Back to Journals » OncoTargets and Therapy » Volume 12
Interaction Of c-Jun And HOTAIR- Increased Expression Of p21 Converge In Polyphyllin I-Inhibited Growth Of Human Lung Cancer Cells
Authors Zhao Y, Tang X , Huang Y , Tang Q, Ma C, Zheng F, Wu W, Hann SS
Received 11 August 2019
Accepted for publication 31 October 2019
Published 25 November 2019 Volume 2019:12 Pages 10115—10127
DOI https://doi.org/10.2147/OTT.S226830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
YueYang Zhao,1,2 XiaoJuan Tang,1 Yuhua Huang,3 Qing Tang,1 ChangJu Ma,1 Fang Zheng,1 WanYin Wu,4 Swei Sunny Hann1
1Laboratory of Tumor Biology; 2Department of Hematology; 3Department of Stomatology; 4Department of Medical Oncology, The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong Province 510120, People’s Republic of China
Correspondence: Swei Sunny Hann; WanYin Wu
Laboratory of Tumor Biology, The Second Clinical College of Guangzhou University of Chinese Medicine, No. 111 Dade Road, Guangzhou, Guangdong Province 510120, People’s Republic of China
Tel +8620-39318472
Email [email protected]; [email protected]
Background: Lung cancer is a leading cause of cancer-related death worldwide. Previously we demonstrated that polyphyllin I (PPI), a bioactive component extracted from Paris polyphylla, inhibited the growth of non-small cell lung cancer (NSCLC) cells through the SAPK/JNK-mediated suppressing p65, DNMT1 and EZH2 expressions. However, the molecular mechanism underlying anti-lung cancer effect by PPI still remain elusive.
Purpose: In this current study, we further explored the molecular mechanism underlying the anti-lung cancer effect of PPI.
Methods: MTT, Cell-LightTM EdU DNA cell proliferation and colony formation assays were used to measure cell growth. Western blot were used to examine protein levels of c-Jun and p21. The expression level of long non-codingth RNA HOX transcript antisense RNA (HOTAIR) was measured by qRT-PCR. The p21 promoter activity was measured by Dual-Luciferase Reporter Assay System. The transient transfection experiments were used to silence and overexpression of c-Jun, p21 and HOTAIR. Tumor xenograft and bioluminescent imaging experiments were carried out to confirm the in vitro findings.
Results: We showed that PPI suppressed growth of NSCLC cells. Mechanistically, we observed that PPI reduced expression of HOTAIR, while increased transcription factor c-Jun protein levels. Additionally, PPI also induced protein expression and promoter activity of p21, a cyclin-dependent kinase inhibitor. While exogenously expressed HOTAIR showed no effect on c-Jun levels, silencing of c-Jun significantly reversed the PPI-inhibited HOTAIR expression. Moreover, excessive expressed c-Jun further enhanced PPI-inhibited HOTAIR expression and PPI-induced p21 protein levels. Intriguingly, overexpression of HOTAIR and silencing of c-Jun overcame the PPI-induced p21 protein and promoter activity. Finally, silencing of p21 neutralized the PPI-inhibited cell proliferation. Similar results were also found in one xenograft mouse model.
Conclusion: Our results demonstrate that PPI inhibits growth of NSCLC cells through regulation of HOTAIR and c-Jun expressions, which lead to induction of p21 gene. The interactions among HOTAIR, c-Jun and p21 regulatory axis converge in the overall anti-lung cancer effect of PPI. This study unveils an additional new mechanism for the anti-lung cancer role of PPI.
Keywords: PPI, NSCLC, HOTAIR, c-Jun, p21
Introduction
Lung cancer, especially non-small cell lung cancer (NSCLC), is the leading cause of cancer-related death worldwide.1 Despite substantial advancement in understanding the mechanisms and improving treatment, the 5-year survival rate remains unfavorable. Thus, enhancing therapeutic outcomes in patients with NSCLC remains an increased challenge. Searching for alternative therapeutic modalities in enhancing the therapeutic efficacy of lung cancer patients is eagerly needed.
Polyphyllin I (PPI), a bioactive constituent extracted from Rhizoma Paridis saponins (RPS), has been shown to have anti-tumor activity in cancers.2–7 By inactivation of the Wnt/β-catenin regulatory signaling axis, PPI inhibited growth, invasion, and migration of osteosarcoma cells in vitro and in vivo.8 Moreover, PPI also reduced the growth, invasion, and epithelial–mesenchymal transition (EMT) of prostate cancer cells via inhibition of the protein phosphatase 2A (CIP2A)/protein phosphatase 2A (PP2A)/extracellular signal-regulated kinase (ERK) signaling cascade.9 We previously showed that PPI inhibited growth of NSCLC cells through stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK)-mediated reduction of transcription factor p65 and DNA methyltransferase 1 (DNMT1) protein levels, and this resulted in suppression of enhancer of zeste homologue 2 (EZH2) gene expression in NSCLC cells.10 We also found that PPI inhibited growth of human castration-resistant prostate cancer (CRPC) cells via suppression of long non-coding RNA (lncRNA) HOX transcript antisense RNA (HOTAIR)/DNMT1/EZH2 signaling regulatory loops.11 These results suggested the therapeutic potential of PPI in cancer treatment. Regardless, the molecular mechanisms underlying the anti-lung cancer effect of PPI remained to be elucidated.
lncRNA has been shown to be involved in cellular and biochemical processes at transcriptional levels, posttranscriptional levels, and epigenetic modifications.12,13 Aberrant lncRNA expression is reported to be involved in tumorigenesis and development in NSCLC.14 Among these, HOTAIR, which is located within the Homeobox C (HOXC) gene cluster on chromosome 12, has been found to be dysregulated in various cancers. Increased expression of HOTAIR was associated with unfavorable prognosis in cancer patients.15 HOTAIR was highly expressed in NSCLC and silencing of HOTAIR reduced growth and induced apoptosis of NSCLC cells. Thus, HOTAIR may be considered as a potential biomarker for patients with NSCLC.16 Nevertheless, the potential links and molecular mechanisms underlying the exact role of HOTAIR in mediating the growth and progression of lung cancer still remain to be elucidated.
Transcription factor activator of protein 1 (AP-1) consists of a variety of members including c-Jun, c-Fos families and binds to specific DNA putative sites. Several studies observed that activity and regulation of AP-1 in cancer mainly depended on c-Jun, which was mostly considered an oncogenic factor and involved in growth, metastasis, and drug resistance.17–19 However, opposite findings have also been reported; one early study found that the proteasome inhibitor PS-341 induced cell cycle arrest and apoptosis of NSCLC cells in conjunction with significant up-regulation of p21 (WAF1/Cip1), a cyclin-dependent kinase inhibitor, and down-regulation of Bcl-2 proteins. Concomitantly, PS-341 also increased phosphorylation of JNK and c-Jun, and DNA binding activities in NSCLC cells, indicating that the JNK/c-Jun/AP-1 regulatory axis could mediate the anti-cancer effects of PS-341.20 Another study also reported that tylophorine, one major medicinal constituent of herb tylophora indica, induced the c-Jun protein level in cancer cells via NF-κB/JNK/mitogen-activated protein kinase kinase 4 (MKK4) and the phosphatidylinositol 3-kinase (PI3-K)/phosphatase 2A (PP2A)/eukaryotic elongation factor 2 (eEF2) dual regulatory cascades, suggesting the tumor suppressor role of c-Jun in the anti-cancer effect of tylophorine.21 Regardless, the true function of c-Jun in the biological process, especially in cancer cells, still needs to be carefully weighted with more experimental approaches.
Cyclin-dependent kinase (CDK) inhibitor p21, a key player in regulating many cellular processes, is involved in cell cycle regulation, apoptosis, and gene regulation.22 p21 acts as a tumor suppressor and concomitantly an inhibitor of apoptosis by interacting with various molecules and transcription factors, suggesting a critical role and potential target in the development and treatment of cancer.23–25 The exogenously expressed carboxyl terminus of Hsc70-interacting protein (CHIP) facilitated degradation of p21 in experiments, showing that p21 is a novel and direct ubiquitylation substrate of CHIP.26 Also, excessive expression of p21 was reported to rescue the effects of miR-95-3p in proliferation and migration in hepatocellular carcinoma cells.27 Moreover, senescent cells with the p21 gene silenced acquired multiple DNA lesions that resulted in activation of ataxia telangiectasia mutated (ATM) and NF-κB signaling, leading to a decrease in cell survival.28 Thus, the controversial roles of the two-faced involvement of p21 in cancer development and a therapeutic target might exist. Therefore, more effort in understanding the mechanism underlying the role of p21 in cancer pathogenesis is still required to be elucidated.24
A number of studies have shown the associations among HOTAIR, c-Jun, and p21 in cancer. One study reported that inhibition of HOTAIR suppressed proliferation and invasion, but promoted the apoptosis of colorectal cancer cells via increased p21 expression.29 The data demonstrated increased SP1/c-Jun complex formation in melanoma cells, resulting in increased activity of p21 gene promoter.30 The chromatin immunoprecipitation experiment also found an association between c-Jun and the activity of the p21 gene promoter.31 Moreover, another study showed that c-Jun was identified as a potential upstream transcript factor for mi302D; c-Jun-mediated miR-302d-3p suppressed the differentiation of retinal pigment epithelium (RPE) cells through directly targeting p21.32 However, little information was available for the links of HOTAIR and c-Jun. Therefore, the interactions and correlations among these (HOTAIR, c-Jun, and p21) involved in biological functions in cancer growth remain unknown.
In this current study, we further explored the molecular mechanism underlying the anti-lung cancer effect of PPI and observed the novel links and associations among HOTAIR, c-Jun, and p21 in this process. We showed that reciprocal regulations and interactions among HOTAIR, c-Jun, and p21 converged in the overall anti-lung cancer effect of PPI.
Materials And Methods
Reagents And Cell Culture
Monoclonal antibodies specific to c-Jun and p21 were purchased from Cell Signaling Technology (Beverly, MA, USA). Lipofectamine 3000 reagent was ordered from Life Technologies (AB & Invitrogen, Carlsbad, CA, USA). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) powder was purchased from Sigma Aldrich (St Louis, MO, USA). Geneticin (G-418 Sulfate) was obtained from Life Technologies (Grand Island, NY, USA) and used for PC9-Luc cells (carrying luciferase reporter gene obtained from the Guangzhou Land Biological Technology Co., Guangzhou, China) at a concentration of 250 μg/mL. The c-Jun, p21, and HOTAIR siRNAs and the Cell-Light™ EdU DNA cell proliferation kit were purchased from RiboBio (Guangzhou, China). The pcDNA3.1-HOTAIR and control (pcDNA3.1) were obtained from GeneCopoeia, Inc. (Rockville, MD, USA). The c-Jun expression plasmid (No.47443, Flag-JunWT-Myc) was purchased from Addgene, Inc. (Cambridge, MA, USA).33 The p21 promoter GV238-CDKN1A was obtained from Genechem Co., Ltd (Shanghai, China). The Dual-Luciferase Reporter Assay Kit was purchased from Promega (Madison, WI, USA). The NSCLC cell lines PC9 and H1650 were obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (Shanghai, China) and authenticated for the absence of mycoplasma, genotypes, drug response, and morphology using a commercial kit provided by Guangzhou Cellcook Biotech Co. Ltd (Guangzhou, China). The cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. The culture medium consisted of RPMI 1640 medium obtained from GIBCO, Life Technologies (Grand Island, NY, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Thermo Fisher Scientific Inc, Waltham, MA, USA), 100 μg/mL streptomycin, and 100 U/mL penicillin. PPI was purchased from Chengdu Must Biotechnology (Chengdu, China) and dissolved in dimethylsufoxide [DMSO, maximum concentration, 0.1% (v/v)], which was then added to complete the cell culture medium.
MTT Assay
Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. The PC9 and H1650 cells seeded in 96-well plates (6×103 cells/well) were incubated at 37 °C with 5% CO2 with increasing concentrations of PPI for up to 72 h. In separate experiments, PC9 and H1650 cells were transfected with the control, pcDNA3.1-HOTAIR for 24 h before exposing to PPI for an additional 24 h. Afterward, absorbance was determined using an automated microplate reader at 490 nm (Victor X5; Perkin Elmer, Waltham, MA, USA). The IC50 values resulting from 50% inhibition of cell viability were calculated as a comparison with that of the control group.
EdU Incorporation Assay
Cell proliferation was assessed by the Cell-Light™ EdU DNA cell proliferation kit (RiboBio ) according to the instructions from the manufacturer. In brief, after treatment with PPI, the PC9 and H1650 cells were exposed to 50 μM of 5-ethynyl-2ʹ-deoxyuridine (EdU) for 2 h at 37 °C, and then fixed in 4% PA–PBS for 30 min. After permeabilization with 0.5% Triton X-100, the cells were stained with 1×Apollo reaction reagent followed by staining with Hoechst 33342. Finally, the pictures were obtained with 200× magnification under microscopy (Ts2RFL; Nikon, Tokyo, Japan). At least five captured fields were randomly selected and the EdU positive cells were calculated: (EdU positive cells/Hoechst stain cells)×100.
Colony Formation Assay
PC9 cells (5×102 cells/6-well plate) in logarithmic growth phase were seeded in 6-well plates and treated with PPI at 37 °C in 5% humidified CO2 for 8 days with continuously changing RPMI 1640 medium supplemented with 10% FBS every 3 days. After that, the cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) and stained by 0.1% crystal violet (Sigma-Aldrich) for a duration of 30 min. Visible colonies from randomly selected five fields were manually counted under a microscope (TI2-E; Nikon). The results were shown as the fold change relative to that of the vehicle control group.
Western Blot Analysis
The cell lysates containing equal amounts of protein were separated on SDS polyacrylamide gels. Membranes (Millipore, Billerica, MA, USA) were incubated with antibodies against c-Jun and p21 (1:1000), respectively. Afterward, following washing and incubating with a secondary antibody raised against rabbit IgG conjugated to horseradish peroxidase (Cell Signaling Technology), the membranes then were transferred to fresh-made ECL solution (Immobilon Western; Millipore), and the signals were documented using the Gel Imagine System (Bio-Rad, Hercules, CA, USA). ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to quantify and compare the intensity of single bands between the control and proteins of interest.
Transfection Experiments
PC9 and H1650 cells were seeded at a density of 2.0×105 cells/6-well dishes and grown to 60% confluence. For each well, 2 μg pcDNA 3.1 control or HOTAIR overexpression plasmids, the control and c-Jun expression plasmid (Flag-JunWT-Myc), the control and p21 siRNAs, or c-Jun siRNAs (50 nM each) were transfected into the cells using the Lipofectamine 3000 reagent according to the instructions from manufacturer for up to 24 h, followed by treatment with PPI for an additional 24 h for all other experiments.
Quantitative Real-Time PCR (qRT-PCR)
A quantitative real-time PCR (qRT-PCR) assay was developed for the detection of HOTAIR transcripts. The primers used in this study were designed as follows: HOTAIR forward 5′-GGTAGAAAAAGCAACCACGAAGC-3′, reverse 5′-ACATAAACCTCTGTCTGTGAGTGCC-3′; GAPDH forward 5′-AAGCCTGCCGGTGACTAAC-3′, reverse 5′-GCGCCCAATACGACCAAATC-3′. The amplification was carried out in a 20 μL mixture containing 2 μL of the cDNA preparation, 10 μL of 2× SYBR Green Premix ExTaq, and 10 μM primers on an ABI 7500 Real-Time PCR System (Applied Biosystems Inc., Grand Island, NY, USA). The qRT-PCR protocol included an initial step of 95 °C for 30 s followed by 40 cycles of 95 °C for 3 s and then annealing at 60 °C for 30 s. Each sample was tested in triplicate. Threshold values were determined for each sample/primer pair and the 2−ΔΔCt method was used to calculate the relative levels of specific HOTAIR.
Dual-Luciferase Assays
PC9 and H1650 cells seeded at a density of 6x104 cells/well in 24-well plates were transfected with p21 promoter constructer linked to the luciferase reporter gene (GV238-CDKN1A) and an internal control (Renilla) for 24 h before treatment with PPI for an additional 24 h. The preparation of cell extracts and measurement of luciferase activities were performed using the Dual-Luciferase Reporter Assay System (Promega) based on the instructions from the manufacturer.
Xenografted Tumor Model
Mouse tumor xenograft studies were carried out according to the guidelines for care, and the protocol was approved by the Animal Care and Use Committee of Guangdong Provincial Hospital of Chinese Medicine (Ethics Approval Number 2018020) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). A total of 30 eight-week-old female nude mice obtained from Beijing Vital River Experimental Animal Co. Ltd (Beijing, China) were maintained at the Animal Center of Guangdong Provincial Hospital of Chinese Medicine. PC9-Luc cells (5×106 cells) carrying the luciferase reporter gene (obtained from the Guangzhou Land Biological Technology Co.) were injected subcutaneously near the axillary fossa region in nude mice. Xenografts were allowed to grow until the initial measurement was made with calipers. Mice were randomly divided into the control, low (1 mg/kg) and high doses (3 mg/kg) of PPI groups, given daily by intraperitoneal injection for up to 21 days (n=10 per group) based on the previous study.10 Next, for the bioluminescence imaging (BLI) procedure, mice were anesthetized by inhalation of 2% isoflurane. The substrate D-luciferin (Caliper Life Sciences, Hopkinton, MA, USA) was injected into the peritoneal cavity with a dose of 150 mg/kg in approximately 100 μL. The intensity of the BLI signal was determined using the IVIS-200 imaging system (Xenogen/Caliper, Alameda, CA, USA). Tumor volume measurements were calculated using the formula for an oblong sphere: volume=(width2×length)×0.5. Quantification of bioluminescence was reported as photons/s. All mice were euthanized on day 21 and the corresponding xenografted tumors were removed and processed for detecting the c-Jun and p21 protein levels by western blot and HOTAIR expression by qRT-PCR, respectively.
Immunohistochemistry (IHC)
Immunohistochemical assay was used to determine c-Jun and p21 protein expressions in lung tumor tissues. Tissues were obtained from nude mice with/without treatment with PPI and fixed in 10% formaldehyde for 24 h, then embedded with paraffin. The specimens were cut into 5-μm sections and roasted at 60 °C for 2 h. Antigenic retrieval was performed in citric acid buffer (pH 6.0) followed by washing with PBS, and treatment with 3% H2O2 and 5% bovine serum albumin. Afterward, the sections were incubated with primary antibodies against p21 (dilutions of 1:50; Cell Signaling Technology) and c-Jun (dilutions of 1:300; Cell Signaling Technology,) at 4 °C overnight. Subsequently, they were incubated with secondary antibody (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing, China) for 30 min. Detection was performed using 3,3ʹ-diaminobenzidine (DAB) chromogen (Maixin Biotech. Co. Ltd, Fuzhou, China) and were counterstained with hematoxylin, then dehydrated through graded alcohols, and cleared in xylene. Finally, the pictures were taken under 200× magnification under a microscope (Ti2-E; Nikon). The immunostaining was evaluated by Image-Pro Plus6.0 image analysis software (Media Cybernetics, lnc., Sliver Spring, MD, USA).
Statistical Analysis
Almost all data were expressed as the mean±SD from three independent experiments. Differences between groups were assessed by one-way ANOVA and significance of difference between treatment groups was analyzed by Tukey’s multiple comparison test using GraphPad Prism software version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). A Palue of <0.05 was deemed significant.
Results
PPI Inhibited Growth Of NSCLC Cells
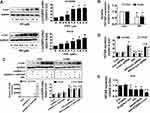
Our previous studies found that PPI can reduce the growth of NSCLC cells.10 In this study, we further detected and confirmed the effect of PPI on growth in PC9 and additional NSCLC H1650 cells by MTT assay. As shown in Figure 1A, PPI inhibited the cell viability in a dose- and time-dependent manner in PC9 and H1650 cells. The IC50 values were 1.644 and 2.172 μM in PC9 and H1650 cells, respectively. This finding was also confirmed using alternative methods for detecting cell proliferation: EdU incorporation (Figure 1B) and colony formation assays (Figure 1C). These results suggested that PPI inhibited the growth of PC9 and H1650 cells.
PPI Suppressed lncRNA HOTAIR Levels And Increased c-Jun Protein Expression And Silencing Of c-Jun Neutralized The PPI-Inhibited HOTAIR Expression
We next search for the potential targets that may be mediated in the inhibitory effect of PPI on cancer cell growth. Oncogenic factor c-Jun has been shown to be involved in tumorigenesis and progression.34 We found that PPI unexpectedly increased c-Jun protein levels in a dose-dependent fashion in PC9 and H1650 cells (Figure 2A). We also observed that PPI reduced lncRNA HOTAIR expression levels (Figure 2B) and that exogenous expression of HOTAIR had no effect on either basal c-Jun or PPI-induced c-Jun protein levels in PC9 and H1650 cells (Figure 2C). However, we showed that silencing of c-Jun resisted the PPI-inhibited HOTAIR expression (Figure 2D). Conversely, excessive expressed c-Jun repressed HOTAIR and further facilitated PPI-inhibited HOTAIR expression (Figure 2E). These findings indicated that c-Jun, acting as an upstream tumor suppressor, regulated the expressions of HOTAIR in this process.
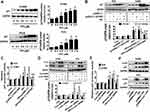
PPI Increased Expression And Promoter Activity Of P21, Which Were Overcome By Excessive Expression Of HOTAIR And Silencing Of C-Jun
To investigate the mechanism underlying the regulation of c-Jun and HOTAIR in mediating the relevant downstream target regulated by PPI, we further delineate the role of p21, the cyclin-dependent kinase inhibitor involved in growth arrest at specific stages in the cell cycle and linked to the functions of HOTAIR and c-Jun in different cancer cells.29,35,36 We found that PPI increased p21 protein expression in a dose-dependent fashion (Figure 3A), which was overcome in cells overexpressing HOTAIR (Figure 3B). As expected, it also increased the promoter activity of p21 (Figure 3C), which was also overcome in cells overexpressing HOTAIR (Figure 3C). As expected, silencing of c-Jun resisted the PPI-induced p21 protein expression and promoter activity in PC9 and H1650 cells (Figure 3D and E). Conversely, excessive expressed c-Jun not only induced, but also enhanced PPI-induced p21 protein expression (Figure 3F). These findings indicated that both c-Jun and HOTAIR, acting as upstream factors, were involved in the regulation of the PPI-induced expression of p21.
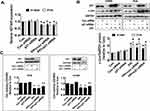
Silencing Of P21 Neutralized The PPI-Inhibited Cell Proliferation But Had No Effect On Feedback Regulations Of HOTAIR And C-Jun Expressions
To further dissect the molecular mechanism by which the interactions among c-Jun, p21, and HOTAIR converge in the overall response of PPI in this setting, we silenced the p21 gene using siRNA methods and examined the relevance and function of c-Jun and HOTAIR that mediated the PPI-inhibited cell growth. We showed that silencing of p21 had no effect on the HOTAIR level and c-Jun protein expression in PC9 and H1650 cells (Figure 4A and B). This result suggested that no feedback regulatory axis existed in this process. Interestingly, silencing of p21 neutralized the PPI-inhibited cell proliferation in PC9 and H1650 cells (Figure 4C). This result demonstrated a critical role of p21 in this process.
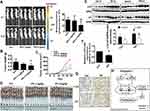
A Mouse Xenograft Model To Further Validate The Effect Of PPI
Finally, we tested the effect of PPI in a xenograft mice model. Mice bearing xenografted lung tumors were treated with the control or PPI via intraperitoneal injection for up to 21 days followed by intraperitoneal injection of D-luciferin. We found that the high doses of (3 mg/kg) PPI-treated mice demonstrated a significant growth-inhibitory effect compared to the control group as assessed by the Xenogen IVIS200 system (Figure 5A). In addition, we observed a substantial reduction of the tumor weight and sizes (volume) in the high dose of PPI-treated group as compared to that in the control group (Figure 5B–D). Moreover, consistent with the results in vitro, the induction of c-Jun and p21 proteins, and reduction of HOTAIR expression levels in fresh tumors harvested from the above experiments were observed in the PPI-treated group as compared to that in the control one, as determined by western blot and qRT-PCR, respectively (Figure 5E and F). In addition, the representative images of IHC staining for c-Jun and p21 showed similar findings (Figure 5G).
Discussion
Polyphyllin I (PPI), a natural compound extracted from the rhizomes of Paris polyphylla, has been used to treat several cancer types via multiple mechanisms.2–6 Previously, we demonstrated that PPI inhibited growth of human lung cancer cells via SAPK/JNK-mediated inhibition of NF-κB subunit p65, DNMT1, and EZH2 expressions in human lung cancer cells.10 We also showed that PPI reduced growth of human CRPC cells through inhibition of the lncRNA HOTAIR/DNMT1/EZH2 signing regulatory axis.11 These results suggested the therapeutic potential of PPI in novel cancer treatment. In the current study, we provided further evidence demonstrating the anti-lung cancer effects of PPI. We showed that reciprocal regulations and interactions among lncRNA HOTAIR, transcription factor c-Jun, and CDK inhibitor p21 converged in the overall anti-lung cancer effect of PPI.
In this study, we demonstrated a potential regulatory role of lncRNA HOTAIR in mediating the anti-lung cancer effect of PPI. HOTAIR was increased in NSCLC cells and involved in growth, invasion, metastasis, and progression.15,37–40 The dysregulation of HOTAIR was correlated with metastasis and poor prognosis, and was expected to be considered as a potential biomarker and therapeutic target for patients with NSCLC.41 Thus, HOTAIR could be a potential target in cancer therapy. In line with this, our results provided and confirmed the notions that HOTAIR may be an important target gene of PPI in the lung cancer cells, and that inhibition of HOTAIR was involved in the PPI-inhibited NSCLC cell growth.
Intriguingly, we also found an important role of c-Jun in this process. Several studies have shown that an enhanced AP-1 activity in cancer is mainly dependent on the c-Jun subunit, which has been considered as an oncogenic factor and involved in growth, differentiation, apoptosis, metastasis, and drug resistance.17–19 However, opposite findings were also noticed,20,21 implying a tumor suppressor role of c-Jun. Consistent with this, our results indicated that the induction of c-Jun mediated the anti-lung cancer effect of PPI, and suggested that c-Jun was also upstream of HOTAIR and modulated expression of HOTAIR affected by PPI in this process. Thus, the potential dual roles of c-Jun in biological functions, especially in cancer cell growth, deserve more attention and future studies are required to carefully weight this with more experimental approaches.
More importantly, we observed the critical role of p21 in this study. The cyclin kinase inhibitor p21 (also known as WAF1, CIP1, SDI1, and MDA-6) interacted with CDK complexes and blocked their kinase activities, thereby causing cell cycle arrest at the G1 phase.42 Overexpression of p21 resulted in G1 arrest and was shown to suppress tumor growth in vitro and in vivo.43 Our results demonstrated that induction of p21 was involved in the inhibitory effect of PPI on lung cancer cell growth, suggesting that p21 is a critical tumor suppressor, although p21 was regarded as a protein with a dual behavior.44 Moreover, we demonstrated that excessive expression of HOTAIR and silencing of c-Jun resisted the PPI-induced p21 protein expression and promoter activity. The association of p21 and HOTAIR has been reported; the regulation and interaction between miR-203 and HOTAIR resulted in the inhibition of epithelial–mesenchymal transition (EMT) and regulation of metastatic downstream genes including induction of tumor suppressor p21 in renal cell carcinoma cells.45 HOTAIR was also involved in regulation of p21 expression in cervical cancer cells. Silencing of HOTAIR induced p21 expression and consequentially increased the radio-sensitivity of cervical cancer cells, suggesting that the effect of HOTAIR was mainly through reduction of p21.46 Inhibition of HOTAIR by siRNAs diminished cigarette smoke extract (CSE)-reduced p21 expression. Thus, HOTAIR epigenetic silencing of p21 via EZH2-mediated H3K27 trimethylation contributed to the cell cycle progression induced by CSE in human bronchial epithelial (HBE) cells.47 We also observed the induction of p21 expression by c-Jun. Analysis of the p21 gene promoter showed an increase in SP1/c-Jun complex formation, suggesting that these complexes were involved in activation of the p21 gene promoter.30 Transcription factors of the AP-1 family (c-Jun and ATF-2) are capable of transactivation of the p21 promoter when overexpressed in human hepatoma HepG2 cells, suggesting that AP-1 proteins played a role in the stimulation of p21 gene expression. In addition, co-expression and functional interaction of c-Jun and SP1 resulted in a strong synergistic transactivation of p21 promoter in several different cells.48 Overall, our findings demonstrated that the regulations and potential mechanisms among HOTAIR, c-Jun, and p21 converge in the overall anti-lung cancer effect of PPI. Of notes, the roles and regulations of HOTAIR, c-Jun, or p21 in mediating PPI-induced cell cycle arrest in different cancer cells were reported previously.2,11,49–51 We believe that investigation of the effect of PPI on lung cancer cell cycle arrest through modulating the interactions among the HOTAIR, c-Jun, and p21 regulatory axis would further unveil the in-depth molecular mechanism underlying this, which needs to be explored further.
Moreover, our in vivo data fit the findings in vitro, further confirming the inhibitory effects of PPI on tumor growth and regulation of HOTAIR, c-Jun, and p21 expressions. The doses of PPI used were based on our previous reports and other studies,10,52,53 which showed substantial inhibitory effects without noticeable toxicity. Our findings suggest that PPI suppressed growth of human lung cancer cells via targeting the HOTAIR, c-Jun, and p21 signaling regulatory axis.
In summary, our results show that PPI inhibits NSCLC cell growth through regulation and interaction of HOTAIR and c-Jun, and this leads to the induction of p21 gene expression. The potential regulatory loops among HOTAIR, c-Jun, and p21 converge in the overall anti-lung cancer effect of PPI (Figure 5H). This study provides further evidence and unveils additional new mechanism for the anti-lung cancer role of PPI.
Datasets Are Available
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Acknowledgments
This work was supported in part by the grants from the National Natural Scientific Foundation of China (No. 81871863), the Major Program of National Natural Science Foundation of Guangdong (No. 2018B030311061), the Special Science and Technology Join fund from Guangdong Provincial Department of Science and Technology – Guangdong Academy of Traditional Chinese Medicine (No. 2014A020221024), the Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (No. YN2015MS19), and the Science and Technology Planning Project of Guangdong Province (No. 2017B030314166).
Author Contributions
YYZ, XJT, YH and SSH initially designed, organized, collected and interpreted data; drafted the manuscript. QT, CJM, FZ, WYW and SSH reorganized, interpreted data and revised it critically for important intellectual content. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Gu L, Feng J, Xu H, Luo M, Su D. Polyphyllin I inhibits proliferation and metastasis of ovarian cancer cell line HO-8910PM in vitro. J Tradit Chin Med. 2013;33(3):325–333. doi:10.1016/S0254-6272(13)60174-0
3. Shi YM, Yang L, Geng YD, Zhang C, Kong LY. Polyphyllin I induced-apoptosis is enhanced by inhibition of autophagy in human hepatocellular carcinoma cells. Phytomedicine. 2015;22(13):1139–1149. doi:10.1016/j.phymed.2015.08.014
4. Li GB, Fu RQ, Shen HM, et al. Polyphyllin I induces mitophagic and apoptotic cell death in human breast cancer cells by increasing mitochondrial PINK1 levels. Oncotarget. 2017;8(6):10359–10374. doi:10.18632/oncotarget.14413
5. Yu S, Wang L, Cao Z, et al. Anticancer effect of Polyphyllin Iota in colorectal cancer cells through ROS-dependent autophagy and G2/M arrest mechanisms. Nat Prod Res. 2017;1–4.
6. Xiang S, Zou P, Wu J, et al. Crosstalk of NF-kappaB/P65 and LncRNA HOTAIR-mediated repression of MUC1 expression contribute to synergistic inhibition of castration-resistant prostate cancer by polyphyllin 1-enzalutamide combination treatment. Cell Physiol Biochem. 2018;47(2):759–773. doi:10.1159/000490028
7. Xiao T, Zhong W, Zhao J, et al. Polyphyllin I suppresses the formation of vasculogenic mimicry via Twist1/VE-cadherin pathway. Cell Death Dis. 2018;9(9):906. doi:10.1038/s41419-018-0902-5
8. Chang J, Li Y, Wang X, et al. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/beta-catenin pathway in vitro and in vivo. Sci Rep. 2017;7(1):7605. doi:10.1038/s41598-017-07194-9
9. Liu X, Sun Z, Deng J, et al. Polyphyllin I inhibits invasion and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling in prostate cancer. Int J Oncol. 2018;53(3):1279–1288. doi:10.3892/ijo.2018.4464
10. Li L, Wu J, Zheng F, Tang Q, Wu W, Hann SS. Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J Exp Clin Cancer Res. 2016;35(1):112. doi:10.1186/s13046-016-0388-x
11. Xiang S, Zou P, Tang Q, et al. HOTAIR-mediated reciprocal regulation of EZH2 and DNMT1 contribute to polyphyllin I-inhibited growth of castration-resistant prostate cancer cells in vitro and in vivo. Biochim Biophys Acta Gen Subj. 2018;1862(3):589–599. doi:10.1016/j.bbagen.2017.12.001
12. Li TT, He RQ, Ma J, Li ZY, Hu XH, Chen G. Long noncoding RNAs in small cell lung cancer: a potential opening to combat the disease (Review). Oncol Rep. 2018;40(4):1831–1842. doi:10.3892/or.2018.6635
13. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi:10.1016/j.ccell.2016.03.010
14. Chen J, Wang R, Zhang K, Chen LB. Long non-coding RNAs in non-small cell lung cancer as biomarkers and therapeutic targets. J Cell Mol Med. 2014;18(12):2425–2436. doi:10.1111/jcmm.12431
15. Loewen G, Jayawickramarajah J, Zhuo Y, Shan B. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7:90. doi:10.1186/s13045-014-0090-4
16. Yang Y, Jiang C, Yang Y, et al. Silencing of LncRNA-HOTAIR decreases drug resistance of non-small cell lung cancer cells by inactivating autophagy via suppressing the phosphorylation of ULK1. Biochem Biophys Res Commun. 2018;497(4):1003–1010. doi:10.1016/j.bbrc.2018.02.141
17. Cao Z, Zhang R, Li J, et al. X-linked inhibitor of apoptosis protein (XIAP) regulation of cyclin D1 protein expression and cancer cell anchorage-independent growth via its E3 ligase-mediated protein phosphatase 2A/c-Jun axis. J Biol Chem. 2013;288(28):20238–20247. doi:10.1074/jbc.M112.448365
18. Meng S, Wang G, Lu Y, Fan Z. Functional cooperation between HIF-1alpha and c-Jun in mediating primary and acquired resistance to gefitinib in NSCLC cells with activating mutation of EGFR. Lung Cancer. 2018;121:82–90. doi:10.1016/j.lungcan.2018.04.024
19. Gao N, Liu J, Liu D, et al. c-Jun transcriptionally regulates alpha 1, 2-fucosyltransferase 1 (FUT1) in ovarian cancer. Biochimie. 2014;107 Pt B:286–292. doi:10.1016/j.biochi.2014.09.015
20. Yang Y, Ikezoe T, Saito T, Kobayashi M, Koeffler HP, Taguchi H. Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling. Cancer Sci. 2004;95(2):176–180. doi:10.1111/j.1349-7006.2004.tb03200.x
21. Yang CW, Lee YZ, Hsu HY, et al. c-Jun-mediated anticancer mechanisms of tylophorine. Carcinogenesis. 2013;34(6):1304–1314. doi:10.1093/carcin/bgt039
22. Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst). 2016;42:63–71. doi:10.1016/j.dnarep.2016.04.008
23. Parveen A, Akash MS, Rehman K, Kyunn WW. Dual role of p21 in the progression of cancer and its treatment. Crit Rev Eukaryot Gene Expr. 2016;26(1):49–62. doi:10.1615/CritRevEukaryotGeneExpr.v26.i1.60
24. Georgakilas AG, Martin OA, Bonner WM. p21: a two-faced genome guardian. Trends Mol Med. 2017;23(4):310–319. doi:10.1016/j.molmed.2017.02.001
25. Chatterjee B, Ghosh K, Kanade SR. Curcumin-mediated demethylation of the proximal promoter CpG island enhances the KLF4 recruitment that leads to increased expression of p21Cip1 in vitro. J Cell Biochem. 2019;120(1):809–820. doi:10.1002/jcb.v120.1
26. Biswas K, Sarkar S, Du K, Brautigan DL, Abbas T, Larner JM. The E3 ligase CHIP mediates p21 degradation to maintain radioresistance. Mol Cancer Res. 2017;15(6):651–659. doi:10.1158/1541-7786.MCR-16-0466
27. Ye J, Yao Y, Song Q, et al. Up-regulation of miR-95-3p in hepatocellular carcinoma promotes tumorigenesis by targeting p21 expression. Sci Rep. 2016;6:34034. doi:10.1038/srep34034
28. Yosef R, Pilpel N, Papismadov N, et al. p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. Embo J. 2017;36(15):2280–2295. doi:10.15252/embj.201695553
29. Lin K, Jiang H, Zhang LL, et al. Down-regulated LncRNA-HOTAIR suppressed colorectal cancer cell proliferation, invasion, and migration by mediating p21. Dig Dis Sci. 2018;63(9):2320–2331. doi:10.1007/s10620-018-5127-z
30. Moussa RS, Kovacevic Z, Bae DH, Lane DJR, Richardson DR. Transcriptional regulation of the cyclin-dependent kinase inhibitor, p21(CIP1/WAF1), by the chelator, Dp44mT. Biochim Biophys Acta. 2018;1862(3):761–774.
31. Kolomeichuk SN, Bene A, Upreti M, et al. Induction of apoptosis by vinblastine via c-Jun autoamplification and p53-independent down-regulation of p21WAF1/CIP1. Mol Pharmacol. 2008;73(1):128–136. doi:10.1124/mol.107.039750
32. Jiang C, Xie P, Sun R, et al. c-Jun-mediated microRNA-302d-3p induces RPE dedifferentiation by targeting p21(Waf1/Cip1). Cell Death Dis. 2018;9(5):451. doi:10.1038/s41419-018-0481-5
33. Aguilera C, Nakagawa K, Sancho R, Chakraborty A, Hendrich B, Behrens A. c-Jun N-terminal phosphorylation antagonises recruitment of the Mbd3/NuRD repressor complex. Nature. 2011;469(7329):231–235. doi:10.1038/nature09607
34. Shaulian E. AP-1–the Jun proteins: oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22(6):894–899. doi:10.1016/j.cellsig.2009.12.008
35. Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–414. doi:10.1038/nrc2657
36. Kim YR, Kang TW, To PK, et al. HOXB13-mediated suppression of p21WAF1/CIP1 regulates JNK/c-Jun signaling in prostate cancer cells. Oncol Rep. 2016;35(4):2011–2016. doi:10.3892/or.2016.4563
37. Nakagawa T, Endo H, Yokoyama M, et al. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436(2):319–324. doi:10.1016/j.bbrc.2013.05.101
38. Zhai N, Xia Y, Yin R, Liu J, Gao F. A negative regulation loop of long noncoding RNA HOTAIR and p53 in non-small-cell lung cancer. Onco Targets Ther. 2016;9:5713–5720. doi:10.2147/OTT.S110219
39. Zhang CG, Yin DD, Sun SY, Han L. The use of lncRNA analysis for stratification management of prognostic risk in patients with NSCLC. Eur Rev Med Pharmacol Sci. 2017;21(1):115–119.
40. Wang R, Yan B, Li Z, et al. Long non-coding RNA HOX transcript antisense RNA promotes expression of 14-3-3sigma in non-small cell lung cancer. Exp Ther Med. 2017;14(5):4503–4508. doi:10.3892/etm.2017.5041
41. Li N, Wang Y, Liu X, et al. Identification of circulating long noncoding RNA HOTAIR as a novel biomarker for diagnosis and monitoring of non-small cell lung cancer. Technol Cancer Res Treat. 2017;1533034617723754.
42. Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36(3):131–149. doi:10.1046/j.1365-2184.2003.00266.x
43. Gartel AL, Serfas MS, Tyner AL. p21–negative regulator of the cell cycle. Proc Soc Exp Biol Med. 1996;213(2):138–149. doi:10.3181/00379727-213-44046
44. Kraljevic Pavelic S, Cacev T, Kralj M. A dual role of p21waf1/cip1 gene in apoptosis of HEp-2 treated with cisplatin or methotrexate. Cancer Gene Ther. 2008;15(9):576–590. doi:10.1038/cgt.2008.28
45. Dasgupta P, Kulkarni P, Majid S, et al. MicroRNA-203 inhibits long noncoding RNA HOTAIR and regulates tumorigenesis through epithelial-to-mesenchymal transition pathway in renal cell carcinoma. Mol Cancer Ther. 2018;17(5):1061–1069. doi:10.1158/1535-7163.MCT-17-0925
46. Jing L, Yuan W, Ruofan D, Jinjin Y, Haifeng Q. HOTAIR enhanced aggressive biological behaviors and induced radio-resistance via inhibiting p21 in cervical cancer. Tumour Biol. 2015;36(5):3611–3619. doi:10.1007/s13277-014-2998-2
47. Liu Y, Wang B, Liu X, et al. Epigenetic silencing of p21 by long non-coding RNA HOTAIR is involved in the cell cycle disorder induced by cigarette smoke extract. Toxicol Lett. 2016;240(1):60–67. doi:10.1016/j.toxlet.2015.10.016
48. Kardassis D, Papakosta P, Pardali K, Moustakas A. c-Jun transactivates the promoter of the human p21(WAF1/Cip1) gene by acting as a superactivator of the ubiquitous transcription factor Sp1. J Biol Chem. 1999;274(41):29572–29581. doi:10.1074/jbc.274.41.29572
49. Gu L, Feng J, Zheng Z, Xu H, Yu W. Polyphyllin I inhibits the growth of ovarian cancer cells in nude mice. Oncol Lett. 2016;12(6):4969–4974. doi:10.3892/ol.2016.5348
50. Chai Y, Si Y, Xu J, et al. Polyphyllin I inhibits proliferation and induces apoptosis by downregulating AML1-ETO and SUPPRESSING C-KIT/Akt signaling in t(8;21) acute myeloid leukemia. Chem Biodivers. 2018;15(11):e1800314. doi:10.1002/cbdv.v15.11
51. Yu S, Wang L, Cao Z, et al. Anticancer effect of Polyphyllin Iota in colorectal cancer cells through ROS-dependent autophagy and G2/M arrest mechanisms. Nat Prod Res. 2018;32(12):1489–1492. doi:10.1080/14786419.2017.1353512
52. Lallous N, Leblanc E, Munuganti RS, et al. Targeting binding function-3 of the androgen receptor blocks its co-chaperone interactions, nuclear translocation, and activation. Mol Cancer Ther. 2016;15(12):2936–2945. doi:10.1158/1535-7163.MCT-16-0354
53. Fong KW, Zhao JC, Kim J, et al. Polycomb-mediated disruption of an androgen receptor feedback loop drives castration-resistant prostate cancer. Cancer Res. 2017;77(2):412–422. doi:10.1158/0008-5472.CAN-16-1949
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.