Back to Journals » Cancer Management and Research » Volume 11
Intake consumption of ginsenoside Rg3, profiling of selected cytokines, and development of rectal polyps
Authors Xie J, Luo S, Mi H, Du Y, Bao G, Zhou J, Xi Y, Li C
Received 5 December 2018
Accepted for publication 27 February 2019
Published 6 May 2019 Volume 2019:11 Pages 4059—4064
DOI https://doi.org/10.2147/CMAR.S197097
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Jian Xie,1 Shicheng Luo,2 Hongying Mi,3 Yibin Du,4 Guohong Bao,1 Jing Zhou,1 Yumei Xi,1 Cichun Li5
1Department of Traditional Chinese Medicine and Anorectum, The First People’s Hospital of Yunnan Province, Kunming 650000, People’s Republic of China; 2Department of General Surgery, The First People’s Hospital of Yunnan Province, Kunming 650000, People’s Republic of China; 3Department of Pediatrics, First People’s Hospital of Yunnan Province, Kunming 650000, People’s Republic of China; 4Department of Geriatrics, The First Affiliated Hospital of Yunnan College of Traditional Chinese Medicine, Kunming 650000, People’s Republic of China; 5Department of Traditional Chinese Medicine and Anorectum, The Second Affiliated Hospital of Kunming Medical University, Kunming 650000, People’s Republic of China
Background: Rectal polyps is a major risk factor for rectal cancer. There is a need to explore a panel of preventive measures, as well as reliable biomarkers for screening of rectal polyps.
Patients and methods: We conducted a case control study which aimed to explore the effects of regular consumption of ginsenoside Rg3, profiling of selected cytokines, and development of rectal polyps in a Chinese population.
Results: Significantly higher levels of IL-4, MIP-1β, FasL, TGF-β1, and RANTES were detected in rectal polyp cases. Further, we found significant dose-response relationships between quartile-categorized levels of IL-4, MIP-1β, FasL, and TGF-β1, and risk of rectal polyps. The strongest associations for IL-4, MIP-1β, FasL, and TGF-β1 were observed for the highest quartile vs the lowest quartile with an OR of 1.78, 2.70, 1.49, and 2.36, respectively. Compared with non-Rg3 consumers, regular Rg3 consumers had a significantly lower risk of rectal polyps (OR =0.71; 95% CI: 0.55–0.92; P=0.009). We also found that Rg3 consumers had significantly lower levels of IL-4, MIP-1β, FasL, and TGF-β1 than non-Rg3 consumers, in both rectal polyp cases and healthy controls.
Conclusion: These results indicate that regular consumption of Rg3 might prevent the occurrence of rectal polyps through decreasing the serum level of selected cytokines, including IL-4, MIP-1β, FasL, and TGF-β1. Further clinical trials and prospective cohort studies with larger sample sizes are warranted to validate the anti-inflammatory activity and the anti-tumorigenic role of Rg3.
Keywords: cytokine, Rg3, rectal polyps, IL-4, MIP-1β, FasL, TGF-β1
Introduction
Rectal polyps occur in 7%–50% of all people, and can be either benign or malignant.1,2 Epidemiological studies have proven that rectal polyps are a major risk factor for rectal cancer and have raised severe public concern.3–5 Thus, there is a need to develop a panel of preventive measures, as well as reliable biomarkers for screening rectal polyps.
Many kinds of cytokines, which act as cell signaling proteins, are involved in the physiologic and pathophysiologic mechanisms of immunity, inflammation, and hematopoiesis of tumorigenesis and carcinogenesis.6,7 Previously, Johdi et al8 explored the profiles of key inflammatory cytokines, chemokines, and other soluble proteins using multiplexing technology in small samples, and found that colorectal polyp patients had high IL-4, MIP-1β, FasL, and TGF-β1 levels, but lower levels of RANTES, compared with healthy controls. However, further validation of their findings with a larger sample size is needed to produce a panel of serum biomarkers for colorectal polyps.
Ginsenoside Rg3, the major active component of ginseng, has been shown to have various therapeutic effects including anti-inflammatory and anti-tumorigenic activity, however, its relationship with level of IL-4, MIP-1β, FasL, TGF-β1, and RANTES remains unexplored.9–12 Multiple experimental studies revealed the inhibitory effect of Rg3 on growth and angiogenesis of tumors, including colorectal cancer, ovarian cancer, gastric cancer, lung cancer, intestinal adenocarcinomas, melanoma, breast cancer, and so on.13–21 Clinical trials have also proven that Rg3 could improve the prognosis of non-small-cell lung cancer and hepatocellular carcinoma, although the possible mechanism and the regulatory network were unclear.22–24 However, the possible beneficial effects of Rg3 consumption on the occurrence of rectal polyps still remain unknown. Considering the fact that Rg3 is widely used and popular in Asian populations for the prevention of many diseases, evaluation of association of regular consumption of Rg3 with serum levels of selected cytokines, and risk of rectal polyps is warranted. Thus, we conducted this case control study which aimed to explore consumption of ginsenoside Rg3, the profiling of selected cytokines (IL-4, MIP-1β, FasL, TGF-β1, and RANTES), and risk of rectal polyps in a Chinese population.
Patients and methods
Study subjects
To improve the reporting quality of our research, we followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. The current study included a total of 600 cases of rectal polyps and 600 frequency-matched healthy controls by age and gender. Both the cases and controls were recruited since January 2014. The cases were diagnosed by two senior gastrointestinal pathologists, respectively. Controls were randomly selected from the volunteers who underwent endoscopy procedures and were diagnosed as healthy. Demographic data and information about the regular consumption of Rg3 were collected face to face using a uniform questionnaire, while body mass index (BMI) was measured at the same time. The frequency of regular consumption of Rg3 was classified as 1–3 times/week, 4–7 times/week, and 7–10 times/week. Ten milliliters of heparinized blood were taken from each participant at the interview and centrifuged at 3,000 g for 10 minutes. Serum samples were stored at −80°C and thawed immediately prior to any laboratory determination. Written informed consent was obtained from all individual participants, and the study was approved by the institutional Ethics Committee of the second affiliated Hospital of Kunming Medical University (IRB-014023). This study was conducted in accordance with the Declaration of Helsinki.
Cytokine analysis
As reported previously, Luminex cytokine bead array technology was utilized for this study using an EMD Millipore (Billerica, MA, USA) HCTYOMAG-60K kit on a BioPlex analyzer (Bio-Rad Laboratories Inc., Hercules, CA, USA).25 Data were acquired using the Bioplex analyzer, and the contents of each well were examined using Luminex xPonent software.
Statistical analysis
All statistical analyses were conducted with STATA (version 13.1). P<0.05 was considered significant. Descriptive statistics were presented as means, SDs, and percentages. Student’s t-tests were used for comparison of continuous variables and chi-squared tests (Fisher’s exact tests) for comparison of categorical variables. To explore the possible dose-response relationship between level of selected cytokines and risk of rectal polyps, we categorized the level of selected cytokines using their interquartile range distributions among the healthy controls. ORs and 95% CIs using logistic regression model were calculated to estimate the relative risks related to the quartile distribution of selected cytokines, regular consumption of Rg3, and risk of rectal polyps.
Results
Characteristics of the study population
As shown in Table 1, 600 cases of rectal polyps and 600 frequency-matched healthy controls by age and gender were included in the current study. No significant differences in the distribution of age, gender, BMI, and smoking status between the cases and controls were observed (P>0.05). However, more subjects with family history of cancer, alcohol status, and regular consumption of Rg3 were found in the rectal polyp cases (P<0.05). The frequency of regular consumption of Rg3 was also presented, which showed that rectal polyp cases had lower consumption of Rg3 as well asless high-frequency consumption. For the selected cytokines, we found that rectal polyp cases had higher serum levels of IL-4, MIP-1β, FasL, TGF-β1, and RANTES.
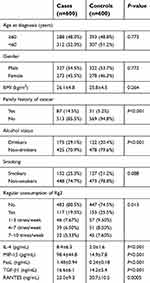 | Table 1 Characteristics of rectal polyp cases and healthy controls |
Quartile distribution of selected cytokines and risk of rectal polyps
To explore the possible dose-response relationship between level of selected cytokines and risk of rectal polyps, the levels of selected cytokines were categorized using their interquartile range distributions among the healthy controls. Overall, there were statistically significant dose-response relationships between IL-4, MIP-1β, FasL, TGF-β1, and risk of rectal polyps (Table 2, P<0.05). The strongest associations for IL-4, MIP-1β, FasL, and TGF-β1 were observed for the highest vs the lowest quartile with an OR of 1.78, 2.70, 1.49, and 2.36, respectively. However, we did not detect a significant dose-response relationship for the quartile distribution of RANTES.
 | Table 2 ORs for rectal polyp cases by quartile distribution of selected cytokines by logistic regression analysis |
Association between the consumption of Rg3 and risk of rectal polyps
As shown in Table 3, we evaluated the association between the consumption of Rg3 with risk of rectal polyps in both qualitative and quantitative models. Compared with non-Rg3 consumers, those whoconsumed Rg3 had significantly lower risk of rectal polyps (OR =0.71; 95% CI: 0.55–0.92; P=0.009). Further, we found a reversed dose-response relationship between frequency of Rg3 consumption and risk of rectal polyps (P for trend =0.015). Those who consumed Rg3 7–10 times/week had a 0.66-fold risk of rectal polyps (OR =0.66; 95% CI: 0.41–1.05), compared with non-Rg3 consumers.
 | Table 3 Adjusted association between the consumption of Rg3 with susceptibility of rectal polyps |
Comparisons of selected cytokines between Rg3 consumers and non-Rg3 consumers in rectal polyp cases and healthy controls
Further, we also evaluated the effect of regular consumption of Rg3 on serum levels of IL-4, MIP-1β, FasL, TGF-β1, and RANTES. As shown in Table 4, we found that Rg3 consumers had significantly lower levels of IL-4, MIP-1β, FasL, and TGF-β1 than non-Rg3 consumers, in both rectal polyp cases and healthy controls.
 | Table 4 Comparison of selected cytokines between Rg3 consumers and non-Rg3 consumers |
Discussion
Based on the preliminary results identified by Johdi,8 we evaluated the associations of profiling of selected cytokines, together with the regular consumption of ginsenoside Rg3 with risk of rectal polyps in a Chinese population. Significantly higher levels of IL-4, MIP-1β, FasL, TGF-β1, and RANTES were detected in rectal polyp cases. We also found significant dose-response relationships between quartile-categorized levels of IL-4, MIP-1β, FasL, TGF-β1, and risk of rectal polyps. Compared with non-Rg3 consumers, Rg3 consumers had significantly lower risk of rectal polyps. We also found that Rg3 consumers had significantly lower levels of IL-4, MIP-1β, FasL, and TGF-β1 than non-Rg3 consumers, in both rectal polyp cases and healthy controls. These results indicate that regular consumption of Rg3 might prevent the occurrence of rectal polyps through decreasing the serum level of selected cytokines, including IL-4, MIP-1β, FasL, and TGF-β1.
Rg3 has a number of pharmacological effects, including inducing the apoptosis of tumor cells, promoting T lymphocyte mitosis and NK cell activity.26 Wang et al26 detected 43 differentially expressed genes, consisting of ten upregulated genes and 33 downregulated genes, in Rg3-consuming diabetic nephropathy patients. Saba et al27 reported the anti-inflammatory activity of Rg3 in a murine model of sepsis through the NF-κB and MAPK pathways. Clinical benefit from EGFR-TKI plus Rg3 in patients with advanced non-small-cell lung cancer harboring EGFR active mutation was also identified.22 Besides, Rg3 was also found to benefit the progression and prognosis of lung cancer, colorectal cancer, and hepatocellular carcinoma.9,23,24,28 Consistent with these findings, we found that regular consumption of Rg3 could significantly reduce the risk of rectal polyps. Furthermore, we also detected a reversed dose-response relationship between the quantity of regularly consumed Rg3 and risk of rectal polyps.
IL-4, MIP-1β, FasL, TGF-β1, and RANTES are important cytokines and are involved in the pathogenesis of many diseases, including cancers.8,29,30 Our results validated the preliminary results identified by Johdi et al,8 and found significantly higher levels of IL-4, MIP-1β, FasL, TGF-β1, and RANTES in rectal polyp cases, although we did not find a significant dose-response relationship between quartile-categorized level of RANTES and risk of rectal polyps. Eissa et al31 found that RANTES was significantly increased in breast cancer cases with no metastasis compared to the control group (p<0.05) and a highly significant increase in metastatic patients compared to controls (p<0.001). Serum FasL level was also found to be associated with esophageal squamous cell carcinoma and follicular thyroid cancer.32,33 Besides, we also found that Rg3 could significantly reduce the levels of IL-4, MIP-1β, FasL, and TGF-β1 in both rectal polyp cases and healthy controls. This also validated the anti-inflammatory activity of Rg3.
In conclusion, our findings indicate that aberrant expression of IL-4, MIP-1β, FasL, and TGF-β1 could contribute to the risk of rectal polyps, while regular consumption of Rg3 could significantly reduce the risk of rectal polyps with a reversed dose-response relationship in a Chinese population. Rg3 could also significantly reduce the levels of IL-4, MIP-1β, FasL, and TGF-β1 in both rectal polyp cases and healthy controls, which validated the anti-inflammatory activity of Rg3. Further clinical trials (for example, randomized controlled trials about the effect of Rg3 consumption on the level of selected cytokines and development of rectal polyps) and prospective cohort studies (comparing the changes in levels of selected cytokines and incidence of rectal polyps between the regular Rg3 consumption group and non-consumption group respectively) with larger sample sizes are warranted to validate the anti-inflammatory activity and the anti-tumorigenic role of Rg3.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Platell C, Denholm E, Makin G. Efficacy of transanal endoscopic microsurgery in the management of rectal polyps. J Gastroenterol Hepatol. 2004;19:767–772. doi:10.1111/j.1440-1746.2004.03364.x
2. Nakajima H, Iwane S, Mikami T, et al. Osseous metaplasia in benign rectal polyps. J Clin Gastroenterol. 1997;25:558–559.
3. Oines M, Helsingen LM, Bretthauer M, Emilsson L. Epidemiology and risk factors of colorectal polyps. Best Pract Res Clin Gastroenterol. 2017;31:419–424. doi:10.1016/j.bpg.2017.06.004
4. Giacosa A, Frascio F, Munizzi F. Epidemiology of colorectal polyps. Tech Coloproctol. 2004;8(Suppl 2):s243–247. doi:10.1007/s10151-004-0169-y
5. Correa P, Strong JP, Reif A, Johnson WD. The epidemiology of colorectal polyps: prevalence in New Orleans and international comparisons. Cancer. 1977;39:2258–2264.
6. La Manna S, Lee E, Ouzounova M, et al. Mimetics of suppressor of cytokine signaling 3: novel potential therapeutics in triple breast cancer. Int J Cancer. 2018;143:2177–2186. doi:10.1002/ijc.31594
7. Chen D, Sha H, Hu T, et al. Cytokine-induced killer cells as a feasible adoptive immunotherapy for the treatment of lung cancer. Cell Death Dis. 2018;9:366. doi:10.1038/s41419-018-0404-5
8. Johdi NA, Mazlan L, Sagap I, Jamal R. Profiling of cytokines, chemokines and other soluble proteins as a potential biomarker in colorectal cancer and polyps. Cytokine. 2017;99:35–42. doi:10.1016/j.cyto.2017.06.015
9. Sun HY, Lee JH, Han YS, et al. Pivotal roles of ginsenoside Rg3 in tumor apoptosis through regulation of reactive oxygen species. Anticancer Res. 2016;36:4647–4654. doi:10.21873/anticanres.11015
10. Anufriev VP, Malinovskaya GV, Denisenko VA, et al. Synthesis of ginsenoside Rg3, a minor constituent of ginseng radix. Carbohydr Res. 1997;304:179–182.
11. Shinkai K, Akedo H, Mukai M, et al. Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn J Cancer Res. 1996;87:357–362.
12. Mochizuki M, Yoo YC, Matsuzawa K, et al. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202.
13. Xu TM, Xin Y, Cui MH, Jiang X, Gu LP. Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chin Med J (Engl). 2007;120:584–588.
14. Chen ZJ, Cheng J, Huang YP, et al. Effect of adjuvant chemotherapy of ginsenoside Rg3 combined with mitomycin C and tegafur in advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10:64–66.
15. Zhang Q, Kang X, Zhao W. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem Biophys Res Commun. 2006;342:824–828. doi:10.1016/j.bbrc.2006.02.044
16. Iishi H, Tatsuta M, Baba M, et al. Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in Wistar rats. Clin Exp Metastasis. 1997;15:603–611. doi:10.1023/A:1018491314066
17. Chen J, Peng H, Ou-Yang X, He X. Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma cells. Melanoma Res. 2008;18:322–329. doi:10.1097/CMR.0b013e32830b3536
18. Zhang Q, Kang X, Yang B, Wang J, Yang F. Antiangiogenic effect of capecitabine combined with ginsenoside Rg3 on breast cancer in mice. Cancer Biother Radiopharm. 2008;23:647–653. doi:10.1089/cbr.2008.0532
19. Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H. Anticancer effects of ginsenoside Rg3 (Review). Int J Mol Med. 2017;39:507–518. doi:10.3892/ijmm.2017.2857
20. Kim SM, Lee SY, Yuk DY, et al. Inhibition of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch Pharm Res. 2009;32:755–765. doi:10.1007/s12272-009-1515-4
21. Lee SY, Kim GT, Roh SH, et al. Proteomic analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human colon cancer cell lines. Biosci Biotechnol Biochem. 2009;73:811–816. doi:10.1271/bbb.80637
22. Li Y, Wang Y, Niu K, et al. Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget. 2016;7:70535–70545. doi:10.18632/oncotarget.12059
23. Zhou B, Yan Z, Liu R, et al. Prospective study of transcatheter arterial chemoembolization (TACE) with ginsenoside Rg3 versus TACE alone for the treatment of patients with advanced hepatocellular carcinoma. Radiology. 2016;280:630–639. doi:10.1148/radiol.2016150719
24. Lu P, Su W, Miao ZH, Niu HR, Liu J, Hua QL. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin J Integr Med. 2008;14:33–36. doi:10.1007/s11655-007-9002
25. Kaskas NM, Moore-Medlin T, McClure GB, Ekshyyan O, Vanchiere JA, Nathan CA. Serum biomarkers in head and neck squamous cell cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:5–11. doi:10.1001/jamaoto.2013.5688
26. Wang J, Cui C, Fu L, et al. Genomic expression profiling and bioinformatics analysis on diabetic nephrology with ginsenoside Rg3. Mol Med Rep. 2016;14:1162–1172. doi:10.3892/mmr.2016.5349
27. Saba E, Jeong D, Irfan M, et al. Anti-inflammatory activity of Rg3-enriched Korean red ginseng extract in murine model of sepsis. Evid Based Complement Alternat Med. 2018;2018:6874692. doi:10.1155/2018/9567061
28. Tang YC, Zhang Y, Zhou J, et al. Ginsenoside Rg3 targets cancer stem cells and tumor angiogenesis to inhibit colorectal cancer progression in vivo. Int J Oncol. 2018;52:127–138. doi:10.3892/ijo.2017.4183
29. Saadalla AM, Osman A, Gurish MF, et al. Mast cells promote small bowel cancer in a tumor stage-specific and cytokine-dependent manner. Proc Natl Acad Sci USA. 2018;115:1588–1592. doi:10.1073/pnas.1716804115
30. Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–789. doi:10.1038/s41577-018-0066-7
31. Eissa SA, Zaki SA, El-Maghraby SM, Kadry DY. Importance of serum IL-18 and RANTES as markers for breast carcinoma progression. J Egypt Natl Canc Inst. 2005;17:51–55.
32. Kozlowski M, Kowalczuk O, Sulewska A, et al. Serum soluble Fas ligand (sFasL) in patients with primary squamous cell carcinoma of the esophagus. Folia histochemica et cytobiologica. 2007;45:199–204.
33. Kolomecki K, Maciaszczyk P, Stepien H, et al. Evaluation of p53 and soluble Fas ligand (sFasL) serum level concentration as indicators of apoptosis in serum of patients with benign and malignant primary follicular thyroid tumors. Endokrynol Pol. 2006;57:320–325.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
