Back to Journals » OncoTargets and Therapy » Volume 12
Integrated Bioinformatics Data Analysis Reveals Prognostic Significance Of SIDT1 In Triple-Negative Breast Cancer
Authors Wang Y, Li H, Ma J, Fang T, Li X, Liu J, Afewerky HK , Li X, Gao Q
Received 16 May 2019
Accepted for publication 14 September 2019
Published 11 October 2019 Volume 2019:12 Pages 8401—8410
DOI https://doi.org/10.2147/OTT.S215898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Federico Perche
Ya Wang,1 Hanning Li,2 Jingjing Ma,1 Tian Fang,1 Xiaoting Li,1 Jiahao Liu,1 Henok Kessete Afewerky,3 Xiong Li,4 Qinglei Gao1
1Cancer Biology Research Center (Key Laboratory of the Ministry of Education), Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Department of Thyroid and Breast Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 3Department of Pathology and Pathophysiology, School of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 4Department of Gynecology and Obstetrics, Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Qinglei Gao
Cancer Biology Research Center (Key Laboratory of the Ministry of Education), Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Ave, Wuhan 430000, People’s Republic of China
Tel/fax +86-27-83662681
Email [email protected]
Background: Triple-negative breast cancer (TNBC) is a heterogeneous disease with a worse prognosis. However, current therapies have rarely improved the outcome of patients with TNBC. Here we sought to identify novel biomarkers or targets for TNBC.
Materials and methods: Patients GSE76275 clinic traits and their corresponding mRNA profiles for 198 TNBC and 67 non-TNBC were obtained from the GEO database. Weighted gene co-expression network analysis (WGCNA) of the GSE76275 keyed out hub genes, and the differentially expressed genes (DEGs) were identified with the cut-off of adjusted P (adj. P) <0.01 and |log2 fold-change (FC)| > 1.5. The hub - DEGs overlapping genes, as key genes, were considered for further study using Kaplan-Meier plotter online analysis. Subsequently, Breast Cancer Gene-Expression Miner v4.0 and tissue microarray analysis were applied to determine the transcriptional and translational levels of every key gene. Following plasmid transfection for overexpression, the proliferation of TNBC cells was determined by CCK8 and colony formation assay. Moreover, xenograft tumor models were canvassed to investigate their effect upon in vivo tumor growth.
Results: Four genes (SIDT1, ANKRD30A, GPR160, and CA12) were found to be associated with relapse-free survival (RFS) in TNBC through WGCNA and DEGs integrated analysis. Patients with a higher level of SIDT1 had significantly better RFS compared to those with lower levels. The transcriptional and translational levels of SIDT1 were validated as downregulated in patients with triple-negative status, negative estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Furthermore, SIDT1 inhibited proliferation of breast cancer cells (MDA-MB-231 and MDA-MB-468) and xenograft studies demonstrated that SIDT1 can suppress tumor growth in vivo.
Conclusion: This study suggests that SIDT1 may play a crucial role in TNBC progression and has the potential as a prognostic biomarker of TNBC.
Keywords: triple-negative breast cancer, WGCNA, prognosis, SIDT1
Introduction
Breast cancer remains the most commonly diagnosed cancer and a leading cause of cancer death among females.1 Approximately 2.1 million new cases were diagnosed with breast cancer in 2018 globally, accounting for 24.2% of total novel cancer cases among females.1 Triple-negative breast cancer (TNBC), which is defined by no expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), accounts for 10–20% of all breast cancers.2,3 Patients with TNBC typically have a higher recurrence and mortality rate than all other breast cancer subtypes owing to biologically more aggressive behavior and the lack of hormone receptors and HER2 expression for targeted therapy.4–7 Hence, identifying reliable biomarkers and effective targets for TNBC is urgently needed for enhancing overall prognosis.
The wealth of molecular information from public database resources, such as The Cancer Genome Atlas (TCGA: https://www.cancer.gov/tcga) and Gene Expression Omnibus (GEO) database,8 offers insight upon the mechanism of cancer progression and an opportunity for discovering new biomarkers. Furthermore, reanalyzing of these data based on bioinformatics methods could also provide new clues for discovering valuable targets. Among these bioinformatics methods, differentially expressed genes (DEGs) analysis is a widely used tool riveted on gene upregulation and downregulation independently. As genome is a complicated and highly interconnected network, there is a need to analyze from different perspectives.9 Weighted gene co-expression network analysis (WGCNA), based on a scale-free network, is used for identifying modules or clusters of highly correlated genes.10 Thus, WGCNA is a powerful tool to identify key genes that contribute to phenotypic traits and referencable candidate biomarkers or therapeutic targets.
In this study, we selected candidate genes related to TNBC by combining DEGs and WGCNA algorithms. We further explored their prognostic value and expression in breast cancer. Then, we chose SIDT1 for further study and verified its correlation with clinicopathological progression by tissue microarray. Moreover, we investigated its effect on tumor growth both in vitro and in vivo.
Materials And Methods
Cell Culture And Transfection
Human breast cancer lines MDA-MB-231 and MDA-MB-468 were purchased from ATCC and cultured in DMEM medium with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C with 5% CO2. A plasmid expressing SIDT1 (pENTER-CMV-SIDT1-flag) and an empty plasmid control (pENTER) were constructed by inserting target gene into plasmid pENTER using restriction enzyme AsisI/MluI obtained from Vigene Bioscience (Shangdong, China). To confirm the insertion, two primers (primerF: CGCAA'ATGGGCGGTAGGCGTG and primerR: CCTCTACAAATGTGGTATGGC) were constructed by sequencing the target gene. The plasmid transfections were performed using X-tremeGENE HP DNA Transfection Reagent (Cat. #739406, Roche) following the manufacturer instructions. To generate the lentiviral vector for overexpression of SIDT1, the FUGW-H1-GFP-neomycin (Cat. #37632, Addgene) was modified by inserting a target gene downstream from HIV-1 flap into a unique EcoRI-BamHI site. The lentivirus was constructed and produced by Taitool Bioscience Co. Ltd. (Shanghai, China).
Animal Studies
Four-week-old 24 female athymic nude (nu/nu) mice were purchased from HFK Bio-Technology Co. Ltd. (Beijing, China). MDA-MB-231 (1×106) and MDA-MB-468 (1×106) cells with indicated lentivirus were suspended in 50 µl of PBS and injected subcutaneously into the caudal thigh of each mouse. Tumor volumes were calculated every 7 days according to the formula: volume = (the longest diameter) × (the maximum width)2/2 and the weight of tumors were recorded at the end of the experiment. The animal experiment was performed in accordance with the Animal Welfare Act, “Guidelines for the Care and Use of Laboratory Animals”, approved by the Committee for Ethics on Animal Experiments of Tongji Hospital, Tongji medical college, Huazhong University of Science and Technology.
Immunohistochemistry
Two human breast cancer tissue chips (BR2082a and BR487c, Alenabio, China) were utilized for SIDT1 immunostaining in accord to the Specimens in Tissue Chips Collection and Use guideline approved by the Ethics Committee of People’s Hospital of Xutong County, Henan Province and subsequent approval by the Ethical Management Committee of Tongji Hospital - Tongji Medical College. A human paraffin-embedded tissue array of BR2082a and BR487c contained 44 cases with benign breast lesion, 52 cases diagnosed with TNBC, and 108 cases with non-TNBC. Tissue sections were subjected to immunohistochemical (IHC) analysis using the Avidin-Biotin Complex (ABC) Vectastain Kit (SP-9001, Zsgb-Bio) according to the manufacturer’s protocol. Anti-human SIDT1 (55352-1-AP, Proteintech) was used as a primary antibody. Further, SIDT1 immunostaining was evaluated independently by two pathologists who were blinded to all clinical information. A semi-quantitative scoring system was used for evaluation as described previously.11 In brief, the staining intensity was marked (0 = absence; 1 = weak; 2 = moderate; 3 = strong). The HSCORE was determined via the following formula: HSCORE = ∑Pi×(i+1), where i is the staining intensity of immunocytes, and Pi is the percentage of corresponding cells at each level of intensity. Hscore ≤ 2 was sorted as a low protein level, and Hscore >2 was sorted as a high protein level.
Western Blot Analysis
Total proteins of pre-treated cells were harvested with RIPA buffer. Immunoblotting was performed as described by the manufacturer’s instructions. Anti-SIDT1 (1:500, 55352-1-AP, Proteintech) and anti-GAPDH (1:2000, GB11002, Servicebio, Inc.) were used as primary antibodies for incubation. Quantification of the relative expression level was performed by Image-Pro Plus.
Cell Viability Assay
Cell viability was assessed by utilizing CCK-8 (Beyotime Institute of Biotechnology, China). Briefly, cells were seeded into 96-well plates (1,000 cells per well) and then incubated for 12, 24, 36 and 48 hrs. The CCK-8 reagent was added into each well with incubation at 37 °C for 4 h. The absorbance at 450 nm was measured using a Microplate Reader.
Colony Formation Assay
A total of 1,000 survived cells per well were incubated in 6-well plates for 10 days. Colonies were treated with 4% paraformaldehyde, stained with crystal violet for 8 min, and counted.
Data Collection And Preprocessing
The mRNA expression profiles and corresponding patient clinic traits were downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE76275.12 In total, 265 samples (198 TNBC and 67 non-TNBC) analyzed on the Affymetrix Human Genome U133 Plus 2.0 Array platforms were included in the dataset. The GEL files of normalized data were downloaded and processed, then preprocessed for background correction using the RMA (Robust Multichip Average) package (http://rdrr.io/bioc/oligo/man/rma-methods.html).
Identification Of Candidate Genes
A coexpression network was constructed using the WGCNA algorithm.10 Module eigengenes (MEs) were utilized to assess module-trait associations. Module significance (MS) and gene significance (GS) were used to calculate the expression patterns of modules associated with clinical traits. The minimum number of genes was set as 30 with a threshold of 0.25 for the high reliability of the results. DEGs in GSE76275 were identified using the limma R package with the Empirical Bayes method, and statistically significant DEGs were defined as adjusted P (adj. P) <0.01 and |log2 fold-change (FC)| >1.5. The candidate genes were screened by taking the intersection of DEGs and hub module.
Prognostic Analysis
The prognostic value of candidate genes was analyzed using the Kaplan-Meier Plotter (http://kmplot.com/analysis/), an online database that integrates gene expression data and clinical data. The correlation between mRNA expression of candidate genes and relapse-free survival (RFS) was assessed in patients with TNBC.
Breast Cancer Gene-Expression Miner v4.0
Breast Cancer Gene-Expression Miner v4.0 (bc-GenExMiner v4.0) is a statistical mining tool, which contains 36 annotated genomic datasets (updated in December 2017).13,14 It was used to evaluate the mRNA expression levels of SIDT1 according to clinical parameters, including triple-negative status, ER, PR, and HER2.
Statistical Analysis
This study used SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis. Data were presented as mean value ± standard error of the mean (SEM) and interpreted by two-tailed, unpaired Student’s t-tests or one-way ANOVA. Fisher’s exact and chi-square tests were performed to evaluate the association of SIDT1 expression with clinical factors. P-value < 0.05 was considered significant for all results.
Results
Construction Of Weighted Coexpression Network And Identification Of Hub Genes
In total, 265 samples with clinical data were included from the coexpression analysis. We chose clinicopathologic features including triple-negative status (TNS), AJCC stage, body mass index (BMI), tumor size, and menopausal status for the WGCNA study. Genes with similar expression patterns were classified into one module. Accordingly, 12 modules were excavated with a cutoff of powers = 13 (scale-free R2 = 0.90) (Figure 1A). Among the modules, the blue and pink module had the highest correlation with the TNS trait (Figure 1B), and 1000 genes were randomly selected for the heatmap (Figure 1C). Subsequently, an intramodular analysis of gene significance (GS) and module membership (MM) of the genes within 12 modules was performed. As GS and MM exhibited a significant correlation, the genes in the blue module were synergistically downregulated and tended to be highly correlated with TNBC (Figure 1D). Therefore, 241 genes in the blue module were selected as hub genes for further screening and analysis.
Identification Of DEGs In TNBC
The DEGs of GSE76275 were analyzed using the limma R package following preprocessing for background correction. With the cut-off of adj. P < 0.05 and |logFC| > 1.5, 92 DEGs were identified, including 34 upregulated genes and 58 downregulated genes in TNBC specimens compared to non-TNBC specimens (Figure 1E). The total DEGs were shown in the volcano map, and the visualized heatmap of 92 DEGs according to the value of |logFC| were also shown (Figure S1).
Key Genes Identified In Hub Genes And DEGs
There were 31 overlapping genes among hub genes and DEGs (Table S1). It suggested that these genes were significantly downregulated in TNBC and were closely related to TNBC. To further investigate their association with TNBC outcomes, prognostic analysis of these genes in TNBC was conducted on the Kaplan-Meier plotter. Briefly, four genes namely SIDT1, ANKRD30A, GPR160, and CA12 were found to be correlated with the RFS of patients in TNBC (HR = 0.62 (0.40–0.95), 0.57 (0.33–1.00), 0.53 (0.31–0.93), and 1.77 (1.15–2.73), respectively) (Figure 2A–D). Patients with a higher level of SIDT1, ANKRD30A, or GPR160 had significantly better RFS compared to those with lower levels; while conversely, upregulated CA12 was significantly associated with poor RFS. ANKRD30A, previously identified as breast cancer antigen NY-BR-1,15 has been generally detected both in normal and tumorous mammary epithelium.16 It has also been found to be preferentially expressed in breast tumors with lower malignant potential, including low grade, estrogen receptor-positive, and lymph node-negative status.17 Moreover, downregulation of NY-BR-1 mRNA and protein levels have been demonstrated in TNBC.18,19 GPR160, an orphan G protein-coupled receptor, is gradually known to play a critical role in the pathogenesis of cancer.20 The overexpression of GPR160 correlates with poor prognosis in nasopharyngeal cancer.21 CA12 is widely expressed in several tumor types, such as renal, colorectal, lung, ovarian, and cervical cancers.22–24 Previous studies have demonstrated that high expression of CA12 predicts good prognosis in breast cancer.25,26 SIDT1 is originally recognized as a transmembrane channel for small RNA.27 A study on IL-4/Stat6 pathway in breast cancer showed that SIDT1 is upregulated by IL4.28 However, there is a lack of research on the relationship between SIDT1 and cancer. Therefore, we plan to explore the expression of SIDT1 in breast cancer and investigate its role in cancer progression.
Expression Of SIDT1 In Patients With Breast Cancer
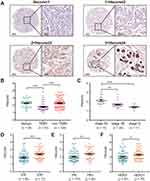
The mRNA expression of SIDT1 in breast cancer was validated using the bc-GenExMiner database. As shown in Figure 2E, SIDT1 mRNA levels were lower in patients diagnosed with TNBC than in those with non-TNBC(P < 0.001). Moreover, the mRNA levels of SIDT1 were significantly decreased in patients with ER, PR, and HER2 negative status compared to the positive status respectively (Figure 2F–H). To further verify the expression of SIDT1 in breast cancer, immunohistochemical analysis was conducted in tissue samples. As shown in Figure 3, positive staining for SIDT1 was distributed in the cytoplasm and plasma membrane of cells (Figure 3A). SIDT1 expression was obviously decreased in TNBC tissues compared to benign breast lesion and non-TNBC tissues (Figure 3B). Notably, later stages of TNBC were detected with downregulated SIDT1 levels (Figure 3C). Specifically, patients diagnosed at stage IIA showed higher expression of SIDT1 compared to those diagnosed at stage IIB (P < 0.01) and stage III (P < 0.001). Consistent with the previous database analysis, decreased expression of SIDT1 was observed in patients with ER, PR, and HER2 negative status at the protein level (Figure 3D–F).
Correlation Between SIDT1 Expression And Clinicopathological Features
TNBC is known to be more aggressive and has a poorer prognosis compared to other breast cancer subtypes.5,29 Given that SIDT1 is significantly downregulated in TNBC and is associated with TNBC progression, we further investigated the value of SIDT1 in overall breast cancer progression. The relationship between SIDT1 expression and clinicopathological features of 160 breast cancer cases was analyzed. As shown in Table 1, most patients with breast cancer in the study were aged ≤ 50 (60%), and a high percentage of patients presented as stage II (62.5%). There was no difference in the SIDT1 expression level between patients aged ≤ 50 and > 50 (P = 0.750) (Table 1). However, SIDT1 expression was negatively correlated with the pathologic grades of breast cancer (P = 0.015) (Table 1). Notably, later stages of breast cancer were detected with downregulated SIDT1 (P = 0.001) (Table 1). These results indicated that a negative correlation exists between SIDT1 and general breast cancer progression.
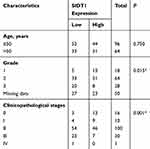 |
Table 1 Association Of SIDT1 Expression With The Clinicopathologic Characteristics Among Breast Cancer Patients |
Effect Of SIDT1 Overexpression On Tumor Progression In Vitro And In Vivo
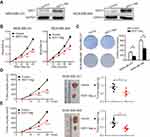
Given the correlation of SIDT1 and TNBC progression and its prognostic value in TNBC, we next investigated the effect of SIDT1 on the proliferation of MDA-MB-231 and MDA-MB-468 cells by CCK8 and colony formation assay. Successful overexpression by SIDT1-plasmid was verified by Western blot in both cell lines (Figure 4A). MDA-MB-231 and MDA-MB-468 cells with SIDT1 overexpression exhibit a decrease in cell viability and colony formation (Figure 4B and C). To further determine the role of SIDT1 in breast cancer, we used siRNA to inhibit SIDT1 expression in MCF-7 cells, a non-TNBC cell line with high-level expression of SIDT1 (Figure S2A and B). Consistently, downregulation of SIDT1 expression markedly enhanced the cell viability and colony formation of MCF-7 cells (Figure S2C and D). Moreover, to confirm whether SIDT1 has a function in vivo, we then used mice xenotransplant models. Tumor xenografts with SIDT1-overexpression MDA-MB-231 and MDA-MB-468 cells both displayed a significant reduction in tumor growth and weight (Figure 4D and E).
Discussion
In the present study, we identified four genes (SIDT1, ANKRD30A, GPR160, and CA12) were downregulated in TNBC and related to TNBC patient prognosis by combining WGCNA and DEGs analysis. Among them, SIDT1 expression was lower in both TNBC specimens and cases with negative ER, PR, and HER2 status; marking a later stage for patients with TNBC. Moreover, we found that SIDT1 exhibited a remarkable anti-tumor effect on TNBC in vitro and in vivo.
To gain valuable insights in TNBC, TNBC and non-TNBC specimens were analyzed by WGCNA and DEGs. WGCNA is a widely used biological data mining method to identify modules of highly correlated genes that may be used for candidate markers or therapeutic targets.10,30 Several prognostic genes have been recognized by WGCNA in breast cancer.31,32 In this study, we identified four downregulated genes (SIDT1, ANKRD30A, GPR160, and CA12) in TNBC by integrating the results of WGCNA and DEGs. These genes were found to be predictive markers for the prognosis of patients with TNBC. ANKRD30A, a commonly used marker of disseminated tumor cells (DTC), has previously been found significantly downregulated in TNBC tissues.18,33 GPR160 is reported to be involved in the pathogenesis of nasopharynx and prostate cancers.20,21 Moreover, GPR160 overexpression was found in metastatic sites of melanoma.34 CA12 is widely investigated in human tumors and has proven to be a valuable prognostic factor for patients with esophageal and breast cancers.25,35 However, to date, the regulation and functional properties of SIDT1 in cancer have been extremely poorly studied.
SIDT1, also known as a human orthologue of SID-1, belongs to the SID family which is a highly conserved group of transmembrane channel-like proteins.36 It can function as a transmembrane channel for dsRNA and was primarily determined following a screen of C. elegans mutants deficient the systemically RNA interference (sysRNAi) phenotype.37,38 As a transmembrane channel for intercellular communication, SIDT1 was found to facilitate small interfering RNA (siRNA) or miRNA uptake and also involved in cholesterol transport in human systems.27,39–41 Remarkably, SIDT1 is essential for normal tissue organization, but it also participates in tumor progression and chemotherapy resistance. Specifically, SIDT1 could act as a mediator of intercellular communication to enhance pancreatic adenocarcinoma chemoresistance to gemcitabine driven by miR-21.36 Moreover, Zhang WJ et al also found that SIDT1 could be regulated by IL-4 in breast cancer cells via Stat6-dependent and/or –independent pathways.28 In this study, we revealed its down-regulation in TNBC. Notably, SIDT1 expression was positively correlated with the expression of ER, PR, and HER2, respectively (p<0.05). Furthermore, we found a strong association between SIDT1 and RFS in patients with TNBC (HR = 0.62 (0.40–0.95)). Upregulated expression of SIDT1 was linked with better RFS. To further explore its potential value in tumor progression, we compared the expression levels of SIDT1 in human tissues by immunohistochemistry. A negative correlation between SIDT1 and FIGO stage was found in breast cancer and TNBC. Considering the limited sample sizes in the TNBC analysis, this result needs further confirmation. Statistical analysis also revealed that the expression of SIDT1 was negatively correlated with breast cancer grade. All of these results suggest a crucial role for SIDT1 in breast cancer.
Although the essence of organism-level sysRNAi in mammals is not yet fully elucidated, SIDT1-dependent intercellular communication by RNA transfer may have several functional genomics and remedial applications in cancer. Across biological processes, a growing body of research has led to an understanding of tumorigenesis, metastasis, and drug resistance, arbitrated by miRNAs in TNBC.42 The role of miRNA is complex and multidirectional, such as oncomiRs and tumor-suppressor microRNAs.43 miRNA levels could increase through a contact-mediated, SIDT1-dependent mechanism, thus intensifying the consequence of posttranscriptional gene regulation and allowing wider adaptive changes within the tumor microenvironment.36 In this study, we observed the strong anti-tumor effect of SIDT1 which not only suppressed the proliferation of two TNBC cell lines but also reduced tumor growth in vivo. This phenomenon may be interpreted by its broad and diverse roles in RNA transfer including certain key signals in TNBC progression. Thus, further studies are required to address the underlying mechanism of SIDT1 in cancer.
In conclusion, to the best of our knowledge, this is the first study evaluating the value of SIDT1 in TNBC. Our findings revealed lower SIDT1 expression in TNBC specimens compared to non-TNBC and also indicated that the upregulated expression of SIDT1 was associated with a longer RFS in patients with TNBC. SIDT1 overexpression exerted an anti-tumor effect by inhibiting cell proliferation. Overall, our study revealed potential implications of SIDT1 for prognostic prediction and therapeutic exploration in TNBC.
Acknowledgments
This study was supported by the National Science and Technology Major Sub-Project (2018ZX10301402-002), Technical Innovation Special Project of Hubei Province (2018ACA138), Wuhan Municipal Health Commission (WX18Q16) and National Natural Science Foundation of China (81572570 and 81772787).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi:10.1158/1078-0432.CCR-06-3045
3. Venkitaraman R. Triple-negative/basal-like breast cancer: clinical, pathologic and molecular features. Expert Rev Anticancer Ther. 2010;10:199–207. doi:10.1586/era.09.189
4. Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–692. doi:10.1038/nrclinonc.2010.154
5. Dietze EC, Chavez TA, Seewaldt VL. Obesity and triple-negative breast cancer: disparities, controversies, and biology. Am J Pathol. 2018;188:280–290. doi:10.1016/j.ajpath.2017.09.018
6. He ZX, Xu Q, Wang X, et al. RPLP1 promotes tumor metastasis and is associated with a poor prognosis in triple-negative breast cancer patients. Cancer Cell Int. 2018;18. doi:10.1186/s12935-018-0658-0
7. Li J, Lai YH, Ma JY, et al. miR-17-5p suppresses cell proliferation and invasion by targeting ETV1 in triple-negative breast cancer. BMC Cancer. 2017;17. doi:10.1186/s12885-017-3674-x
8. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets-update. Nucleic Acids Res. 2013;41:D991–D995. doi:10.1093/nar/gks1193
9. Jeong H, Mason SP, Barabasi AL, Oltvai ZN. Lethality and centrality in protein networks. Nature. 2001;411:41–42. doi:10.1038/35075138
10. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi:10.1186/1471-2105-9-559
11. Liu D, Li L, Zhang XX, et al. SIX1 promotes tumor lymphangiogenesis by coordinating TGFbeta signals that increase expression of VEGF-C. Cancer Res. 2014;74:5597–5607. doi:10.1158/0008-5472.CAN-13-3598
12. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. doi:10.1158/1078-0432.CCR-14-0432
13. Jezequel P, Campone M, Gouraud W, et al. bc-GenExMiner: an easy-to-use online platform for gene prognostic analyses in breast cancer. Breast Cancer Res Treat. 2012;131:765–775. doi:10.1007/s10549-011-1457-7
14. Jezequel P, Frenel JS, Campion L, et al. bc-GenExMiner 3.0: new mining module computes breast cancer gene expression correlation analyses. Database (Oxford). 2013;2013:bas060. doi:10.1093/database/bat081
15. Jager D, Stockert E, Gure AO, et al. Identification of a tissue-specific putative transcription factor in breast tissue by serological screening of a breast cancer library. Cancer Res. 2001;61:2055–2061.
16. Jager D, Filonenko V, Gout I, et al. NY-BR-1 is a differentiation antigen of the mammary gland. Appl Immunohisto M M. 2007;15:77–83. doi:10.1097/01.pai.0000213111.05108.a0
17. Varga Z, Theurillat JP, Filonenko V, et al. Preferential nuclear and cytoplasmic NY-BR-1 protein expression in primary breast cancer and lymph node metastases. Clin Cancer Res. 2006;12:2745–2751. doi:10.1158/1078-0432.CCR-05-2192
18. Chen C, Li ZL, Yang Y, Xiang TX, Song WH, Liu SC. Microarray expression profiling of dysregulated long non-coding RNAs in triple-negative breast cancer. Cancer Biol Ther. 2015;16:856–865. doi:10.1080/15384047.2015.1040957
19. Zombori T, Cserni G. Immunohistochemical analysis of the expression of breast markers in basal-like breast carcinomas defined as triple negative cancers expressing keratin 5. Pathol Oncol Res. 2018;24:259–267. doi:10.1007/s12253-017-0246-y
20. Zhou CH, Dai XC, Chen Y, et al. G protein-coupled receptor GPR160 is associated with apoptosis and cell cycle arrest of prostate cancer cells. Oncotarget. 2016;7:12823–12839. doi:10.18632/oncotarget.7313
21. Sheu JJC, Lee CH, Ko JY, et al. Chromosome 3p12.3-p14.2 and 3q26.2-q26.32 are genomic markers for prognosis of advanced nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2709–2716. doi:10.1158/1055-9965.EPI-09-0349
22. Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi:10.1016/S0002-9440(10)64038-2
23. Kivela A, Parkkila S, Saarnio J, et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol. 2000;156:577–584. doi:10.1016/S0002-9440(10)64762-1
24. Tureci O, Sahin U, Vollmar E, et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci U S A. 1998;95:7608–7613. doi:10.1073/pnas.95.13.7608
25. Li Y, Lei B, Zou J, et al. High expression of carbonic anhydrase 12 (CA12) is associated with good prognosis in breast cancer. Neoplasma. 2019;66:420–426. doi:10.4149/neo_2018_180819N624
26. Watson PH, Chia SK, Wykoff CC, et al. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br J Cancer. 2003;88:1065–1070. doi:10.1038/sj.bjc.6600796
27. Duxbury MS, Ashley SW, Whang EE. RNA interference: a mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem Biophys Res Commun. 2005;331:459–463. doi:10.1016/j.bbrc.2005.03.199
28. Zhang WJ, Li BH, Yang XZ, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine. 2008;42:39–47. doi:10.1016/j.cyto.2008.01.016
29. He Z, Xu Q, Wang X, et al. RPLP1 promotes tumor metastasis and is associated with a poor prognosis in triple-negative breast cancer patients. Cancer Cell Int. 2018;18:170. doi:10.1186/s12935-018-0658-0
30. Zhao W, Langfelder P, Fuller T, Dong J, Li A, Hovarth S. Weighted gene coexpression network analysis: state of the art. J Biopharm Stat. 2010;20:281–300. doi:10.1080/10543400903572753
31. Tang J, Kong D, Cui Q, et al. Prognostic genes of breast cancer identified by gene co-expression network analysis. Front Oncol. 2018;8:374. doi:10.3389/fonc.2018.00374
32. Shi H, Zhang L, Qu Y, Hou L, Wang L, Zheng M. Prognostic genes of breast cancer revealed by gene co-expression network analysis. Oncol Lett. 2017;14:4535–4542. doi:10.3892/ol.2017.6779
33. Lacroix M. Significance, detection and markers of disseminated breast cancer cells. Endocr Relat Cancer. 2006;13:1033–1067. doi:10.1677/ERC-06-0001
34. Qin Y, Verdegaal EM, Siderius M, et al. Quantitative expression profiling of G-protein-coupled receptors (GPCRs) in metastatic melanoma: the constitutively active orphan GPCR GPR18 as novel drug target. Pigment Cell Melanoma Res. 2011;24:207–218. doi:10.1111/j.1755-148X.2010.00781.x
35. Ochi F, Shiozaki A, Ichikawa D, et al. Carbonic anhydrase XII as an independent prognostic factor in advanced esophageal squamous cell carcinoma. J Cancer. 2015;6:922–929. doi:10.7150/jca.11269
36. Elhassan MO, Christie J, Duxbury MS. Homo sapiens systemic RNA interference-defective-1 transmembrane family member 1 (SIDT1) protein mediates contact-dependent small RNA transfer and microRNA-21-driven chemoresistance. J Biol Chem. 2012;287:5267–5277. doi:10.1074/jbc.M111.318865
37. Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi:10.1126/science.1068836
38. Feinberg EH, Hunter CP. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003;301:1545–1547. doi:10.1126/science.1087117
39. Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157. doi:10.1038/nbt1339
40. Tsang SY, Moore JC, Huizen RV, Chan CW, Li RA. Ectopic expression of systemic RNA interference defective protein in embryonic stem cells. Biochem Biophys Res Commun. 2007;357:480–486. doi:10.1016/j.bbrc.2007.03.187
41. Mendez-Acevedo KM, Valdes VJ, Asanov A, Vaca L. A novel family of mammalian transmembrane proteins involved in cholesterol transport. Sci Rep. 2017;7:7450. doi:10.1038/s41598-017-07077-z
42. Malla RR, Kumari S, Gavara MM, et al. A perspective on the diagnostics, prognostics, and therapeutics of microRNAs of triple-negative breast cancer. Biophys Rev. 2019;11:227–234. doi:10.1007/s12551-019-00503-8
43. Khordadmehr M, Shahbazi R, Ezzati H, Jigari-Asl F, Sadreddini S, Baradaran B. Key microRNAs in the biology of breast cancer; emerging evidence in the last decade. J Cell Physiol. 2019;234:8316–8326. doi:10.1002/jcp.27716
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.