Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Integration of Chinese Herbal Medicine into Routine Care Was Related to Lower Risk of Chronic Kidney Disease in Patients with Rheumatoid Arthritis: A Population-Based Nested Case–Control Study in Taiwan
Authors Liao HH , Chen HT, Livneh H , Huang HL, Lai NS, Lu MC , Yeh CC , Tsai TY
Received 26 December 2022
Accepted for publication 22 February 2023
Published 29 April 2023 Volume 2023:16 Pages 1191—1201
DOI https://doi.org/10.2147/JMDH.S400917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hou-Hsun Liao,1– 3 Hsiao-Tien Chen,4 Hanoch Livneh,5 Hua-Lung Huang,6 Ning-Sheng Lai,7,8 Ming-Chi Lu,7,8 Chia-Chou Yeh,1,2,9 Tzung-Yi Tsai3,10,11
1Graduate Institute of Chinese Medicine, School of Chinese Medicine, College of Chinese Medicine, China Medical University, Taichung, Taiwan; 2Department of Chinese Medicine, Dalin Tzuchi Hospital, The Buddhist Tzuchi Medical Foundation, Chiayi, Taiwan; 3Department of Nursing, Tzu Chi University of Science and Technology, Hualien, Taiwan; 4Department of Chinese Medicine, Chi Mei Medical Center, Tainan, Taiwan; 5Rehabilitation Counseling Program, Portland State University, Portland, OR, USA; 6Department of Rehabilitation, Dalin Tzuchi Hospital, The Buddhist Tzuchi Medical Foundation, Chiayi, Taiwan; 7School of Medicine, Tzu Chi University, Hualien, Taiwan; 8Division of Allergy, Immunology and Rheumatology, Dalin Tzuchi Hospital, The Buddhist Tzuchi Medical Foundation, Chiayi, Taiwan; 9School of Post-Baccalaureate Chinese Medicine, Tzu Chi University, Hualien, Taiwan; 10Department of Environmental and Occupational Health, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 11Department of Medical Research, Dalin Tzuchi Hospital, The Buddhist Tzuchi Medical Foundation, Chiayi, Taiwan
Correspondence: Chia-Chou Yeh; Tzung-Yi Tsai, Tel +886-5-2648000-8713 ; +886-5-2648000 ext. 3209, Fax +886-5-2648006, Email [email protected]; [email protected]
Objective: Non-steroidal anti-inflammatory drugs (NSAIDs) are frequently used as the first-line agents for the symptomatic relief of rheumatoid arthritis (RA), but it may insidiously provoke the onset of renal diseases, especially chronic kidney disease (CKD). While Chinese herbal medicine (CHM) has become an increasingly popular adjunctive therapy among RA groups, there are currently no available data on the effect of CHM use towards risk of CKD. This study aimed to explore on a population-level whether CHM use decreases sequent CKD risk among them.
Methods: In this nested case–control study retrieved from the nationwide insurance database of Taiwan from 2000 to 2012, we looked at the association between CHM use and the likelihood of developing CKD, with a focus on usage intensity. Cases with CKD claims were defined and matched to one randomly selected control case. Conditional logistic regression was then applied to estimate odds ratio (OR) of CKD from CHM treatment measured before the index date. For each OR, we calculated a 95% confidence interval for CHM use relative to the matched control.
Results: This nested case–control study included 5464 patients with RA, where after matching comprised 2712 cases and 2712 controls. Among them, there were 706 and 1199 cases that ever received CHM treatment, respectively. After the adjustment, CHM use in RA individuals was related to a lower likelihood of CKD, with an adjusted OR of 0.49 (95% CI: 0.44– 0.56). Additionally, a dose-dependent, reverse association was found between the cumulative duration of CHM use and risk of CKD.
Conclusion: Integrating CHM into conventional therapy may reduce the likelihood of developing CKD, which could be a reference in instituting novel preventive strategies to improve treatment outcomes and reduce related fatalities for RA subjects.
Keywords: rheumatoid arthritis, chronic kidney disease, Chinese herbal medicine, nested case–control study
Introduction
Rheumatoid arthritis (RA) is a chronic disabling illness characterized by pain and joint inflammation. Recent studies have shown that RA patients also have a high incidence of chronic kidney disease (CKD), which may be attributed to the systemic chronic inflammation, and renal toxicity due to uses of non-steroidal anti-inflammatory drugs (NSAIDs).1 NSAIDs are often recommended as first-line therapy for the symptomatic management of RA to block the production of prostaglandins through the inhibition of two cyclooxygenase enzymes, like COX-1 and COX-2,2 yet the inhibition of cyclooxygenase enzymes with subsequent reduction in prostaglandin synthesis may lead to reversible kidney ischemia, such as a decline in glomerular hydraulic pressure and acute kidney injury.3
In this case, subjects taking higher dose of NSAIDs were found to have a higher risk of CKD than their counterparts. Möller et al investigated the effects of NSAID use on long-term decline of renal function in a cohort of RA subjects, and found that high cumulative NSAID usage substantially induced the rapid CKD progression.4 Notably, once RA patients developed end-stage renal disease, they may be at higher risk of severe disease and death.5 Considering the high level of morbidity caused by these conditions, implementing a novel treatment notion may be of importance during early stages of RA than during late disease stages in which irreversible physiological manifestations may have already taken place.
Today, use of Chinese Herbal Medicine (CHM) products has become one of the fastest growing forms of health care proven beneficial in treating a variety of muscle and joint disorders.6 Similarly, ample evidence suggests that the herbal products can be used to prevent CKD progression and ameliorate the impacts of CKD.7,8 Using a rodent model, Zhang et al mentioned a major compound purified from Rheum officinale, could provide renal protective properties by reversing Klotho repression via promoter demethylation.9 Additionally, one recent meta-analysis of 27 studies indicated that the administration of Rheum officinale Baill. was beneficial in lowering serum creatinine and blood urine nitrogen,10 both of these products were commonly used for the RA treatment so far.11 In this context, there is an urgent need to explore whether adding CHM to conventional RA treatment may be beneficial in preventing or delaying CKD onset.
After a detailed literature review, we discovered that few studies, if any, have been conducted to explore the long-term effect of use of CHM treatment in diminishing the subsequent risk of CKD among RA groups. To take a close look at this question, we conducted a nested case–control study to address this issue on the basis of a random sample from a nationwide claims database.
Methods
Data Source
We performed a nested case–control study using 2000–2012 data from the Longitudinal Health Insurance Database (LHID) held by the Bureau of National Health Insurance (NHI) in Taiwan.12 At present, more than 95% of Taiwan’s healthcare providers have contracted with the NHI and nearly 99% of Taiwan’s residents have enrolled in the National Health Insurance Administration Ministry of the Health and Welfare’s program. The implementation of the national insurance program has enabled residents to receive universal participation and equal-opportunity medical care.12 LHID comprises a data subset of the NHI program and incorporates the claims of 1 million beneficiaries, randomly selected from all beneficiaries under the NHI program. This database contains all NHI enrollment files, inpatient and outpatient claims data, primary and secondary diagnoses, procedures, prescription drugs and medical costs that provide comprehensive information on all insured subjects.
Underlying Cohort Establishment
Using the LHID, we identified subjects 20–80 years of age who had more than 2 outpatient, or a single inpatient, visits due to RA diagnosis from January 2000 to December 2010 (The International Classification of Diseases, 9th Revision, Clinical Modification, ICD-9-CM 714.0). Afterwards, all enrolled cases were linked to the catastrophic illness registry to ensure diagnostic validity. Under the NHI program, the beneficiaries with major diseases, such as autoimmune disorders, are exempt from the required cost sharing policy. This approach allowed us to strictly define RA cases and reduce any potential misclassification bias. We then utilized the date of approval for catastrophic illness registration as the starting point for the time at risk with RA. Meanwhile, we are bound to obey the rule that subjects would be excluded if they had any history of CKD prior to RA onset. All enrollees were followed up until the earliest CKD incident (defined in the case ascertainment), death, withdrawal, or the end of this study (December 31, 2012), whichever came first. This research project was approved by the Ethics Committee of the Buddhist Dalin Tzu Chi Hospital (No. B10004021-3) and was conducted with consideration of the Helsinki Declaration in all phases of the study. Additionally, the institutional review board waived the need for informed consent for this study since an encrypted database was fully used.
Case and Control Ascertainment
The study outcome measure was first-time diagnosis of CKD, which occurred between 2001 and 2012. The documentation of the CKD code was regarded as valid if the enrollee has incurred at least twice in the records of outpatient clinics within 1 year, or at least once during hospitalization (ICD-9-CM code of 585).8 The date of the first diagnosis of CKD onset was deemed the index date. Of the study cohort, each CKD case was matched, according to age (within 2 years) and sex, using a risk set sampling of 1:1, with a control subject who was not diagnosed with CKD (Figure 1). After matching, the outcome date for each case group was assigned as the index date to the control group, for case and control groups with the same probability to occur of CKD outcome during the follow-up period.
 |
Figure 1 Flowchart of patient screening. |
Exposure Assessment of CHM Use
To define CHM exposure of subjects, we examined the individual CHM treatment records occurring from the cohort entry date to the index date. In this study, CHM users were defined as those who ever received the relevant CHM treatment for more than 30 days due to diagnosis of RA or its associated symptoms. In contrast, those who were treated by Western medical physicians were classified as non-CHM users.13 Following this step, we reviewed the study period for claims of CHM treatments and summed up the total days of CHM sessions. For CHM users, their prescription days were further categorized into low, medium, or high sub-periods, based on the tertile distribution of the length (in days) of time of receiving CHM treatments due to RA. This procedure allowed us to clearly shed light on the impact of CHM on prevention of CKD among them.
Measurement of Covariates
Of the covariates considered for the study, it comprised gender, age, income for estimating insurance payment, urbanization of the subject’s residential area and former comorbidities. Regarding income, we used the premium category as a proxy and it was transformed to ordinal variables, namely New Taiwan Dollars [NTD] ≦17,880, 17,881–40,000, and ≥400,001. Furthermore, we adopted the urbanization system of insured zones, studied by former scholars, and these ranged from level 1 (highly urban) to level 7 (highly rural), as the standard to assess personal urbanization.14 Baseline comorbidities considered in our analysis included several chronic illnesses, such as cancer (ICD-9-CM 140–208), hypertension (ICD-9-CM 401–405), depression (ICD-9-CM 296.2, 296.3, 300.4 and 311), diabetes (ICD-9-CM 250) and heart disease (ICD-9-CM 410–429).
Statistical Modeling
For baseline characteristics, we reported continuous variables using means (and standard deviation, SD) and categorical variables with frequencies (and percentages). Comparisons between groups were made using Student’s t-test and Chi-square test. Univariate conditional logistic regression analysis was employed to estimate the crude OR of CKD events among CHM users. We then utilized the multivariate conditional logistic regression to adjust all covariates that were measured in one year preceding the index date, which included age, gender, urbanization level, income and comorbidities. All analyses were done using SAS version 9.3 for Windows (SAS Institute Inc., Cary, NC, USA), and all statistical tests were performed at the two-tails significance level of 0.05.
Results
We identified 11,071 RA subjects who met the selection criteria within the study period. Among those, 2,712 matched pairs of RA patients with and without CKD were recruited. Baseline characteristics are shown in Table 1. The mean age was 50.5 years (SD = 13.1), and the majority were female (68.0%). Also, the majority of patients had a monthly income of NTD 17,881–43,900 (51.6%) and lived in urbanized areas (57.9%). The most common comorbidities in both groups were hypertension (23.0%), followed by heart disease (12.2%) and diabetes (10.1%). Collectively, there were no differences in initial demographic data and comorbidities between two groups.
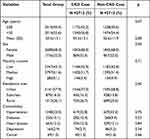 |
Table 1 Demographic Data and Selected Comorbidities Between Two Groups |
Of the whole study cohort, nearly 26.0% (706/2712) and 44.2% (1199/2712) cases ever received CHM therapy in the CKD group and non-CKD group, respectively. After using multivariable logistic regression models to identify any association between previous CHM use and CKD risk (Table 2), we observed that those who ever used CHM had a lower risk of CKD than those who did not use CHM (adjusted OR, 0.49; 95% CI = 0.44–0.56). Notably, the high-intensity CHM use was remarkably associated with a nearly 60% lower risk of developing CKD, which suggests a dose-dependent inverse relation between CHM use and risk of developing CKD (adjusted OR, 0.42; 95% CI = 0.36–0.49). Of the commonly prescribed CHM formulas for RA (Table 3), we found that eight prescriptions were associated with a lower risk of CKD, and these included Yan-Hu-Suo, Tian-Hua-Fen, Ge-Gen, Da-Huang, Shao-Yao-Gan-Cao-Tang (SYGCT), Jia-Wei-Xiao-Yao-San (JWXYS), Chuan-Xiong-Cha-Tiao-San (CXCTS), and Gan-Lu-Yin (GLY) (Figure 2).
 |
Table 2 The Association Between CKD Onset and Use of CHM Treatment |
 |
Figure 2 Risk of CKD in relation to the 10 most-used single-herb and multi-herb CHM products for RA patients. |
 |
Table 3 The Ingredient Herbs Contained in the Most-Used Single-Herb and Multi-Herb Products Among Participants |
Discussion
Few studies, if any, have been conducted to explore the long-term effects of CHM on the reduction of CKD risk in RA patients. A total of 5464 patients with RA were included in this nested case–control study. After one-to-one frequency matching, the study found that the incidence of CKD was substantially lower in CHM users, with an adjusted OR of 0.49 (95% CI = 0.44–0.56). In addition, the longer use of CHM treatments, the more positive prevention against incident CKD. We observed that RA patients with a high-intensity CHM use experienced a nearly 60% reduced risk of CKD, which coincided with the earlier reports.7,15 A variety of natural products from traditional Chinese medicine have been shown to safely regulate proinflammatory pathways and control inflammation-associated diseases.16 In addition, some of the ingredients extracted from medicinal herbs have demonstrated effective anti-inflammatory along with antiarthritic activities,17,18 which may explain the beneficial effect of CHM found in our work.
Of the commonly used single-herb products to treat RA, we found that Yan-Hu-Suo would reduce the risk of CKD by nearly 40%. This result was similar to that of from another retrospective population-based cohort study among hepatitis patients.19 Its components are believed to reduce the blood pressure and exert anti-oxidative, anti-fibrotic, and anti-thrombotic effects. Tetrahydropalmatine, one of the active components isolated from Yan-Hu-Suo, was found to possess antihypertensive activity by decreasing hypothalamic serotonin and noradrenaline release in the rodent model.20,21 Anti-platelet aggregative and anti-oxidative effects have also been detected from Tetrahydro berberine, a major compound purified from this formula,22–24 all of which may support its therapeutic benefit.
The current study pointed to a lower incidence of CKD among RA patients who received Da-Huang. A human clinical study addressed that Da-Huang supplementation could improve the renal function in the CKD patients with stage 3 or 4.25 Clinically, though Da-Huang has been traditionally used as laxative, several recent studies noted that Da-Huang possessed various renal protective effects including diuretic, purgative, anti-inflammatory, anti-oxidative and anti-fibrosis activities.26,27 In addition, Chrysophanol, purified from Da-Huang, has been indicated to alleviate renal interstitial fibrosis by inhibiting Smad3 phosphorylation and suppressing the progression of diabetic nephropathy via the TGF-β signaling pathway inactivation.28,29
Our findings depicted the protective effect of Tian-Hua-Fen and Ge-Gen in preventing the onset of CKD as well. This reaction may have several scientific explanations behind it. For example, the dry root tuber of Tian-Hua-Fen has been shown to possess antioxidant and anti-inflammatory properties both in vitro and in vivo.30,31 Furthermore, a recent experiment based on the murine model indicated that this herbal formula might significantly enhance serum creatinine, blood urea nitrogen, and 24-h urinary albumin through the inhibition of cell apoptosis.32 All of these may improve renal tissue function to lower the vulnerability of CKD. Regarding Ge Gen, this herbal product also appears to lower the risk of CKD among RA patients. Several priori studies have found that Ge-Gen produces possessed the antioxidative, antifibrotic and anti-inflammatory effects in reducing kidney damage,33,34 which may point to the possible mechanism of this herbal medicine.
Of the commonly prescribed multi-herb products, use of SYGCT may reduce the risk of CKD. Several previous animal experiments described that the compounds in SYGCT may produce anti-inflammatory, anti-oxidant and anti-thrombotic effects in the kidney.35–37 Furthermore, the antihypertensive activity of Radix Paeoniae Alba, a major component of this formula, had been proved to improve vascular health and reduce endothelial dysfunction in a rat model.38,39 Those with endothelial dysfunction were more likely to progress to renal failure to suffer cardiovascular malfunctioning.
Compatible with recent scientific findings, use of JWXYS could improve both short-term and long-term renal outcomes.7,40 One experiment based on the animal model disclosed that the herb JWXYS attenuated kidney fibrosis via inhibition of the Hedgehog pathway.41 Additionally, other frequently used CHM therapies targeting RA, such as GLY and CXCTS, were remarkably associated with the predisposition of CKD. Both human and animal studies postulated that these remedies could decrease the levels of IL-6 and TNF-α via the suppression of nuclear factor kappa beta (NF-κB) activation.42–44 As a whole, we inferred that the anti-inflammatory effects derived from these polyherbal formulations may account for its beneficial impact in reducing incident CKD.
Despite its promising findings, several important limitations may restrict the generalizability of our study’s findings. First, several sources of data regarding family history, lifestyle, body weight, exercise regimen, and laboratory parameters were not recorded in the database. Thus, residual confounding might occur in the observed association of CHM use to the CKD risk. A randomized controlled trial to validate these findings is warranted. Second, this work was merely based on a retrospective cohort design that used ICD-9-CM diagnostic codes. Bias due to miscoding and misclassification may arise. To deal with this concern, we capitalized on procedural claims data to confirm ambulatory diagnostic codes, as well as use of inpatient claims data to minimize the possibility of misclassification. It should also be acknowledged that the NHI of Taiwan makes an effort to prevent false diagnoses by performing quarterly expert reviews of submitted diagnoses and imposes severe penalties for false diagnosis. On this note, the probability of individuals being misclassified is equal across groups in this investigation, so any misclassification was prone to be random, likely providing more conservative estimates. Third, data regarding RA severity are unavailable in the database, so we utilized the prescription of biological agents to be another proxy indicator to confirm RA severity, which contained adalimumab, etanercept, infliximab, rituximab and tocilizumab. Firstly, we separated all enrollees based on whether they received biological agents for more, or less, than 6 months after RA onset. Biological agents were used by 38.2% of the CHM users (1036/2712) and 40.1% (1088/2712) of the non-CHM users. After adjusting for this surrogate variable in the multivariate analysis, RA patients who used CHM still had a lower risk for CKD (adjusted OR, 0.50; 95% CI = 0.42–0.59), implying that the severity of RA may not affect the reported results herein. Despite our careful efforts to control for confounding factors in this study, we consent that the evidence from any observational cohort study is generally less robust than that from randomized trials due to the potential biases that may arise from unmeasured or unknown confounders. This study, however, offers several strengths that should be mentioned. First, we performed this investigation using a large population database. Over 90% of the Taiwanese population and their healthcare providers are covered under the NHI program, which includes a representative RA sample and leaves little room for non-response or loss to follow-up. Second, over 10-year follow-up period utilized allowed for ample opportunity to document the primary outcome. Last, the nested case–control approach used is a rival alternative to cohort analysis when studying time-dependent exposure, like the use of CHM treatments. Hence, our study could reflect real-world data that would be found in a clinical situation, as opposed to a randomized controlled trial.
In conclusion, findings of this study revealed that RA patients receiving CHM, in addition to conventional treatment, experienced a lower risk of developing CKD by 51%. As a consequence, more attention should be paid to preventing and managing symptoms of RA, especially the prevention of CKD. Future prospective randomized trials are, therefore, warranted to provide more robust evidence to support and guide the use of CHM in clinical practice targeting those diagnosed with rheumatic diseases.
Acknowledgment
This study obtained data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, managed by the Department of Health and Welfare, Taiwan. The main mission of the authority is to ensure that universal participation and equal-opportunity of medical care are being fulfilled. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or of the National Health Research Institutes. HHL, HTC, HL and HLH equally contributed to this work.
Author Contributions
All authors made a significant contribution to the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by Dalin Tzuchi Hospital (Grant Number DTCRD 110-E-19). The funders had no participation in any aspect of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chiu HY, Huang HL, Li CH, et al. Increased risk of chronic kidney disease in rheumatoid arthritis associated with cardiovascular complications - A national population-based cohort study. PLoS One. 2015;10(9):e0136508. doi:10.1371/journal.pone.0136508
2. Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(Suppl 3):S2. doi:10.1186/ar4174
3. Lucas GNC, Leitão ACC, Alencar RL, et al. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. J Bras Nefrol. 2019;41:124–130. doi:10.1590/2175-8239-jbn-2018-0107
4. Moller B, Pruijm M, Adler S, et al. Chronic NSAID use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann Rheum Dis. 2015;74(4):718–723. doi:10.1136/annrheumdis-2013-204078
5. Lai KM, Chen TL, Chang CC, Chen HH, Lee YW. Association between NSAID use and mortality risk in patients with end-stage renal disease: a population-based cohort study. Clin Epidemiol. 2019;11:429–441. doi:10.2147/CLEP.S204322
6. Zhang Z, Cao H, Shen P, et al. Ping weisan alleviates chronic colitis in mice by regulating intestinal microbiota composition. J Ethnopharmacol. 2020;255:112715. doi:10.1016/j.jep.2020.112715
7. Lin MY, Chiu YW, Chang JS, et al. Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int. 2015;88(6):1365–1373. doi:10.1038/ki.2015.226
8. Huang KC, Su YC, Sun MF, Huang ST. Chinese herbal medicine improves the long-term survival rate of patients with chronic kidney disease in Taiwan: a nationwide retrospective population-based cohort study. Front Pharmacol. 2018;9:1117. doi:10.3389/fphar.2018.01117
9. Zhang Q, Liu L, Lin W, et al. Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2017;91(1):144–156. doi:10.1016/j.kint.2016.07.040
10. Zeng JY, Wang Y, Miao M, Bao XR. The effects of rhubarb for the treatment of diabetic nephropathy in animals: a systematic review and meta-analysis. Front Pharmacol. 2021;12:602816. doi:10.3389/fphar.2021.602816
11. Pathan E, Gaitonde S, Rajadhyaksha S, et al. A longitudinal study of serum creatinine levels in patients of rheumatoid arthritis on long term NSAID therapy. J Assoc Physicians India. 2003;51:1045–1049.
12. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Patients with catastrophic illnesses or rare disease. Available from: https://www.nhi.gov.tw/English/Content_List.aspx?n=F5B8E49CB4548C60&topn=1D1ECC54F86E9050.
13. Chiao YW, Livneh H, Guo HR, et al. Use of Chinese herbal medicines is related to a reduction in depression risk among patients with insomnia: a matched cohort study. Front Neurol. 2020;11:583485. doi:10.3389/fneur.2020.583485
14. Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4(1):1–22.
15. Lai NS, Livneh H, Fan YH, et al. Use of Chinese herbal medicines by rheumatoid arthritis patients was associated with lower risk of stroke: a retrospective cohort study. Complement Ther Med. 2019;45:124–129. doi:10.1016/j.ctim.2019.05.029
16. Pan MH, Chiou YS, Tsai ML, Ho CT. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med. 2011;1(1):8–24. doi:10.1016/S2225-4110(16)30052-9
17. Tang Y, Liu Q, Feng Y, et al. Tripterygium ingredients for pathogenicity cells in rheumatoid arthritis. Front Pharmacol. 2020;11:583171. doi:10.3389/fphar.2020.583171
18. He YT, Ou AH, Yang XB, et al. Traditional Chinese medicine versus western medicine as used in China in the management of rheumatoid arthritis: a randomized, single-blind, 24-week study. Rheumatol Int. 2014;34(12):1647–1655. doi:10.1007/s00296-014-3010-6
19. Chang CP, Su YC, Lin MC, Huang ST. Chinese herbal medicine ameliorated the development of chronic kidney disease in patients with chronic hepatitis C: a retrospective population-based cohort study. Evid Based Complement Alternat Med. 2019;2019:5319456. doi:10.1155/2019/5319456
20. Bellomo R, Giantomasso DD. Noradrenaline and the kidney: friends or foes? Crit Care. 2001;5(6):294–298. doi:10.1186/cc1052
21. Tian B, Tian M, Huang SM. Advances in phytochemical and modern pharmacological research of Rhizoma corydalis. Pharm Biol. 2020;58(1):265–275. doi:10.1080/13880209.2020.1741651
22. Wu H, Waldbauer K, Tang L, et al. Influence of vinegar and wine processing on the alkaloid content and composition of the traditional Chinese medicine Corydalis Rhizoma (Yanhusuo). Molecules. 2014;19(8):11487–11504. doi:10.3390/molecules190811487
23. Pingali S, Donahue JP, Payton-Stewart F. Tetrahydroberberine, a pharmacologically active naturally occurring alkaloid. Acta Crystallogr C Struct Chem. 2015;71(Pt 4):262–265. doi:10.1107/S2053229615004076
24. Chen C, Wang F, Xiao W, et al. Effect on platelet aggregation activity: extracts from 31 traditional Chinese medicines with the property of activating blood and resolving stasis. J Tradit Chin Med. 2017;37(1):64–75. doi:10.1016/S0254-6272(17)30028-6
25. Khan IA, Nasiruddin M, Haque SF, Khan RA. Evaluation of rhubarb supplementation in stages 3 and 4 of chronic kidney disease: a randomized clinical trial. Int J Chronic Dis. 2014;2014:789340. doi:10.1155/2014/789340
26. Cao YJ, Pu ZJ, Tang YP, et al. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin Med. 2017;12:36. doi:10.1186/s13020-017-0158-5
27. Xiang H, Zuo J, Guo F, Dong D. What we already know about rhubarb: a comprehensive review. Chin Med. 2020;15:88. doi:10.1186/s13020-020-00370-6
28. Dou F, Ding Y, Wang C, et al. Chrysophanol ameliorates renal interstitial fibrosis by inhibiting the TGF-beta/Smad signaling pathway. Biochem Pharmacol. 2020;180:114079. doi:10.1016/j.bcp.2020.114079
29. Guo C, Wang Y, Piao Y, Rao X, Yin D. Chrysophanol inhibits the progression of diabetic nephropathy via inactivation of TGF-beta pathway. Drug Des Devel Ther. 2020;14:4951–4962. doi:10.2147/DDDT.S274191
30. Chen Y, Miao Y, Huang L, et al. Antioxidant activities of saponins extracted from Radix trichosanthis: an in vivo and in vitro evaluation. BMC Complement Altern Med. 2014;14:86. doi:10.1186/1472-6882-14-86
31. Jiandong L, Yang Y, Peng J, et al. Trichosanthes kirilowii lectin ameliorates streptozocin-induced kidney injury via modulation of the balance between M1/M2 phenotype macrophage. Biomed Pharmacother. 2019;109:93–102. doi:10.1016/j.biopha.2018.10.060
32. Zhang Q, Xiao X, Zheng J, et al. Shenqi jiangtang granule ameliorates kidney function by inhibiting apoptosis in a diabetic rat model. Evid Based Complement Alternat Med. 2019;2019:3240618. doi:10.1155/2019/3240618
33. Xu X, Zheng N, Chen Z, et al. Puerarin, isolated from pueraria lobata (Willd.), protects against diabetic nephropathy by attenuating oxidative stress. Gene. 2016;591(2):411–416. doi:10.1016/j.gene.2016.06.032
34. Jin SE, Son YK, Min B-S, et al. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch Pharm Res. 2012;35(5):823–837. doi:10.1007/s12272-012-0508-x
35. Tan Y-Q, Chen H-W, Li J, Wu Q-J. Efficacy, chemical constituents, and pharmacological actions of radix paeoniae rubra and radix paeoniae alba. Front Pharmacol. 2020;11:1054. doi:10.3389/fphar.2020.01054
36. Okubo T, Nagai F, Seto T, et al. The inhibition of phenylhydroquinone-induced oxidative DNA cleavage by constituents of moutan cortex and paeoniae radix. Biol Pharm Bull. 2000;23(2):199–203. doi:10.1248/bpb.23.199
37. Ye J, Duan H, Yang X, Yan W, Zheng X. Anti-thrombosis effect of paeoniflorin: evaluated in a photochemical reaction thrombosis model in vivo. Planta Med. 2001;67(8):766–767. doi:10.1055/s-2001-18364
38. Li B, Yang ZB, Lei SS, et al. Combined antihypertensive effect of paeoniflorin enriched extract and metoprolol in spontaneously hypertensive rats. Pharmacogn Mag. 2018;14(53):44–52. doi:10.4103/pm.pm_483_16
39. Su-Hong C, Qi C, Bo L, et al. Antihypertensive effect of radix paeoniae alba in spontaneously hypertensive rats and excessive alcohol intake and high fat diet induced hypertensive rats. Evid Based Complement Alternat Med. 2015;2015:731237. doi:10.1155/2015/731237
40. Chen W, Chen HY, Yang YH, et al. An investigation of the prescription patterns of Chinese herbal products for chronic glomerulonephritis patients: a hospital-based cross-sectional study. Evid Based Complement Alternat Med. 2018;2018:5080764. doi:10.1155/2018/5080764
41. Ren D, Luo J, Li Y, et al. Saikosaponin B2 attenuates kidney fibrosis via inhibiting the hedgehog pathway. Phytomedicine. 2020;67:153163. doi:10.1016/j.phymed.2019.153163
42. Yasui T, Yamada M, Uemura H, et al. Changes in circulating cytokine levels in midlife women with psychological symptoms with selective serotonin reuptake inhibitor and Japanese traditional medicine. Maturitas. 2009;62(2):146–152. doi:10.1016/j.maturitas.2008.12.007
43. Chen YH, Luo R, Lei SS, et al. Anti-inflammatory effect of ganluyin, a Chinese classic prescription, in chronic pharyngitis rat model. BMC Complement Med Ther. 2020;20(1):265. doi:10.1186/s12906-020-03057-5
44. Guo RB, Wang GF, Zhao AP, et al. Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-IoB-mediated inflammatory responses. PLoS One. 2012;7(11):e49701. doi:10.1371/journal.pone.0049701
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
