial Agents” was issued to enhance the use of the surgical pathway.13
In terms of the quantitative evaluation of antimicrobial stewardship, several authors have used AUDs. Niwa et al used AUDs in 2012 to assess their intervention.14 In 2014, Yoon and others used AUDs for carbapenems to measure the effect of an intervention with carbapenems on the susceptibility of Acinetobacter baumannii.15 In 2015, Borde et al used AUDs for third-generation cephalosporin and fluoroquinolone as a metric.16 Hohn and others assessed AUDs before and after their intervention with surgical prophylactic antimicrobials.17 Likewise, Kawamura and associates used AUDs for CEZ and the rate of surgical site infection with methicillin-resistant Staphylococcus aureus to evaluate their intervention.18 In 2018, Hagiwara et al reported that their intervention decreased AUDs for carbapenems and PIPC/TAZ with a decrease in resistant strains of Pseudomonas aeruginosa.19 Therefore, the use of AUDs as a metric following an intervention has been established, but had not yet been described in the context of CDI cases.
Next arises a question as to why AUDs affect CDIs. In 2016, Freedberg and others reported that receipt of antimicrobials in inpatients was a risk for CDI in subsequent patients in the same bed.20 In addition, Tarrant et al described the survival of C. difficile spores on hospital bed sheets following a laundry process.21 Therefore, AUDs may represent antibiotic pressure in the ward environment.
In terms of isolation policies for infection control, García-Lecona et al reported that CDI patients in a common isolation unit showed a decreased mortality rate but an increased rate of recurrence than those managed alone.22 Recently, ultraviolet disinfection has been trialed in this context with promising outcomes.23,24 We have not employed this disinfection strategy but a previous study supports its future utilization by our team.
The idea of an interprofessional approach to antimicrobial stewardship was reviewed at the turn of the 21st century.25 In 2015, the Centers for Disease Control and Prevention issued the “Core Elements” of antimicrobial stewardship, stressing the importance of a multidisciplinary approach.26 Subsequently in 2017, Dik et al reported an antimicrobial, infection control, and diagnostic approach to reduce CDIs.27 Our ipAS method has merits in the measurement of AUDs to document a reduction in the use of broad-spectrum antimicrobials.
The limitations of our study include a retrospective comparison of AUDs before and after the establishment of ipAS, a graded initiation time in 2013 because of a multidisciplinary approach and the use of PDCA cycles, historical bias in vendor difference in CDTs, and the repeat counting of recurrent CDIs for the same patient over numerous years. The last issue has been addressed elsewhere.28 Our results, however, await future validation in prospective studies.
Conclusion
The integrated antimicrobial, diagnostic, infection control approach represented by ipAS may reduce CDIs.
Disclosure
Junichi Yoshida reports funds from Sanofi Aventis, MSD, and Astellas Co. for the clinical trials of CDI. Masao Tanaka reports grants from Olympus Inc., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Bui C, Zhu E, Donnelley MA, et al. Antimicrobial stewardship programs that target only high-cost, broad-spectrum antimicrobials miss opportunities to reduce Clostridium difficile infections. Am J Infect Control. 2016;44(12):1684–1686. doi:10.1016/j.ajic.2016.06.025
2. Patton A, Davey P, Harbarth S, et al. Impact of antimicrobial stewardship interventions on Clostridium difficile infection and clinical outcomes: segmented regression analyses. J Antimicrob Chemother. 2018;73(2):517–526. doi:10.1093/jac/dkx413
3. Yanke E, Moriarty H, Carayon P, Safdar N. A qualitative, interprofessional analysis of barriers to and facilitators of implementation of the Department of Veterans Affairs’ Clostridium difficile prevention bundle using a human factors engineering approach. Am J Infect Control. 2018;46(3):276–284. doi:10.1016/j.ajic.2017.08.027
4. De Backer D, Dorman T. Surviving sepsis guidelines: a continuous move toward better care of patients with sepsis. JAMA. 2017;317(8):807–808. doi:10.1001/jama.2017.0059
5. Japanese Association of Infectious Diseases and Japanese Society of Chemotherapy. Kansensho Chiryo Guide [Guide for Treatment of Infectious diseases]. Tokyo: JAID/JSC; 2014.
6. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. centers for disease control and prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. 1999;27(2):97–132. doi:10.1016/S0196-6553(99)70088-X
7. Fujibayashi A, Niwa T, Tsuchiya M, et al. Antimicrobial stewardship intervention for the clinical pathways improves antimicrobial prophylaxis in surgical or non-surgical invasive therapies. Int J Clin Pract. 2018;10:e13293.
8. Updated Guidance on the Management and Treatment of Clostridium Difficile Infection [Homepage on the internet]. London: Public Health England; 2013. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/321891/Clostridium_difficile_management_and_treatment.pdf.
9. ATC/DDD Index 2019 [Homepage on the internet]. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2019. Available from: https://www.whocc.no/atc_ddd_index/.
10. de With K, Allerberger F, Amann S, et al. Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases. Infection. 2016;44(3):395–439.
11. Huttner B, Harbarth S, Nathwani D, ESCMID Study Group for Antibiotic Policies (ESGAP). Success stories of implementation of antimicrobial stewardship: a narrative review. Clin Microbiol Infect. 2014;20(10):954–962. doi:10.1111/1469-0691.12803
12. Balch A, Wendelboe AM, Vesely SK, Bratzler DW. Antibiotic prophylaxis for surgical site infections as a risk factor for infection with Clostridium difficile. PLoS One. 2017;12(6):e0179117. doi:10.1371/journal.pone.0179117
13. Sartelli M, Kluger Y, et al; Global Alliance for Infections in Surgery Working Group. A global declaration on appropriate use of antimicrobial agents across the surgical pathway. Surg Infect (Larchmt). 2017;18(8):846–853. doi:10.1089/sur.2017.219
14. Niwa T, Shinoda Y, Suzuki A, et al. Outcome measurement of extensive implementation of antimicrobial stewardship in patients receiving intravenous antibiotics in a Japanese university hospital. Int J Clin Pract. 2012;66(10):999–1008. doi:10.1111/j.1742-1241.2012.02999.x
15. Yoon YK, Yang KS, Lee SE, Kim HJ, Sohn JW, Kim MJ. Effects of Group 1 versus Group 2 carbapenems on the susceptibility of Acinetobacter baumannii to carbapenems: a before and after intervention study of carbapenem-use stewardship. PLoS One. 2014;9(6):e99101. doi:10.1371/journal.pone.0099101
16. Borde JP, Kern WV, Hug M, et al. Implementation of an intensified antibiotic stewardship programme targeting third-generation cephalosporin and fluoroquinolone use in an emergency medicine department. Emerg Med J. 2015;32(7):509–515. doi:10.1136/emermed-2014-204067
17. Hohn A, Heising B, Hertel S, Baumgarten G, Hochreiter M, Schroeder S. Antibiotic consumption after implementation of a procalcitonin-guided antimicrobial stewardship programme in surgical patients admitted to an intensive care unit: a retrospective before-and-after analysis. Infection. 2015;43(4):405–412. doi:10.1007/s15010-014-0718-x
18. Kawamura H, Matsumoto K, Shigemi A, et al. A bundle that includes active surveillance, contact precaution for carriers, and cefazolin-based antimicrobial prophylaxis prevents methicillin-resistant Staphylococcus aureus infections in clean orthopedic surgery. Am J Infect Control. 2016;44(2):210–214. doi:10.1016/j.ajic.2015.09.014
19. Hagiwara D, Sato K, Miyazaki M, et al. The impact of earlier intervention by an antimicrobial stewardship team for specific antimicrobials in a single weekly intervention. Int J Infect Dis. 2018;77:34–39. doi:10.1016/j.ijid.2018.09.025
20. Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med. 2016;176(12):1801–1808. doi:10.1001/jamainternmed.2016.6193
21. Tarrant J, Jenkins RO, Laird KT. From ward to washer: the survival of Clostridium difficile spores on hospital bed sheets through a commercial UK NHS healthcare laundry process. Infect Control Hosp Epidemiol. 2018;39(12):1406–1411. doi:10.1017/ice.2018.255
22. García-Lecona DA, Garza-González E, Padilla-Orozco M, et al. Outcomes of Clostridium difficile-infected patients managed in a common isolation unit compared with isolation in their bed of diagnosis. Am J Infect Control. 2018;46(1):103–104. doi:10.1016/j.ajic.2017.06.006
23. Sampathkumar P, Folkert C, Barth JE, et al. A trial of pulsed xenon ultraviolet disinfection to reduce Clostridioides difficile infection. Am J Infect Control. 2019;47(4):406–408. doi:10.1016/j.ajic.2018.09.018
24. Turner MC, Behrens SL, Webster W, et al. Multidisciplinary approach to clostridium difficile infection in adult surgical patients. J Am Coll Surg. 2019;228(4):570–580. doi:10.1016/j.jamcollsurg.2018.12.045
25. Paskovaty A, Pflomm JM, Myke N, et al. A multidisciplinary approach to antimicrobial stewardship: evolution into the 21st century. Int J Antimicrob Agents. 2005;25(1):1–10. doi:10.1016/j.ijantimicag.2004.09.001
26. Core Elements of Hospital Antibiotic Stewardship Programs [Homepage on the internet]. Atlanta: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP); 2015. Available from: https://www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html#_ENREF_33.
27. Dik JH, Poelman R, Friedrich AW, Niesters HGM, Rossen JWA, Sinha B. Integrated stewardship model comprising antimicrobial, infection prevention, and diagnostic stewardship (AID stewardship). J Clin Microbiol. 2017;55(11):3306–3307. doi:10.1128/JCM.01283-17
28. Yoshida J, Kikuchi K, Ueno T, Mataga A, Asano I, Tamura T. Recurrent Clostridioides difficile infection in surgery and outpatient units: a 12-year study. J Jpn Soc Surg Infect. 2019;16(3):173–179.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.