Back to Journals » Orthopedic Research and Reviews » Volume 11
Interesting effectiveness of ozone injection for carpal tunnel syndrome treatment: a randomized controlled trial
Authors Bahrami MH, Raeissadat SA, Nezamabadi M, Hojjati F , Rahimi-Dehgolan S
Received 24 January 2019
Accepted for publication 10 April 2019
Published 6 May 2019 Volume 2019:11 Pages 61—67
DOI https://doi.org/10.2147/ORR.S202780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Clark Hung
Mohammad Hasan Bahrami,1 Seyed Ahmad Raeissadat,2 Mohammadrasoul Nezamabadi,1 Fateme Hojjati,1 Shahram Rahimi-Dehgolan3
1Physical Medicine and Rehabilitation Department and Research Center, Shohada-e-Tajrish Hospital, Shahid Beheshti University of Medical Sciences, School of Medicine, Tehran, Iran; 2Clinical Development Research Center of Shahid Modarres Hospital, Physical Medicine and Rehabilitation Department and Research Center, Shahid Beheshti University of Medical Sciences, School of Medicine, Tehran, Iran; 3Physical Medicine and Rehabilitation Department, Tehran University of Medical Sciences (TUMS), School of Medicine, Tehran, Iran
Purpose: Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy in the upper limb. Local injection of different substances has been increasingly used as an acceptable conservative treatment in non-severe cases. This study aimed to evaluate the efficacy and safety of local ozone injection in the management of non-severe CTS.
Methods: In the current randomized controlled trial (RCT), 40 patients with mild or moderate CTS were included in two parallel groups. Both of them used the resting volar wrist splint for 8 weeks; while the intervention group received a single dose of local ozone injection, except for the same splinting protocol. The main outcome measures including visual analog scale (VAS) for pain; symptom severity or functional status, based on Boston questionnaire (BQ); and median nerve conduction study, were reassessed 10 weeks after the treatment.
Results: All of the measures including VAS, symptom severity, functional status and EDX improved significantly in both groups with the maximal changes in VAS. The VAS reduction was more remarkable in the ozone group than the control group [64% versus 45.3%, respectively]. Moreover, both of the BQ subscales showed significantly higher improvement in the ozone group compared to the control group (P=0.01 and 0.02, respectively). Although the improvement of EDX parameters was slightly better in the ozone group, the difference was not significant. Neither minor nor major side effects were reported.
Conclusion: Ozone therapy as a safe and low-cost method, could provide promising results among women with mild to moderate CTS, at least for short-term treatment.
Clinical trial registration: IRCT2016040913442N9.
Keywords: local corticosteroid injections, oxygen-ozone, wrist splints
Introduction
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy in the upper limb which is caused by compression of the median nerve as it travels through the carpal tunnel within the flexor retinaculum of the wrist.1,2 The more commonly suggested mechanism explains that increased mechanical pressure in the carpal tunnel can lead to compression, inflammation and decreased blood supply to the nerve which altogether can lead to nerve lesion; thereby producing typical symptoms such as pain, numbness, weakness in grip and tingling usually in the thumb, index and middle fingers.2–4 The incidence of CTS has been reported to be 1–3 per 100 people per year; CTS is associated with the second longest average time away from work and its cost is estimated to be US$30,000 per worker in the United States of America.4,5
Although the surgical release of the retinaculum in advanced cases has been proposed as the method of choice,6 there is not a definite consensus for choosing a single conservative treatment as the best therapeutic option.7 Surgery resolves CTS in 70% of cases with clinical remission lasting up to 30 months. Sometimes severe complications may occur such as nerve injury, infection, etc. Also the recurrence of symptoms is not uncommon, particularly among the secondary cases of CTS.8 Considering the burden and possible complications of surgery, many patients with mild to moderate grades might prefer to select one of the conservative treatments including: oral non-steroidal anti-inflammatory drugs (NSAIDs), diuretics, pyridoxine, wrist splinting, physical agent modalities like LASER, ultrasound (US),9–12 and the most recent method, ie, local injections of corticosteroid, progesterone, platelet-rich plasma (PRP) etc.13–16
Wrist splinting, as a method to avoid further injury, is one of the most common treatments among CTS patients. On the other hand, local corticosteroid injections have been used as a standard conservative treatment in cases of mild or moderate CTS who are resistant to other non-invasive methods; these two therapeutic methods have been endorsed by the American Academy of Orthopedic Surgeons (AAOS) clinical practice guideline published in 2009.5,13 It has been also proved by previous researches that corticosteroid injections cause significant symptom relief, but are not persistent in the long-term. However, it is still the most rapid option for relieving the symptoms. Similarly, splinting is significantly effective on symptoms improvement, functional status, and nerve conduction velocity.13–16
Recent studies have also suggested local ozone injection as a therapeutic option in some musculoskeletal conditions; ozone (O3) gas is a molecule consisting of three oxygen atoms in a dynamically unstable structure. Ozone therapy has been utilized and studied for more than a century. Ozone is a re-emerging substance that has many biological effects such as bactericidal, immune-modulatory, analgesic, anti-inflammatory, anti-oxidative, as well as enhancing the blood circulation. The most established therapeutic mechanisms are as follows: A) Indirect mechanical decompression in the site of nerve entrapment by increasing tissue oxygenation with reduced venous or lymphatic stasis. B) Suppression of the cell-mediated immune response by inhibiting macrophages from the release of proteinase, and also by induction of inhibitory mediators such as interleukin-10 and TGF-beta. C) Suppression of the humoral immune system, by decreasing the prostaglandins release and pro-inflammatory bradykinins.8 Moreover, the concept of using ozone to heal of infected wounds, necrotic, or poorly oxygenated tissue has been explored in orthopedics and dentistry.17,18
Previous studies in the field of neuromuscular disorders have shown that ozone therapy may be useful in the management of select patients with back pain, knee osteoarthritis, myofascial pain syndrome (MPS), tendon injuries, plantar fasciitis, facial nerve regeneration and De Quervain’s disease.19–23 However, only a few studies have been published about the application of ozone in the management of CTS.8 Despite the extensive variety of treatments and also their combinations in CTS, it is not clear yet which to select as the most efficient substance. The main aim of this RCT was to evaluate the efficacy and safety of local ozone injection in CTS treatment.
Material and methods
This prospective randomized controlled study was a parallel non-blinded (=open) trial, conducted at Shahid Modarres hospital in Tehran. The protocol was approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences (No. IR.SBMU.MSP.REC.1395.116); Also it was registered in the Iranian Registry of Clinical Trials (IRCT) with No. IRCT2016040913442N9. Before the commencement, according to the Declaration of Helsinki, all patients were informed about the aim and procedure; then a written informed consent was obtained for all participants. A total of 110 patients presenting to physical medicine and rehabilitation (PM&R) clinic, with non-severe CTS symptoms lasting for 3–12 months were enrolled in October 2017. Among the participants, 40 eligible patients included who were all female [intentionally, because of a higher incidence of CTS among women], aged between 30–60 years with the confirmed diagnosis of mild or moderate CTS (grades 2 or 3).24 In order to confirm CTS diagnosis, and evaluate the severity grade, electro-diagnostic study (EDX) was performed by an experienced physiatrist using a Caldwell Sierra® Wave electromyography machine. Patients with underlying diseases such as thyroid deficiency, diabetes mellitus and rheumatoid arthritis, those with a history of local corticosteroid injection, thenar atrophy, a concomitant peripheral nerve lesion in the upper limb, polyneuropathy or radiculopathy, were excluded from the study. Considering the ozone contraindications, we also excluded the pregnant women and participants with a history of glucose-6-phosphate dehydrogenase (G6PD) deficiency, hyperthyroidism, thrombocytopenia, serious cardio-vascular instability and those who were under treatment with Angiotensin-converting enzyme (ACE) inhibitors.25 Furthermore, if electro-diagnostic studies did not confirm the presence of CTS, the patient was excluded. In the case of bilateral involvement, only the side with more severity was included.
Demographic characteristics of the patients such as age, gender, height, body weight, body mass index (BMI), and the severity grade of disease were recorded. The clinical outcome-measuring tools were as follows: 1) The pain intensity using a 10-score visual analog scale (VAS) in which 0 indicated no pain while 10 indicated the maximal imaginable pain. 2) Persian version of Boston CTS Questionnaire (BCTQ) which had two sections;26 symptoms severity scale (SSS) in 11 items and functional status scale (FSS) in 8 items. Each of these 19 questions had 5 choices based on the severity of involvement; higher scores indicated the more severity of the condition. 3) Median nerve conduction study (NCS) including measuring the latency of compound motor action potential (CMAP), and sensory nerve action potential (SNAP). Median sensory nerve action potential (SNAP) evaluation was antidromically obtained based on the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) in 2002.27 Also median compound motor action potential (CMAP) was assessed using abductor pollicis brevis (APB) muscle via the standard techniques. CTS grading was determined based on a famous neurophysiological scale.24 The first two variables (VAS and BCTQ) were the primary outcome measuring tools, but the EDX parameters were also evaluated as the secondary outcome.
Participants were then randomly divided into two groups using computer software for random-sequence generation, with 20 participants in each group. Patients in the control group used a prefabricated wrist-based resting splint with a metal bar on the volar side for eight weeks (during the night and at most of the awakening time) to keep their wrists in the neutral position (0–5-degree angle extension). In addition to splinting, the participants of intervention group received a single local injection of 4 ml ozone (10 micrograms/dl) plus to 1 ml lidocaine (1%) using a 25 G needle.
An expert physician (the first author, SA. R), with 15-year experience in musculoskeletal interventions, performed the ozone injections for all of them. Needle insertion was on the volar side (conventional midline approach), one finger-breadth proximal to distal wrist crease (between the tendons of flexor carpi radialis and palmaris longus) with a 45-degree angle between needle and skin. Participants were allowed to use acetaminophen in the case of possible post injection pain during the first 48 hrs. Also, the number and frequency of pain-killers used were recorded. Patients in both groups were reevaluated by another PM&R specialist (M.N) after 10 weeks, using VAS for pain, two parts of BCTQ, and NCS.
Finally, data gathered were analyzed using SPSS V.22. Normal distribution of data was assessed using Shapiro-Wilk and Kolmogorov-Smirnov methods. To compare the differences between the two groups; Chi2, independent t-test, and Mann-Whitney method were applied accordingly. Also, the paired t-test was used to declare within-group changes. Throughout all analyses, 0.05 was considered as a significant level.
Results
A total of 40 eligible patients with a mean age of 47 years were treated in two equal groups: a single ozone injection along with splinting versus splinting alone. The majority of participants belonged to mild grade and the rest had moderate CTS. Two patients discontinued the study (both belonged to the ozone group, one due to family immigration and another because of personal problems). Thirty-eight participants remained until the end of our study [Figure 1]. From the demographic and clinical point of view, two groups were relatively comparable [Table 1].
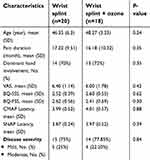 | Table 1 Comparison of demographic characteristics, and baseline level of clinical variables including pain severity, disease severity, BCTQ status between the two groups at baseline |
 | Figure 1 Flowchart of the study population. |
Intra-group changes
Pain severity (measured using VAS), symptom severity (BQ-SSS) and functional status (BQ-FSS) all improved significantly in both groups in comparison to pre-treatment scores (P<0.05) with the maximum changes in VAS scores for both groups [45.3% and 64% for control and ozone groups, respectively]. Likewise, EDX measures including SNAP and CMAP latency showed significant improvement compared to the pre-treatment level in both case and control groups (P<0.05). However, the effect size was much lower in EDX changes, especially in the control group with 2.2% and 3.8% improvement for SNAP and CMAP, respectively [Table 2]. Among two parts of BCTQ, symptom severity (SS) showed higher improvement compared to functional status (FS) in ozone group [43.6% versus 39.1%, respectively], whereas the SS revealed smaller changes than FS in control group [27.8% versus 33.2%, respectively].
 | Table 2 Pain severity, disease severity, symptom severity and functional status 10 weeks after the therapy in the two groups |
Between-groups comparison
Two groups were compared using Chi2 and independent samples t-test. The VAS reduction was more remarkable in the ozone group than the control group [64% versus 45.3%, respectively with P=0.01]. Moreover, BQ-SSS and BQ-FSS showed significantly higher improvement in the ozone group compared to the control group (P=0.01 and 0.02, respectively). Also, it should be noted that the improvement of EDX parameters was slightly better in ozone group; however, no statistically significant difference was detected between two groups [P=0.32 and 0.55 for CMAP and SNAP latency, respectively; Table 3]. Interestingly according to EDX, seven participants (35%) in the control group and eight patients (44.4%) in the intervention group reached to normal value ranges. Eventually, it is also noteworthy that neither minor nor major side effects such as skin atrophy or depigmentation were reported in our participants.
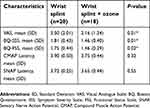 | Table 3 Comparison the mean value of pain severity, symptom severity and functional status 10 weeks after the therapy between the two groups |
Discussion
Present findings based on pain and functional measuring tools showed that ozone injection added to wrist splinting, was effective in improving signs and symptoms of CTS in mild to moderate cases; and this combined method was significantly more successful in comparison to splinting alone. However, there was no significant difference between two groups in EDX evaluation; it might be due to lack of enough follow-up time to detect the small significant differences.
Many trials have studied the effectiveness of different injections in CTS treatment, with some variation in their injection protocols, medications, and follow-up periods.13–16 Therefore undoubtedly, they have not yielded a single conclusion. A similar study performed by Zambello et al had evaluated the efficacy of ozone injection in 112 CTS patients; a total of 186 hands underwent the administration of an oxygen-ozone solution (2–3 mL with 10 mg/ml concentration) injection twice a week for five consequent weeks (10 sessions), and then two more injections with a 14 day interval.8 The outcome measuring tool for pain/function in this study was patient-reported, and consisted of 4 qualitative grades: “excellent, good, satisfactory, and absent” Finally, the short-term results were remarkable; pain reduction at the level of excellent/good was about 90% immediately after injections, which was almost maintained at long-term follow-up after one year (87%). Only 6% of participants reported the absent efficacy and underwent surgical release. Similarly, our findings demonstrated that ozone therapy could be useful in CTS treatment. As a crucial limitation, that study had no control group and was actually a single-arm study; and unfortunately, no objective measurement to carefully evaluate the patient’s pain and functional status. On the other hand, they performed a lot of injections; although this could make the therapy unfavorable, it also could be a reason to achieve more powerful results than ours.8
In addition, some other recent original studies and reviews have demonstrated the usefulness of oxygen-ozone therapy, in the treatment of other musculoskeletal conditions.19–23,28–30 Based on the previous researches, ozone has no serious side effect. In human studies, ozone therapy has had no toxicity; with the exception of possible post-injection flare reaction that can be seen after any soft tissue injection and is within normal ranges of similar methods like corticosteroids, etc. Neither minor nor major side effects were reported in the current study.
Limitations
Since the ozone injection was not a well-defined treatment, in the present study we compared its effect against only a basic simple treatment (splinting alone); however, further trials with stronger control groups such as normal-saline injection are needed to confirm the therapeutic efficacy of ozone in CTS management. A major limitation of our trial was that patients in two groups essentially could not be blinded, so that was a potential source of bias in the results. The acceptance of such great difference between two treatments allocated to groups was another problem; therefore from 110 participants, only 40 eligible patients who accepted the protocol were obtained [Figure 1]. Nevertheless, administering different objective scales to assess the symptoms severity of CTS, including VAS, BQ-SSS, BQ-FSS and EDX parameters was our main strength to compensate for some of the limitations. Also as mentioned before, we used a single injection; future studies could evaluate the different numbers of injections or various doses which may be helpful in reaching more accurate and long-lasting results.
Conclusion
To be summarized, the local ozone injection as a safe and low-cost method could provide promising results among women with mild to moderate CTS, at least for short-term treatment up to 10 weeks. Adding such a method to baseline conservative treatment seems wise, as the combination of ozone injection and splinting was more successful than splinting alone.
Data Sharing Statement
The authors do not intend to share substantial data of this study; but they are ready to share the de-identified file of substantial data in excel format and all other study-related documents, at any specific time for any period, if the editorial board requires.
Acknowledgments
This article has been extracted from the thesis written by Dr. Mohammadrasoul Nezamabadi in School of Medicine, Shahid Beheshti University of Medical Sciences. It was also approved by the Physical Medicine and Rehabilitation Research Center of Shohada-e-Tajrish Educational Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alfonso C, Jann S, Massa R, Torreggiani A. Diagnosis, treatment and follow-up of the carpal tunnel syndrome: a review. Neurol Sci. 2010;31(3):243–252. doi:10.1007/s10072-009-0213-9
2. Kim PT, Lee HJ, Kim TG, Jeon IH. Current approaches for carpal tunnel syndrome. Clin Orthop Surg. 2014;6(3):253–257. doi:10.4055/cios.2014.6.3.253
3. Ibrahim I, Khan WS, Goddard N, Smitham P. Carpal tunnel syndrome: a review of the recent literature. Open Orthop J. 2012;6:69–76. doi:10.2174/1874325001206010069
4. Dale AM, Harris-Adamson C, Rempel D, et al. Prevalence and incidence of carpal tunnel syndrome in US working populations: pooled analysis of six prospective studies. Scand J Work Environ Health. 2013;39(5):495–505. doi:10.5271/sjweh.3351
5. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3:255–261.
6. Turner A, Kimble F, Gulyas K, Ball J. Can the outcome of open carpal tunnel release be predicted?: a review of the literature. ANZ J Surg. 2010;80(1–2):50–54. doi:10.1111/j.1445-2197.2009.05175.x
7. Huisstede BM, Friden J, Coert JH, Hoogvliet P, European HG. Carpal tunnel syndrome: hand surgeons, hand therapists, and physical medicine and rehabilitation physicians agree on a multidisciplinary treatment guideline-results from the European HANDGUIDE study. Arch Phys Med Rehabil. 2014;95(12):2253–2263. doi:10.1016/j.apmr.2014.06.022
8. Zambello A, Fumagalli L, Fara B, Bianchi M. Oxygen-ozone treatment of carpal tunnel syndrome. Retrospective study and literature review of conservative and surgical techniques. Int J Ozone Ther. 2008;7(1):45–48.
9. Raeissadat SA, Rayegani SM, Rezaei S, et al. The effect of polarized polychromatic noncoherent light (bioptron) therapy on patients with carpal tunnel syndrome. J Lasers Med Sci. 2014;5(1):39–46.
10. Rayegani SM, Raeissadat SA, Heidari S, Moradi-Joo M. Safety and effectiveness of low-level laser therapy in patients with knee osteoarthritis: a systematic review and meta-analysis. J Lasers Med Sci. 2017;8(Suppl 1):S12–S19. doi:10.15171/jlms.2017.s3
11. Raeissadat A, Reza Soltani Z. Study of long term effects of laser therapy versus local corticosteroid injection in patients with carpal tunnel syndrome. J Lasers Med Sci. 2010;1:24–30.
12. Dinarvand V, Abdollahi I, Raeissadat SA, Mohseni Bandpei MA, Babaee M, Talimkhani A. The effect of scaphoid and hamate mobilization on treatment of patients with carpal tunnel syndrome. Anesthesiology Pain Med. 2017;7(5):e14621. doi:10.5812/aapm.
13. Karimzadeh A, Bagheri S, Raeissadat SA, et al. Comparing different doses of local corticosteroid injection in carpal tunnel syndrome: a double blind randomized clinical trial. J Pain Res. 2019;12:579–584. doi:10.2147/JPR.S190652
14. Bahrami MH, Shahraeeni S, Raeissadat SA. Comparison between the effects of progesterone versus corticosteroid local injections in mild and moderate carpal tunnel syndrome: a randomized clinical trial. BMC Musculoskelet Disord. 2015;16:322.
15. Raeissadat SA, Karimzadeh A, Hashemi M, Bagherzadeh L. Safety and efficacy of platelet-rich plasma in treatment of carpal tunnel syndrome; a randomized controlled trial. BMC Musculoskelet Disord. 2018;19:49. doi:10.1186/s12891-018-1963-4
16. Chen P-C, Chuang C-H, Tu Y-K, Bai C-H, Chen C-F, Liaw M-Y. A Bayesian network meta-analysis: comparing the clinical effectiveness of local corticosteroid injections using different treatment strategies for carpal tunnel syndrome. BMC Musculoskelet Disord. 2015;16:363.
17. Lin Q, Chen H, Lu C, et al. Effects of ozone on sciatic nerve in rat. Interventional Neuroradiology. 2011;17(3):281–285. doi:10.1177/159101991101700301
18. Borrelli E, Alexandre A, Iliakis E, Alexandre A, Bocci V. Disc herniation and knee arthritis as chronic oxidative stress diseases: the therapeutic role of oxygen ozone therapy. J Arthritis. 2015. doi:10.4172/2167-7921.1000161
19. Raeissadat SA, Rayegani SM, Forogh B, Hassan Abadi P, Moridnia M, Rahimi-Dehgolan S. Intra-articular ozone or hyaluronic acid injection: which one is superior in patients with knee osteoarthritis? A 6-month randomized clinical trial. J Pain Res. 2018;11:111–117. doi:10.2147/JPR.S142755
20. Moretti M. Effectiveness of oxygen-ozone and hyaluronic acid injections in De Quervain‘s syndrome. Int J Ozone Ther. 2012;11(1):31–33.
21. Muto M, Ambrosanio G, Guarnieri G, et al. Low back pain and sciatica: treatment with intradiscal-intraforaminal O(2)-O (3) injection. Our experience. Radiol Med. 2008;113(5):695–706. doi:10.1007/s11547-008-0302-5
22. Raeissadat SA, Rayegani SM, Sadeghi F, Rahimi-Dehgolan S. Comparison of ozone and lidocaine injection efficacy vs dry needling in myofascial pain syndrome patients. J Pain Res. 2018;11:1273–1279. doi:10.2147/JPR.S164629
23. Raeissadat SA, Tabibian E, Rayegani SM, Rahimi-Dehgolan S, Babaei-Ghazani A. An investigation into the efficacy of intra-articular ozone (O2–O3) injection in patients with knee osteoarthritis: a systematic review and meta-analysis. J Pain Res. 2018;11:2537–2550. doi:10.2147/JPR.S175441
24. Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23(8):1280–1283.
25. Velio Alvaro Bocci VA. Scientific and medical aspects of ozone therapy: state of the art. Arch Med Res. 2006;37(4):425–435. doi:10.1016/j.arcmed.2005.08.006
26. Rezazadeh A, Bakhtiary AH, Samaei A, Moghimi J. Validity and reliability of the persian boston questionnaire in Iranian patients with carpal tunnel syndrome. Koomesh. 2014;15(2):138–145.
27.
28. Babaei-Ghazani A, Karimi N, Forogh B, et al. Comparison of ultrasound-guided local ozone (O2-O3) Injection vs corticosteroid injection in the treatment of chronic plantar fasciitis: a randomized clinical trial. Pain Med. 2018. [Epub ahead of print]. doi:10.1093/pm/pny066
29. Ozbay I, Ital I, Kucur C, et al. Effects of ozone therapy on facial nerve regeneration. Braz J Otorhinolaryngology. 2017;83(2):168–175. doi:10.1016/j.bjorl.2016.02.009
30. Moretti B, Lanzisera R, Sisti G, et al. 02–03 therapy in tendinopathies and entrapment syndromes. Rivisita Italiana di Ossigeno-Ozonterapia. 2005;4:20–29.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
