Back to Journals » Journal of Blood Medicine » Volume 10
Interleukin-27 and interleukin-35 in de novo acute myeloid leukemia: expression and significance as biological markers
Authors Ahmed HA , Maklad AM , Khaled SAA , Elyamany A
Received 29 June 2019
Accepted for publication 13 August 2019
Published 1 October 2019 Volume 2019:10 Pages 341—349
DOI https://doi.org/10.2147/JBM.S221301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Video abstract presented by Safaa AA Khaled
Views: 223
Heba A Ahmed,1 Ahmed M Maklad,2,3 Safaa AA Khaled,4 Ashraf Elyamany5,6
1Department of Clinical Pathology, Faculty of Medicine, Sohag University, Sohag, Egypt; 2Department of Clinical Oncology and Nuclear Medicine, Sohag University Hospitals, Sohag, Egypt; 3Department of Radiation Oncology, King Fahad Medical City, Riyadh, KSA; 4Department of Internal Medicine, Clinical Hematology Unit, Faculty of Medicine/Unit of Bone Marrow Transplantation, South Egypt Cancer Institute, Assiut University, Assiut, Egypt; 5Department of Medical Oncology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt; 6Department of Medical Oncology, King Saud Medical City, Riyadh, KSA
Correspondence: Safaa AA Khaled
Department of Internal Medicine, Clinical Hematology Unit, Faculty of Medicine/Unit of Bone Marrow Transplantation, South Egypt Cancer Institute, Assiut University Hospital, 8 th floor, PO Box 71111, Assiut, Egypt
Tel +20 88 241 3826
Email [email protected]
Background and objectives: IL27 and IL35 are regulatory T cells (T-regs) related cytokines; they were accused in eukemogenesis of acute myeloid leukemia (AML). This study aimed to assess the expression of these cytokines in de novo AML and investigate their role as biomarkers.
Subjects and methods: Seventy newly diagnosed patients with primary AML and 30 matched healthy volunteers were recruited. AML diagnosis was confirmed with flowcytometric and immunophenotypic analyses, while ELISA was used to assess serum levels of IL27 and IL35 in patients and controls. Receiver operating characteristic curve analysis was used to estimate IL27 and IL35 optimum cutoff values for predicting AML.
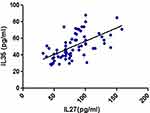
Results: Serum levels of both cytokines were significantly higher in AML patients than controls (P<0.001), with no effect of gender or French-American-British subtypes. Significant correlations of IL27 and IL35 with poor prognostic factors and with each other were detected in patients only. IL27 optimum cutoff for predicting AML was >43, AUC (0.926) with a sensitivity 74% and specificity 96.6% (P<0.001), while for IL35>27.8, AUC (0.972) with 88% and 98% sensitivity and specificity, respectively (P<0.001).
Conclusion: Conclusively, this study proved that IL27and IL35 could identify AML patients from healthy subjects, and their overexpression denotes poor prognosis. Based on the simplicity and wide availability of their detection technique we recommend the inclusion of IL27 and IL35 in the diagnostic/prognostic workup of AML; however, further longitudinal research is needed to prove their exact prognostic value.
Keywords: acute myeloid leukemia (AML), regulatory T cells, flowcytometry, IL27, IL35
Introduction
AML is a hematopoietic neoplasm derived from early progenitor myeloid cells; it is characterized by clonal expansion of myeloblasts, which are immature myeloid progenitors that accumulate in the bone marrow (BM). AML can arise in patients with antecedent hematological disorder and named secondary AML, or as primary disorder in normal individuals (de novo AML) or as a consequence of prior therapy (therapy-related AML). Even with the use chemotherapy, 70% of patients 65 years old, or more, may die because of their disease or its complications, within 1 year of diagnosis.1–4 Immune system impairment and immunetolerance were reported in patients with AML. T cells are the most active part of the immune system, nevertheless they were found to be functionally and numerically defective in AML.5
Tregs suppress the function and multiplication of T helper (Th) cells. AML patients show paradox high level of T-regs in their BM and peripheral blood compared with healthy subjects.5 Tumorgenesis of AML has not been fully clarified, tumor immune escape mechanism controlled by CD4+ & CD25+ Tregs was proposed as the hull mark of AML pathogenesis.6 Circulating T-regs in AML patients mediate vigorous suppression, through contact dependent and independent mechanisms. Furthermore, increased expression of T-reg might enhance tumor development or growth and affects the natural history of the disease. Better response to induction regimens was found in patients with lower T-regs at diagnosis. Based on these findings, depletion of T-regs was suggested as a future therapy that could offer great hope for AML patients.7,8
The family IL12 are heterodimeric cytokines characterized by three alpha subunits (p19, p28, p35) and two beta subunits (EBI3 and p40), that led to six different pairings. There is concluding evidence that the pairing occurs in vivo for IL12 (p35/p40), IL23 (p19/p40) and IL-27 (p28/EBI3).9 Structure similarity within the IL12 family leads to very different biological properties. In general, IL27 and IL35 function mainly as anti-inflammatory or immunoregulatory cytokines.10 IL27 has antitumor effects in different cancer models, therefore it has been proposed as a potential agent in cancer immunotherapy studies.11 IL27 also displays pleiotropic functions, which comprise both immune-enhancing and immune-regulatory effects.12 Several studies found correlation between increased serum levels of IL27 and disease progression or cancer growth in different types of tumors.13–16
IL35 is specifically expressed by resting and activated CD4+ Foxp3+ T-regs and is considered to be a crucial anti-inflammatory cytokine, which could suppress Th1, Th2 and Th17 cell-responses in a context-dependent manner.17 IL35 induces the conversion of naïve T cells into regulatory T cells and downregulates Th17 cell development and differentiation as well suppression of autoimmune inflammation.18 Tao et al found that IL35 exerted a pro-proliferative and an anti-apoptotic effect on AML blasts. These findings pointed to the role of IL35 in the mechanism of immune escape in AML.19
This study aimed to assess serum levels of IL27 and IL35 in patients with primary AML, and to investigate their role as biological markers.
Subjects and methods
Study settings and subjects
AML patients were recruited among those attending the Hematology/Oncology Clinics at Sohag University Hospital, during the period from January 2016 to June 2018. They were included after fulfilling the following inclusion criteria:
- Newly diagnosed (ND) AML patients.
- De novo AML.
- Willingness for participation in the study.
Another 30 healthy volunteers were collected as controls they were age and sex matched with the patients, and were among medical students, medical and paramedical professionals.
Study tools
Medical history, clinical examination and laboratory investigations
Both patients and controls were subjected to thorough history and physical examination, next blood samples were withdrawn for complete blood count (CBC) using Cell-Dyn 3700, automated cell counter (Abbott diagnostic, Dallas, USA).
The diagnosis of AML in the study patients was according to the WHO recommended criteria,20 accordingly patients were subjected to further investigations as:
- Leishman-stained peripheral blood smears.
- BM aspirates or occasionally trephine biopsy.
- Cytochemical staining of BM specimens using myeloperoxidase (MPO), Esterase and periodic acid schiff.
- Immunophenotyping (IPT): monoclonal antibodies (MoAb) used for staining of blast cells were labeled with fluorescein Isothiocyanate, phycoerythrin. Markers looked for to prove or disprove diagnosis of AML including: myeloid markers: CD13, CD33, MPO. Monocytic markers: CD14. B cell markers: CD19, CD20, CD22 and CD79a. T cell markers: CD3, CD5, CD2 and CD7. Common progenitor marker: HLA-DR, CD34. All Moab were purchased from Becton Dickinson Bioscience, San Jose, CA, USA. FACS acquisition and analysis were performed with FACS Cell Quest software (BD Biosciences).
The diagnosis of AML subtype was based on the standard morphological and IPT criteria.21
Karyotyping and assessment of other molecular prognostic markers were not available for all patients in our center so they were not included in the current research.
Measurement of serum levels of IL27 and IL35 in the study subjects
Serum was obtained from plain blood sample tubes that were kept at −80°C by centrifugation. Serum levels of IL-27 and IL-35 were acquired by ELISA, for both patients and controls, using the commercially available kits (Elabscience Systems, Houston, Texas, USA, catalogue numbers E-EL-H2338 and E-EL-H2443, respectively). Absorbance was recorded at 495 nm, IL-27 assay range was 31.25–2000 pg/mL, and IL-35 assay range was 15.63–1000 pg/mL.
Data analysis
Data were analyzed using IBM SPSS Statistics for Windows version 24 and Medcalc version 18.1 Quantitative data were expressed as means±standard deviations, while qualitative data as numbers and percentages. Shapiro–Wilk test was used to test for normality of quantitative data, on the other hand Mann–Whitney U test, Wilcoxon signed-ranks test and Spearman’s correlation were used for not normally distributed data. Independent Samples t-test and Pearson’s correlation were used for assessing differences and associations among normally distributed data and Chi-square (χ2) test for categorical variables. Receiver operating characteristic (ROC) curve was constructed to detect IL27 and IL35 optimum cutoff points in predicting AML, and the area under the ROC curve with 95% CI was calculated, sensitivity and specificity were also calculated. A 5% level was chosen as a level of significance in all statistical tests used in the study.
Ethical considerations
The study was consistent with the World Medical Association declaration of Helsinki, and the study design was approved by the Research Ethical Committee of Faculty of Medicine, Sohag University. The study design protocol and significance was explained to the study participants. All the study participants signed an informed consent and confidentiality was addressed.
Results
Characteristics of the study participants
A total of 100 subjects were enrolled in the study, 70 ND patients with de novo AML and 30 age and sex matched healthy controls. Table 1 shows a comparison between patients and controls regarding demographic, clinical and CBC data. There were no significant differences between patients and controls as regard age and gender distribution (P=0.552 and 0.513, respectively), which ensured perfect matching. The median age of the study patients was 36 years and the range was 18–80 years. Females comprised 52.9% of the patient group with a female to male ratio 1:1. There were significant differences between AML patients and controls (P=<0.001) in CBC parameters (WBCs, hemoglobin and platelets).
 |
Table 1 Comparison between AML patients and the control group as regarding age, gender, clinical and laboratory criteria |
Figure 1 illustrates the distribution of the French-American-British (FAB) subtypes of AML patients which was, 3 patients with M0 (4.2%), 7 with M1 (10.1%), 22 with M2 (31.4%), 20 with M3 (28.6%), 10 with M4 (14.3) and 8 with M5 (11.4%).
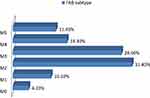 |
Figure 1 Distribution of the French-American-British (FAB) subtypes in the study patients. |
Serum levels of IL27 and IL35 in AML patients compared with the controls
Table 2 shows a significantly higher levels (P<0.001) of IL27 and IL35 in AML patients compared with the control subjects, with median values of 77 and 51 pg/mL compared to 37 and 22.2 pg/mL, respectively.
 |
Table 2 Comparison of serum levels of IL27 (pg/mL) and IL35 (pg/mL) among AML patients and the control group |
On the other hand, there were no significant differences in levels of IL27 and IL35 between males and females, whether in patients or the controls, as in Table 3. Figure 2 illustrates no significant differences in mean values of IL27 and IL35 among different FAB subtypes of the study patients (F=0.93 and 0.25; P=047 and 0.94, respectively).
 |
Table 3 Comparison of IL27 (pg/mL) and IL35 (pg/mL) serum levels among males and females of AML patients and the control group |
Associations between serum levels of IL27 and IL35 and other variables in the study subjects
Table 4 shows the correlations between serum levels of IL27 and IL3 in AML patients (n=70), it revealed a significant positive correlation between IL27 with, WBCs count, peripheral blasts percent and MPO positivity (r=0.269, 0.328 and 0.237; P=0.024, 0.030 and 0.048, respectively). However, IL35 levels showed a significant negative correlation with platelets count (r=−0.267, P=0.025) and significant positive correlations with age, CD45, HLA-DR, CD34 (r=0.294, 0.273, 0.240 and 0.259; P=0.038, 0.022, 0.045 and 0.030, respectively).
 |
Table 4 Correlations of IL 27 (pg/mL) and IL35 (pg/mL) serum levels with age, laboratory and immunophenotypic parameters of AML patients (n=70) |
Table 5 shows no significant correlations between cytokine levels and other quantitative variables of the control group.
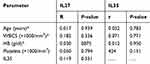 |
Table 5 Correlations between IL27 (pg/mL) and IL35 (pg/mL) with age and laboratory parameters of the control group (n=30) |
Figure 3 and Table 5 reveal a significant positive correlation between IL27 and IL35 levels with each other (r=0.253 and P=0.042) for the patient group only.
Predictive values of IL27 and IL35 in de novo AML
For detection of optimum IL27 and IL35 cutoff level that is needed for predicting AML a ROC curve analysis was done that demonstrated that IL27 optimum cutoff is >43, AUC 0.926 with 74% of 96.6% sensitivity and specificity 100% P-value <0.001. IL35 optimum cutoff is >27.8 AUC 0.972 with 88% of 98% sensitivity and specificity 100%, as shown in Figure 4. Table 6 shows the results of the logistic regression analysis of predictors in de novo AML, P-value <0.001.
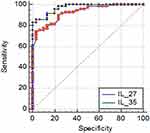 |
Figure 4 Receiver operating characteristic (ROC) curve of IL27 and IL35 for optimum cutoff point in predicting AML. |
 |
Table 6 Logistic regression analysis of predictors in de novo acute myeloid leukemia |
Discussion
AML is a type of blood cancer that mostly has poor consequences. Over the past 10 years the image of AML was albeit changed, this was in part due to advances in understanding AML tumorgenesis and detection of new molecular markers.22–24 Nevertheless there is a lack in the literature regarding the exact pathogenetic mechanism of AML development and pathogenesis. Recently, the T-regs related cytokines, IL27 and IL35 were implicated in pathogenesis of AML either directly or indirectly. However, their role in AML is similar to a buzzle where some researchers proven their antileukemic effects whereas others reported their leukemogenic potential.25–28 Therefore, the role of cytokines IL27 and IL35 in AML is a fertile research area, particularly after the success of immunotherapy in other hematological malignancies.29,30
This study aimed to assess the expression of IL27 and IL35 in de novo AML and explore their role as biological markers of the disease, to do so 70 ND AML patients and 30 matched controls were included in the study. Both patients and controls were subjected to a panel of clinical and laboratory investigations. Analysis of the obtained results ensured perfect matching between patients and controls. Strikingly, this study showed a younger age female predominant AML, compared to most research in AML.31,32 The explanation for these conflicting results that willingness for participation was a prerequisite before enrollment in the current study.
To investigate the expression of our target cytokines in AML patients, we compared serum levels of IL27 and IL35 in ND patients with AML with those in the controls. Notably, the serum levels of cytokines in patients were in the reference range supplied by the kit. Results of the current study showed significantly higher levels of IL27 and IL35 in ND AML patients compared with the control group. However, there were no significant effect of gender or FAB subtypes on cytokine values. Absence of gender effect was further confirmed when we compare interleukin values in males and females of the control group and find similar results. Our findings were in agreement with Zorzoli et al, Tao et al and Wu et al who estimated IL27 in AML pediatric patients and IL35 in adult patients, respectively, they demonstrated a significantly higher serum concentration of IL27 and IL35 in patients compared to the controls.19,27,33 These findings demonstrated the value of IL27 and IL35 as diagnostic markers in ND patients with AML.
To explore the value of IL27 and IL35 as prognostic markers in AML, we analyzed their correlation with other prognostic factors in the study patients. To validate our results we studied the correlation between IL27 and IL35 levels with age and CBC parameters of the controls. Interestingly, our results showed no significant correlation of cytokine levels with any parameter in the control group. On the contrary, we noted significant positive correlations of IL27 and IL35 with poor prognostic factors in AML as WBCs count, MPO and peripheral blasts %, and age, platelets count and CD34, respectively.
In the current study IL27 level seems to be higher in M3 subgroup, despite the usually low WBC/blast number at onset in this FAB group. This could be explained by the different source of origin of the cytokine other than blast cells, furthermore differences in cytokines were not significantly different among FAB subtypes.
The above-mentioned results suggested the value of IL27 and IL35 as prognostic markers in AML and accused them in development and disease progression of AML. In addition, they reinforced the work of Shenghu et al, who found that high levels of IL35 increased the suppressive function of T-reg cells in peripheral blood and BM in patients with AML, this was suggested to play a role in immunetolerance in AML.34 Also the work of Tao et al, who concluded that increased IL35 levels facilitated the immune escape of AML blasts, adult AML blasts, enhanced blast proliferation and inhibited blast apoptosis, as such they suggested IL35 to be a future therapeutic target in AML.19 On the contrary, our findings contradicted with Nasreldin et al who found no significant association between IL35 and platelets count,7 this difference could be explained by the difference in type of AML patients between the two studies, wherein Nasreldin et al's study included ND patients with AML and those in complete remission.
Next, we go further and analyzed the correlation of IL27 and IL35 with each other in both patients and controls. Our results revealed, for the first time, a significant positive correlation of IL27 with IL35 serum levels in AML patients which was obviously absent in the control group. The most logical explanation for these results relies on the findings of Ersvaer et al, who revealed that in ND patients with AML, frequencies of T-regs are higher compared to the controls, and remain high during treatment. They believed that high levels of T-regs at AML diagnosis could denote poor prognosis.35 IL27 and IL35 were found to be T-regs related cytokines that belonged to the same family and performed albeit similar biological functions.9,10 Accordingly, upregulation of T-regs in AML will subsequently result in increased expression of both IL27 and IL35.
IL35 was found to be produced by B-cells (B-regs) as well as T-regs.36,37 This means that serum levels of IL35 could be affected by up- or downregulation of both T-regs and B-regs. This could explain the modest increase of IL35 levels noted in AML patients.
In order to check for the effectiveness of both IL27 and IL35 as diagnostic markers in ND AML, we follow other researchers and use ROC curve analysis.38,39 Interestingly, our results reported a new finding that both IL27 and IL35 have a low false negative rate (specificity), which mean that their use as diagnostic tool in AML could help rule in the disease.
This study proved the value of IL27 and IL35 as biomarkers in de novo AML, and generated a cutoff value for IL27 and IL35 as predictor in AML, from ROC curve, it was 43 (pg/mL) and 27.8 (pg/mL), respectively with high statistical significance.
Conclusion
From this study, we proved, for the first time, that serum levels of IL27 and IL35 might be served as highly sensitive and specific diagnostic/prognostic markers in patients with de novo AML. Also, we suggested that IL27 and IL35 overexpression in ND AML would be associated with poor prognosis. In addition, we reported strong association between the two cytokines in AML, and we generated cutoff values for IL27 and IL35 as predictors for AML. As such we claim IL27 and IL35 in AML development and pathogenesis.
On the light of these results, we recommended assay of IL27 and IL35 in ND AML, as the method for their measurement is relatively cheap, simple and widely available. Furthermore, based on our finding of the intimate association between IL27 and IL35, we assumed measuring one cytokine only to save time and reduce cost. However, further wide scale longitudinal studies are mandatory to link certain cytokine values to disease progression, response to treatment and survival analyses.
Based on the results of this study it was indirectly suggested that cytokine levels can predict relapse probability but unfortunately we focused only in de novo cases to elicit diagnostic/prognostic value of cytokines. Accordingly, future research is needed to investigate cytokine levels in relapsed patients.
Acknowledgments
The authors wish to thank all patients and controls who voluntarily participated in the study. Great thanks to Jennifer H. Mesde the English Language teacher at Shaqra University, for revising English language of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stone RM, O’Donnell MR, Sekeres MA. Acute myeloid leukemia. Hematol Am Soc Hematol Educ Program. 2004;98–117. doi:10.1182/asheducation-2004.1.98
2. Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88:318–327. doi:10.1002/ajh.23404
3. Sill H, Olipitz W, Zebisch A, et al. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br J Pharmacol. 2011;162:792–805. doi:10.1111/j.1476-5381.2010.01100.x
4. Meyers J, Yu Y, Kaye JA, et al. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy. 2013;11:275–286. doi:10.1007/s40258-013-0032-2
5. Ustun C, Miller JS, Munn DH, et al. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118(19):5084–5095. doi:10.1182/blood-2011-02-334870
6. Szczepanski MJ, Szajnik M, Czystowska M, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15:3325–3332. doi:10.1158/1078-0432.CCR-08-3010
7. Nasreldin E, Safwat DM, Hamed HB, et al. T-regulatory and T-helper type17 cells associated cytokines (IL-35, IL-17) as potential diagnostic and prognostic biomarkers in egyptian acute myeloid leukemia patients. J Clin Cell Immunol. 2016;7:478. doi:10.4172/2155-9899
8. Yang W, Xu Y. Clinical significance of Treg cell frequency in acute myeloid leukemia. Int J Hematol. 2013;98(5):558–562. doi:10.1007/s12185-013-1436-3
9. Hasegawa H, Mizoguchi I, Chiba Y, et al. Expanding diversity in molecular structures and functions of the IL-6/IL-12 heterodimeric cytokine family. Front Immunol. 2016;7:479. doi:10.3389/fimmu.2016.00479
10. Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–728. doi:10.1038/ni.2366
11. Yoshimoto T, Chiba Y, Furusawa J-I, et al. Potential clinical application of interleukin-27 as an antitumor agent. Cancer Sci. 2015;106(9):1103–1110. doi:10.1111/cas.12731
12. Li MS, Liu Z, Liu JQ, et al. The Yin and Yang aspects of IL-27 in induction of cancer-specific T-cell responses and immunotherapy. Immunotherapy. 2015;7(2):191–200. doi:10.2217/imt.14.95
13. Diakowska D, Lewandowski A, Markocka-Mączka K, et al. Concentration of serum interleukin-27 increase in patients with lymph node metastatic gastroesophageal cancer. Adv Clin Exp Med. 2013;22:683–691.
14. Gonin J, Carlotti A, Dietrich C, et al. Expression of IL-27 by tumor cells in invasive cutaneous and metastatic melanomas. PLoS One. 2013;8:e75694. doi:10.1371/journal.pone.0075694
15. Lu D, Zhou X, Yao L, et al. Clinical implications of the interleukin 27 serum level in breast cancer. J Investig Med. 2014;62:627–631. doi:10.2310/JIM.0000000000000046
16. Larousserie F, Bardel E, Pflanz S, et al. Analysis of interleukin-27 (EBI3/p28) expression in Epstein-Barr virus- and human T-cell leukemia virus type 1-associated lymphomas: heterogeneous expression of EBI3 subunit by tumoral cells. Am J Pathol. 2005;166:1217–1228. doi:10.1016/S0002-9440(10)62340-1
17. Collison LW, Chaturvedi V, Henderson AL, et al. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. J Immunol. 2011;187:462–471. doi:10.4049/jimmunol.1100967
18. Workman CJ, Brown SA, Rehg JE, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi:10.1038/ni.1952
19. Tao Q, Pan Y, Wang Y, et al. Regulatory T cells-derived IL-35 promotes the growth of adult acute myeloid leukemia blasts. Int J Cancer. 2015;137:2384–2393. doi:10.1002/ijc.29563
20. Appelbaum FR. Chapter 98: acute leukemias in adults. In: Niederhuber JE, Armitage JO, Dorshow JH, Kastan MB, Tepper JE, editors. Abeloff’s Clinical Oncology.
21. Kebriaei P, de Lima M, Estey EH, et al. Chapter 107: management of acute leukemias. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology.
22. Khaled SA, Burthem J, Abo ElNoor E, et al. Differences between nucleophosmin isoforms in de-novo acute myeloid leukemia: possible implications in developing targeted therapy for acute myeloid leukemia with normal karyotype. Egypt J Hematol. 2015;40(41):190–194. doi:10.4103/1110-1067.170220
23. Schlenk RF, Döhner K, Kneba M, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG Trial AML HD98B. Haematol`ogica. 2009;94(1):54–60. doi:10.3324/haematol.13378
24. Khaled SA, Burthem J, Abo ElNoor E, et al. Role of nucleophosmin gene mutation in leukemogenesis of acute myeloid leukemia. J Hematol. 2018;7(1):7–13. doi:10.14740/jh365w
25. Wang Z, Liu JQ, Liu Z, et al. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol. 2013;190:2415–2423. doi:10.4049/jimmunol.1202535
26. Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23:2233–2241. doi:10.1038/leu.2009.175
27. Zorzoli A, Di Carlo E, Cocco C, et al. Interleukin-27 inhibits the growth of pediatric acute myeloid leukemia in NOD/SCID/Il2rg-/- mice. Clin Cancer Res. 2012;18(6):1630–1640. doi:10.1158/1078-0432.CCR-11-2432
28. Yoshida H, Hunter A. The immunobiology of interleukin27. Annu Rev Immunol. 2015;33:417–443. doi:10.1146/annurev-immunol-032414-112134
29. Wei G, Wang J, Huang H, Zhao Y. Novel immunotherapies for adult patients with B-lineage acute lymphoblastic leukemia. J Hematol Oncol. 2017;10(1):150. doi:10.1186/s13045-017-0516-x
30. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi:10.1056/NEJMoa1407222
31. Phekoo KJ, Richards MA, Moller H, Schey SA. The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica. 2006;91(10):1400–1404.
32. Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi:10.1182/blood-2005-09-3724
33. Wu H, Li P, Shao N, et al. Aberrant expression of Treg-associated cytokine IL-35 along with IL-10 and TGF-Î2 in acute myeloid leukemia. Oncol Lett. 2012;3:1119–1123. doi:10.3892/ol.2012.614
34. Shenghui Z, Yixiang H, Jianbo W, et al. Elevated frequencies of CD4⺠CD25⺠CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer. 2011;129:1373–1381. doi:10.1002/ijc.25791
35. Ersvaer E, Liseth K, Skavland J, et al. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC, TH, TH17 and TREG cells. BMC Immunol. 2010;9:11–38.
36. Wang R-X, Yu C-R, Dambeza IM, et al. Interleukin-35 induces regulatory B-cells that suppress autoimmune disease. Nat Med. 2014;20:633–641. doi:10.1038/nm.3554
37. Shen P, Roch T, Lampropoul V, et al. IL35 producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;567:366–370. doi:10.1038/nature12979
38. Zweig MH, Camphell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577.
39. Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi:10.1126/science.3287615
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.