Back to Journals » Cancer Management and Research » Volume 12
Intercalary Reconstruction of the “Ultra-Critical Sized Bone Defect” by 3D-Printed Porous Prosthesis After Resection of Tibial Malignant Tumor
Authors Zhao D, Tang F, Min L, Lu M, Wang J, Zhang Y, Zhao K , Zhou Y, Luo Y, Tu C
Received 14 January 2020
Accepted for publication 13 March 2020
Published 8 April 2020 Volume 2020:12 Pages 2503—2512
DOI https://doi.org/10.2147/CMAR.S245949
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Dingyun Zhao,1,* Fan Tang,1,* Li Min,1 Minxun Lu,1 Jie Wang,1 Yuqi Zhang,1 Kun Zhao,1,2 Yong Zhou,1 Yi Luo,1 Chongqi Tu1
1Department of Orthopeadics, West China Hospital of Sichuan University, Chengdu, Sichuan 610041, People’s Republic of China; 2Department of Orthopeadics, Tianjin Fifth Central Hospital, Tianjin 300450, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chongqi Tu
Department of Orthopedics, West China Hospital of Sichuan University, No. 37 Guo Xue Xiang, Chengdu, Sichuan 610041, People’s Republic of China
Tel +86-189-8060-1387
Fax +86-28-8542-2246
Email [email protected]
Purpose: This study aimed to evaluate the early stability, limb function, and mechanical complications of 3D-printed porous prosthetic reconstruction for “ultra-critical sized bone defects” following intercalary tibial tumor resections.
Methods: This study defined an “ultra-critical sized bone defect” in the tibia when the length of segmental defect in the tibia was > 15.0 cm or > 60% of the full tibia and the length of the residual fragment in proximal or distal tibia was between 0.5 cm and 4.0 cm. Thus, five patients with “ultra-critical sized bone defects” following an intercalary tibial malignant tumor resection treated with 3D-printed porous prosthesis between June 2014 and June 2018 were retrospectively reviewed. Patient information, implants design and fabrication, surgical procedures, and early clinical outcome data were collected and evaluated.
Results: Among the five patients, three were male and two were female, with an average age of 30.2 years. Pathological diagnoses were two osteosarcomas, one Ewing sarcoma, one pseudo-myogenic hemangioendothelioma, and one undifferentiated pleomorphic sarcoma . The average length of the bone defects following tumor resection was 22.8cm, and the average length of ultra-short residual bone was 2.65cm (range=0.6cm– 3.8cm). The mean follow-up time was 27.6 months (range=14.0– 62.0 months). Early biological fixation was achieved in all five patients. The average time of clinical osseointegration at the bone–porous interface was 3.2 months. All patients were reported to be pain free and have no limitations in their walking distance. No prosthetic mechanical complications were observed.
Conclusion: Reconstruction of the “ultra-critical sized bone defect” after an intercalary tibial tumor resection using 3D-printed porous prosthesis achieved satisfactory overall early biological fixation and limb function. Excellent primary stability and the following rigid biological fixation were key factors for success. The outcomes of this study were supposed to support further clinical application and evaluation of 3D-printed porous prosthetic reconstruction for “ultra-critical sized bone defects” in the tibia.
Keywords: 3D-printed porous, prosthetic reconstruction, intercalary tibial resection, critical sized bone defect
Introduction
Critical-sized bone defects vary with the anatomical location of the defect as well as the state of the surrounding soft tissues.1 Tumor-associated defects arising in long bones are frequently associated with considerable segmental bone loss and extensive infiltration of the surrounding soft tissues, fulfilling the criteria of a “critical-sized bone defect”. The tibia is the second most common site for primary bone malignancies following the distal femur, and may also arise as a site of metastatic disease.2 For epiphyseal sarcomas in the tibia, it is possible to consider an intercalary resection when more than 1.0 cm of epiphyseal tissue is retained beyond the tumor.3 There are three intercalary tibial tumor resection types: epi-diaphyseal resections with one osteotomy in the epiphyseal bone and one in the diaphyseal bone; meta-diaphyseal resections with one osteotomy in the metaphyseal bone and one in the diaphyseal bone; or diaphyseal resection with both osteotomies in diaphyseal bone.4 However, reconstructions after intercalary tibial tumor resection are quite a challenge, and biological reconstructions seem to remainthe main option.
Biological reconstructions of intercalary defects include vascularized fibula grafts, allografts, and reinsertions of extra-corporally irradiated autografts.5 However, the obvious drawbacks of each method and high complication rates limit their clinical application when there are massive segmental bone defects. For example, the smaller cross-sectional areas associated with fibula grafts leaves the graft weaker than the originally resected long bone and requires longer periods of non-weight-bearing; allografts have a high rate of immune rejection and non-union; extracorporeally devitalized autografts are contraindicated in marked osteolytic lesions and histological analysis of the whole specimen is not possible.6 The recent introduction of the induced-membrane technique, developed by Masquelet et al, has demonstrated promising preliminary results when applied in the reconstruction of bone-tumor defects.7 However, the risk of graft resorption and a two-stage surgery with a minimum of 6 months duration of treatment has limited its application. For distraction osteogenesis, the length of preserved epiphysis should be ≥1.0 cm after tumor resection, and the complications significantly outweigh the benefits when bone defects are >15.0 cm.8
Intercalary reconstruction using prosthesis allows for early prosthetic stability, rapid rehabilitation and weight-bearing, when compared with biological reconstructions.9 However, the fixation of the stem if there is only a very short bone stock left after intercalary resection is quite difficult. The high stress concentration at the bone–stem interface heralds the high loosening probability in the future. So, it is commonly accepted for conventional prosthetic stems insertion that the adjacent joint needs to be sacrificed when the residual bone is ultra-short, potentially leading to increased instability but permanent reduction in the limb function.10 Ahlmann et al11 have suggested that the shortest length of bone required for fixation of the implant stem is 5.0 cm. And fixation in a short segment <4.0 cm was reported as hazardous owing to the possibility of early loosening and peri-prosthetic fracture.12 Thus, in addition to producing a critical-sized bone defect after intercalary tumor resection of long bones, an ultra-short residual bone also poses many challenges for the reconstruction. Hence, we proposed that the concept that an “ultra-critical sized bone defect” may be more precise, reflecting the clinical intervention of some tumor defects after intercalary resection. An “ultra-critical sized bone defect” in tibia should basically follow the two clinical criteria: the length of segmental defect in tibia >15.0 cm or >60% of full tibia;13 and the length of residual fragment in proximal or distal tibia was between 0.5–4.0 cm.
3D-printed porous prosthesis is regarded as a new and exciting generation of uncemented implants which are demonstrating marked clinical success.14,15 For the reconstruction large-bone defects, 3D-printed porous titanium scaffolds supply adequate mechanical support and facilitate bone formation, which results in high mechanical integrity.16 Clinically, implants with 3D-printed porous prosthesis had shown feasible primary stability for massive segmental bone tumoral defects.17 And the first application of 3D printed porous prosthesis for massive proximal tibia defect was successful.18 Thus, we were highly interested in the efficacy of prosthesis with 3D-printed porous for intercalary tibia tumoral defects. In this study, we evaluated the early clinical outcome of patients with “ultra-critical sized bone defect” in tibia receiving 3D-printed porous prosthetic reconstruction.
Methods
Patients
This retrospective study was conducted with the approval of the ethics committee of West China Hospital, Sichuan University. Informed consent was obtained from all individual participants included in the study.
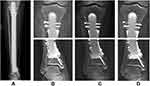
Between June 2014 and June 2018, five patients with “ultra-critical sized bone defects” after intercalary tibial tumor resection received 3D-printed porous prosthetic reconstruction (Figure 1), including one previously reported case.18 Biopsy was performed for each patient before undergoing definitive surgery. The surgical stage was determined according to the Enneking bone and soft tissue sarcoma staging system,19 and the AJCC stating system.20 Among the five patients, three were male and two were female, with an average age of 30.2 years. Pathological diagnoses were osteosarcoma in two cases, Ewing sarcoma, pseudo-myogenic hemangioendothelioma, and undifferentiated pleomorphic sarcoma in one case each (Table 1). Four patients who were Enneking and AJCC stage IIB at the time of diagnosis received the standard pre-operative chemotherapy regimen in use at the time of treatment. Clinical characteristics of patients,including age, gender, tumor size, defects length, and the length of residual proximal or distal tibia, were collected.
 |
Table 1 Basic Information and Follow-Up Metrics of the Included Five Patients |
Implants Properties
All prostheses were designed by our clinical team and fabricated by Chunli Co, Ltd., Tongzhou, Beijing, China. The porosity of printed titanium alloy was 60–70% with a size of 600 µm.21 3D computed tomography (CT) scan data 2 weeks before the planned surgery was obtained and imported into the Mimics V17.0 software (Materialise Corp., Belgium) to evaluate the parameters of the defects. Then, all design of the prosthesis was comprehensively considered based on the specific defect of each patient to achieve the best stability (Figure 2). Specifically, for residual tibia <1.0 cm (case 3), two strategies were adopted to increase the primary stability: (a) Cross screws fixation was designed according to the 3D space anatomical distribution; (b) Due to the extremely limited space for stem insertion, a strain was produced by a 2.0–3.0 mm exceeded length of implants over the defect (Figure 3A). When the length of proximal tibia was between 1.0 cm and 4.0 cm, a conformal design complementary to the residual proximal tibia with cross screws fixation was adopted (case 1 and case 4). For distal tibia, when bone stock was between 1.0 cm and 2.0 cm in length, an at least 0.5 cm insertion porous portion was fabricated to increase the interface of bone–porous (Figure 3B). This special design supplied the capability of against rotation force and bend force that can apparently increase the stability of implantation. When residual distal bone was between 2.0 cm and 4.0 cm in length, the designed plan was determined according to the proximal ultra-short component design (case 1 and 3). Generally, short stem with cross screws fixation was maximumly adopted at this condition (Figure 3C). Based on the comparative long term survival of porous prosthesis,22 for residual bone between 4.0 cm and 10.0 cm in length, a short curve 3D-pritned porous stem with transvers screws fixation was adopted (Figure 3D) (case 2). For residual bone over 10.0 cm in length, we chose a short uncemented casting stem with hydroxyapatite (HA) coating according to our previous study (case 4 and case 5).23 All 3D-printed porous component can be combined with the modular endoprosthesis system used at our hospital (Chunli Co, Ltd., Tongzhou, Beijing, China.). The designing and fabricating of these custom prostheses takes about 7–10 days.
 |
Figure 2 The designing flow diagram of 3D-printed porous prosthesis for “ultra-critical sized bone defect” in the tibia. |
Surgical Procedure
Resection margin was determined according to preoperative magnetic resonance imaging (MRI) and CT scans which were obtained to evaluate the extent of soft tissue extension of tumor and bone destruction. Segmental resection was under the principle to achieve a safe margin for osteogenic sarcoma. Generally, the osteotomy level was determined at least 2.0 cm beyond the boundary, and a custom cutting guide was made corresponding to the resection principle. During the osteotomy, bone markers were customized according to the guide plate: the guide plate for proximal tibial osteotomy was riveted with the tibial tubercle; For distal tibia osteotomy, the guide plate was riveted with the median point of the leading edge of tibia and ankle acupoint. After all osteotomies were completed, intra-operative X-ray was used for verification. Tibial tuberosity was maximumly preserved during osteotomy in all patients (Figure 4). The mean width of the distal tibiofibular syndesmosis anteriorly between the tip of the anterior tibial tubercle and the nearest point of the fibula was 2.0 mm.24 So, we preserved at least 1.0–1.5 mm width of distal tibiofibular syndesmosis to guarantee the stability of the ankle joint.
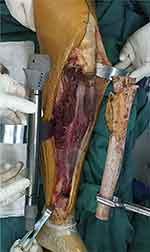 |
Figure 4 Precise osteotomy during resection procedure. In-operative image of case 1 showed the tibial tuberosity was preserved under precise osteotomy with a safe resection margin. |
Reconstruction procedure was performed from the side with the longer residual bone to the shorter one. The residual tibia of proximal or distal parts was reamed to fit the 3D-printed porous component with our customed reaming instruments. Implanting the porous part of the prosthesis exactly at the original position was relatively demanding. Stems were press-fit inserted into the reamed tibia medulla cavity. After confirming both locations of the proximal and distal parts of the prosthesis, screws were inserted to enhance primary stability according to the pre-operative designing. Intraoperative X-ray was adopted to confirm the placed position of these components. For soft tissue coverage, as the volume of prosthesis was smaller than the host bone, so directly suturing the residual gastrocnemius and tibialis anterior muscle were performed in the majority of cases. And the medial head of the gastrocnemius muscle flap was used to cover the upper part of the prosthesis when necessary. If soft tissue defects were quite large, free skin grafting was considered.
Post-Operative Management
The rehabilitation protocol was customized to the patient depending on the rigidity of biological fixation. Generally, patients were informed about non-weight-bearing standing and walking with crutches for the first 6 weeks after surgery. Active flexion of the knee and dorsal extension of the ankle were encouraged when the patient could walk with partial weight-bearing 6 weeks after surgery. The patient was informed about partial weight bearing at 6 weeks and then full weight bearing from 3 months postoperatively. Patients were followed up every month for the first 3 months, then every 2–6 months to date. Osseointegration was evaluated by Tomosynthesis-Shimadzu metal artefact reduction technology (T-SMART), which reduced the metal artifacts. Enneking functional evaluation score for limb salvage, the revised Oxford Knee Score (OKS) for knee function and the American Orthopaedic Foot and Ankle Society (AOFAS) for ankle function were assessed at each follow-up visit. Rigid biological fixation was considered when there were at least three solid bony conjunctions on T-SMART images and should be in accordance with the clinical symptoms during weight-bearing activities.13
Results
The average length of bone defects after tumor resection was 22.8 cm, while the average length of “ultra-short” bone was 2.65 cm. The average percentage of defects on full tibia length was 66.7% (Table 1). The mean follow-up was 27.6 months, ranging from 14 months to 62 months. The average time of rigid biological fixation achieving was 3.2 months (Figure 5). For case 2, in which we did not perform transverse screw fixation for proximal stem as good primary stability was required during in-operative evaluation, a hardened area was seen around the stem. This patient was told to be non-weight-bearing for another 4 weeks until biological fixation was achieved. All patients reported to be pain-free and have no limitations in walking distances (Figure 6). The average MSTS score was 26.8/30 at last follow-up visit. The mean OKS and AOFAS score were 44.8/48 and 89.8/100, respectively (Table 1). No prosthetic mechanical complications such as aseptic loosing, peri-prosthesis fracture, or implants breakage were observed. Also, there were no tumor recurrence, deep infection, or wound healing problems during the follow-up.
Discussion
With the development of neoadjuvant systemic therapy, the improved life expectancy of patients with malignant tumors of bone has led to an increased emphasis on limb salvage as well as the function preservation. Also, image technology improvement optimized the precise osteotomy procedure to achieve a clear resection margin. However, surgeons choose reconstruction type for an “ultra-critical sized bone defect” on individual patient and oncologic factors as well as on their own surgical experiences. Metallic prosthesis replacement allowed rapid rehabilitation and early weight-bearing activities after surgery. However, when >60% of the tibia was resected, the overall implant survival was much poorer, possibly due to the long arm of force that increased the instability after implantation.25 Biological reconstruction for a massive segmental defect over 15.0 cm or over 60% of tibia required a large amount of bone mass. A two-stage surgery of induced-membrane technical with at least 6-month duration and the bone absorption risk limited its application at this situation.7 Parameters critical for the success of distraction osteogenesis include a lesion ≤15.0 cm in length with at least 0.5 cm of subchondral bone and sufficient metaphyseal cortex retained after excision of tumors.26 The above two biological reconstructions sacrificing time to fix massive bone defect largely increased the difficulty of post-operative management. In addition, post-operative chemotherapy lowers the success rate of biological reconstructions as the adverse effect on bone healing and is associated with high rates of fracture and infection.27 At this condition, prosthetic reconstruction with rigid biological fixation from 3D-printed porous implant may be one of the solutions. Thus, we set the length of intercalary tibial defects over 15.0 cm or 60% of tibia as the first essential condition of an “ultra-critical sized bone defect”.
For regular limb sparing prosthesis replacement, the use of HA-coating grooved collars may lead to osteointegration of the implant collar and may reduce the rate of aseptic loosening.28 The early implant using cement fixation technique requires an at least 5.0 cm medullar cavity for fixation.11 When the length of the short-segment intramedullary stem is <4.0 cm, there may be a higher rate of aseptic loosening and peri-prosthetic fracture.12 HA-coating plate and anchorage system are the current two effective options for short residual bone segment less than 4.0 cm (Table 2). HA-coated extracortical plates have been used in joint-sparing surgery with excellent osseointegration at the prosthesis–proximal bone interface and formation of new bone around the HA collar.29 For proximal tibial replacement, using HA-coated extracortical plate fixation when short-segment intramedullary fixation is less than 4.0 cm allows preservation of the knee joint with good function and no early evidence of loosening.30 Guder et al31 developed an ultra-short stem anchorage for intercalary tibia reconstruction, but at least 3.0 cm of tibia plateau should remain after tumor resection. The main complication of the two systems is peri-prosthesis fracture, possibly due to the local high stress and relatively weak biological fixation. However, the individual design and excellent biological fixation of 3D-printed porous implants make the reconstruction of intercalary defects with residual bone less than 1.0 cm available. Thus, with the collected data in literature and our own clinical evidence, we set 0.5–4.0 cm as the second essential requirement for an “ultra-short” residual bone after intercalary tibia tumor resection.
 |
Table 2 Custom Implants for Ultra-Short Bone Fixation After Intercalary Tibial Tumoral Resection |
Osteoarticular allograft has better post-operative limb function when compared with metallic prosthesis reconstruction.32 This indicates the benefits of biological reconstruction for function restore, and the importance of adjacent residual bone preservation. We preserved the distal tibiofibular syndesmosis in case 3 with 0.6 cm distal tibia left after segmental resection. Preserving the distal tibiofibular syndesmosis and other ankle-stabilizing ligaments ensures an early stabilization of implants and a satisfactory function of the affected limb.33 Tibial tuberosity was preserved in all five patients under the wide resection principle. Avulsion and/or extensor lag may lead to poor function. Titus et al34 reattached the patellar ligament to the tibial tuberosity of the proximal tibial mega-prosthesis with a porous surface, and the repair was protected with a cerclage wire through the patella and the prosthesis. This technique resulted in good quadriceps function and a low incidence of complications. Surgeons also directly repaired the patellar tendon on to the transposed medial head of gastrocnemius if the attachment was sacrificed during the tumor resection procedure.30
3D printed technology shows promising potential in medical areas.35,36 And novel 3D-printed implants with highly porous surface achieved fair biological fixation in orthopedics surgery15,37,39 (Table 3). However, a high primary mechanical stability is essential for a successful osseointegration of implants.40 The application of transvers or cross screws fixation was rational in our study as rigid stable fixation was associated with a lower failure rate of reconstruction, and was also the precondition of well bone ingrowth and the following rigid biological fixation.13 We proposed that a micromotion of the stem delayed the bone healing in case 2. When the ends of a fractured long bone are reduced, there should be absolutely no movement between the fragments to endorse fracture healing.41 This happens because movements, even at the micrometer range, can induce a stress or strain that may hinder the formation of new cells in the gap. The same phenomenon is applied at the bone-to-implant interface.42 In addition, the induction of micromotion during functional loading may also be responsible for failure of osseointegration. Micromotions above 50–100 μm negatively influence osseointegration and bone remodeling by forming fibrous tissues and inducing bone resorption at the bone-to-implant interface.43 Thus, all cases in our study were restricted to no weight bearing within the first 6 weeks after surgery, during which time the primary bone ingrowth processed.
 |
Table 3 Current Clinical Applications of 3D-Pritned Porous Implants in Orthopeadics Surgery (Case Series Over 3) |
There were some limitations of this study. First, the number of cases involved in this study was quite small, due to the limited patients with “ultra-critical sized bone defect” after intercalary tibial resection; Second, the duration of follow-up of the five patients was not long, as we aimed here to evaluate the early stability of 3D-printed porous prosthesis for reconstructing the “ultra-critical sized bone defect” in the tibia. So, long-term follow-up is needed; Third, the mechanical analysis of the reconstruction using 3D-printed porous prosthesis for “ultra-critical sized bone defect” should be performed in the future.
Conclusion
Overall, the early clinical outcome in this study was supposed to support the further clinical application of 3D-printed porous prosthesis for “ultra-critical sized bone defect” after intercalary tibial tumor resection. Excellent primary stability and the following rigid biological fixation were the key factors for success if the 3D-printed porous prosthesis was applied under the situation of “ultra-critical sized bone defect”. With the rapid development of adjuvant therapy and image management technics for malignant bone tumors, more patients get favorable tumor control with a relatively long life-expectation. They would like to receive the surgery aiming at functional reconstruction and precise tumor resection. Thus, the concept “ultra-critical-sized bone defect” has practical clinical value in the future. Here, we presented the definition and the two essential conditions of “ultra-critical sized bone defect” in the tibia after intercalary tibial malignant tumor resection. However, due to the differences of anatomy structures, limb function, and mechanical conduction, the length of defects and residual bone for an “ultra-critical sized bone defect” in the femur and humerus varies with tibia. Thus, the future related work to define this concept in these long bones and the mechanical analysis of the 3D-printed porous prosthetic reconstructions are imperative.
Funding
This work was funded by the National Key Research and Development Program of China (No. 2016YFC1102003), the National Natural Science Foundation of China (No. 81801852), the Science and Technology Research Program of Sichuan Province (No 2017SZ0095) and the Chengdu Science and Technology Project (2017-CY02-00032-GX).
Disclosure
The authors declare that they have no conflict of interest in this work.
References
1. Nauth A, McKee MD, Einhorn TA, Watson JT, Li R, Schemitsch EH. Managing bone defects. J Orthop Trauma. 2011;25(8):462–466. doi:10.1097/BOT.0b013e318224caf0
2. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;415(415 Suppl):S219–229. doi:10.1097/01.blo0000093843.72468.3a
3. Kumta SM, Chow TC, Griffith J, Li CK, Kew J, Leung PC. Classifying the location of osteosarcoma with reference to the epiphyseal plate helps determine the optimal skeletal resection in limb salvage procedures. Arch Orthop Trauma Surg. 1999;119(5–6):327–331. doi:10.1007/s004020050420
4. Deijkers RL, Bloem RM, Kroon HM, Van Lent JB, Brand R, Taminiau AH. Epidiaphyseal versus other intercalary allografts for tumors of the lower limb. Clin Orthop Relat Res. 2005;439:151–160. doi:10.1097/00003086-200510000-00029
5. Fuchs B, Ossendorf C, Leerapun T, Sim FH. Intercalary segmental reconstruction after bone tumor resection. Eur J Surg Oncol. 2008;34(12):1271–1276. doi:10.1016/j.ejso.2007.11.010
6. Zekry KM, Yamamoto N, Hayashi K, et al. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J Orthop Surg (Hong Kong). 2019;27(1):2309499019832970.
7. Accadbled F, Mazeau P, Chotel F, Cottalorda J, Sales de Gauzy J, Kohler R. Induced-membrane femur reconstruction after resection of bone malignancies: three cases of massive graft resorption in children. Orthopaedics Traumatol Surg Res. 2013;99(4):479–483. doi:10.1016/j.otsr.2013.01.008
8. Tsuchiya H, Tomita K. Distraction osteogenesis for treatment of bone loss in the lower extremity. J Orthopaedic Sci. 2003;8(1):116–124. doi:10.1007/s007760300020
9. Panagopoulos GN, Mavrogenis AF, Mauffrey C, et al. Intercalary reconstructions after bone tumor resections: a review of treatments. Eur J Orthopaedic Surg Traumatol. 2017;27(6):737–746. doi:10.1007/s00590-017-1985-x
10. Hardes J, Henrichs MP, Gosheger G, et al. Endoprosthetic replacement after extra-articular resection of bone and soft-tissue tumours around the knee. Bone Joint J. 2013;95-b(10):1425–1431. doi:10.1302/0301-620X.95B10.31740
11. Ahlmann ER, Menendez LR. Intercalary endoprosthetic reconstruction for diaphyseal bone tumours. J Bone Joint Surg Br Vol. 2006;88(11):1487–1491. doi:10.1302/0301-620X.88B11.18038
12. Sewell MD, Hanna SA, McGrath A, et al. Intercalary diaphyseal endoprosthetic reconstruction for malignant tibial bone tumours. J Bone Joint Surg Br Vol. 2011;93(8):1111–1117. doi:10.1302/0301-620X.93B8.25750
13. Agarwal M, Puri A, Gulia A, Reddy K. Joint-sparing or physeal-sparing diaphyseal resections: the challenge of holding small fragments. Clin Orthop Relat Res. 2010;468(11):2924–2932. doi:10.1007/s11999-010-1458-6
14. Arabnejad S, Johnston B, Tanzer M, Pasini D. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J Orthopaedic Res. 2017;35(8):1774–1783. doi:10.1002/jor.v35.8
15. Sultan AA, Mahmood B, Samuel LT, et al. Cementless 3D printed highly porous titanium-coated baseplate total knee arthroplasty: survivorship and outcomes at 2-year minimum follow-up. J Knee Surg. 2020;33(3):279–283. doi:10.1055/s-0039-1677842
16. Van der Stok J, Van der Jagt OP, Amin Yavari S, et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J Orthopaedic Res. 2013;31(5):792–799. doi:10.1002/jor.v31.5
17. Lu M, Min L, Xiao C, et al. Uncemented three-dimensional-printed prosthetic replacement for giant cell tumor of distal radius: a new design of prosthesis and surgical techniques. Cancer Manag Res. 2018;10:265–277. doi:10.2147/CMAR.S146434
18. Lu M, Li Y, Luo Y, Zhang W, Zhou Y, Tu C. Uncemented three-dimensional-printed prosthetic reconstruction for massive bone defects of the proximal tibia. World J Surg Oncol. 2018;16(1):47. doi:10.1186/s12957-018-1333-6
19. Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9–24.
20. Edge SB, Edge SB. AJCC Cancer Staging Manual.
21. Taniguchi N, Fujibayashi S, Takemoto M, et al. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: an in vivo experiment. Mater Sci Eng C Mater Biol Appl. 2016;59:690–701. doi:10.1016/j.msec.2015.10.069
22. Tudor FS, Donaldson JR, Rodriguez-Elizalde SR, Cameron HU. Long-term comparison of porous versus hydroxyapatite coated sleeve of a modular cementless femoral stem (SROM) in primary total hip arthroplasty. J Arthroplasty. 2015;30(10):1777–1780. doi:10.1016/j.arth.2015.04.031
23. Lu M, Wang J, Xiao C, et al. Uncemented, curved, short endoprosthesis stem for distal femoral reconstruction: early follow-up outcomes. World J Surg Oncol. 2018;16(1):183. doi:10.1186/s12957-018-1486-3
24. Elgafy H, Semaan HB, Blessinger B, Wassef A, Ebraheim NA. Computed tomography of normal distal tibiofibular syndesmosis. Skeletal Radiol. 2010;39(6):559–564. doi:10.1007/s00256-009-0809-4
25. Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br Vol. 1996;78(1):5–13. doi:10.1302/0301-620X.78B1.0780005
26. Watanabe K, Tsuchiya H, Yamamoto N, et al. Over 10-year follow-up of functional outcome in patients with bone tumors reconstructed using distraction osteogenesis. J Orthopaedic Sci. 2013;18(1):101–109. doi:10.1007/s00776-012-0327-4
27. Ippolito JA, Martinez M, Thomson JE, et al. Complications following allograft reconstruction for primary bone tumors: considerations for management. J Orthopaedics. 2019;16(1):49–54. doi:10.1016/j.jor.2018.12.013
28. Coathup MJ, Batta V, Pollock RC, et al. Long-term survival of cemented distal femoral endoprostheses with a hydroxyapatite-coated collar: a histological study and a radiographic follow-up. JBJS. 2013;95(17):1569–1575. doi:10.2106/JBJS.L.00362
29. Gupta A, Pollock R, Cannon SR, Briggs TW, Skinner J, Blunn G. A knee-sparing distal femoral endoprosthesis using hydroxyapatite-coated extracortical plates. Preliminary results. J Bone Joint Surg Br Vol. 2006;88(10):1367–1372. doi:10.1302/0301-620X.88B10.17756
30. Spiegelberg BG, Sewell MD, Aston WJ, et al. The early results of joint-sparing proximal tibial replacement for primary bone tumours, using extracortical plate fixation. J Bone Joint Surg Br Vol. 2009;91(10):1373–1377. doi:10.1302/0301-620X.91B10.22076
31. Guder WK, Hardes J, Gosheger G, Nottrott M, Streitburger A. Ultra-short stem anchorage in the proximal tibial epiphysis after intercalary tumor resections: analysis of reconstruction survival in four patients at a mean follow-up of 56 months. Arch Orthop Trauma Surg. 2017;137(4):481–488. doi:10.1007/s00402-017-2637-7
32. Summers SH, Zachwieja EC, Butler AJ, Mohile NV, Pretell-Mazzini J. Proximal tibial reconstruction after tumor resection: a systematic review of the literature. JBJS Rev. 2019.
33. Hermans JJ, Beumer A, de Jong TA, Kleinrensink GJ. Anatomy of the distal tibiofibular syndesmosis in adults: a pictorial essay with a multimodality approach. J Anat. 2010;217(6):633–645. doi:10.1111/j.1469-7580.2010.01302.x
34. Titus V, Clayer M. Protecting a patellar ligament reconstruction after proximal tibial resection: a simplified approach. Clin Orthop Relat Res. 2008;466(7):1749–1754. doi:10.1007/s11999-008-0239-y
35. Haleem A, Javaid M. 3D scanning applications in medical field: a literature-based review. Clin Epidemiol Global Health. 2019;7(2):199–210. doi:10.1016/j.cegh.2018.05.006
36. Javaid M, Haleem A. 4D printing applications in medical field: a brief review. Clin Epidemiol Global Health. 2019;7(3):317–321. doi:10.1016/j.cegh.2018.09.007
37. Fan H, Fu J, Li X, et al. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: a case series and review of the literature. World J Surg Oncol. 2015;13(1):308. doi:10.1186/s12957-015-0723-2
38. Sporer S, MacLean L, Burger A, Moric M. Evaluation of a 3D-printed total knee arthroplasty using radiostereometric analysis: assessment of highly porous biological fixation of the tibial baseplate and metal-backed patellar component. Bone Joint J. 2019;101-b(7_Supple_C):40–47. doi:10.1302/0301-620X.101B7.BJJ-2018-1466.R1
39. Wei R, Guo W, Yang R, et al. Reconstruction of the pelvic ring after total en bloc sacrectomy using a 3D-printed sacral endoprosthesis with re-establishment of spinopelvic stability: a retrospective comparative study. Bone Joint J. 2019;101–b(7):880–888. doi:10.1302/0301-620X.101B7.BJJ-2018-1010.R2
40. Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50(3):399–410. doi:10.1016/S0022-3913(83)80101-2
41. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br Vol. 2002;84(8):1093–1110. doi:10.1302/0301-620X.84B8.0841093
42. Chang PK, Chen YC, Huang CC, Lu WH, Chen YC, Tsai HH. Distribution of micromotion in implants and alveolar bone with different thread profiles in immediate loading: a finite element study. Int J Oral Maxillofac Implants. 2012;27(6):e96–101.
43. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;208:108–113.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.