Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Insula H-coil deep transcranial magnetic stimulation in severe and enduring anorexia nervosa (SE-AN): a pilot study
Authors Knyahnytska YO, Blumberger DM, Daskalakis ZJ, Zomorrodi R, Kaplan AS
Received 10 March 2019
Accepted for publication 2 July 2019
Published 6 August 2019 Volume 2019:15 Pages 2247—2256
DOI https://doi.org/10.2147/NDT.S207630
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Yuliya O Knyahnytska,1,2 Daniel M Blumberger,1–3 Zafiris J Daskalakis,1,2 Reza Zomorrodi,1 Allan S Kaplan1,2
1Temerty Centre for Therapeutic- Brain Intervention, Centre for Addiction and Mental Health, Toronto, Ontario, Canada; 2Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada; 3Geriatric Division, Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada
Introduction: Anorexia nervosa (AN) is a complex disorder of unknown etiology, characterized by obsessions and compulsions around body shape, weight, and calorie intake. In the course of AN, 10%–30% will recover, while the rest will develop a treatment-resistant course with a high mortality rate due to AN-related complications. The insula is a region in the brain of considerable interest to its role in gustatory modulation, feeding behavior, and processing of interoceptive stimuli.
Objective: Recent advances in the neurophysiology of AN suggest insula dysfunction as a potential biomarker for people with severe and enduring AN (SE-AN). Deep transcranial magnetic stimulation (dTMS) is of particular interest in SE-AN because of its ability to target deep areas of the brain.
Design: We conducted a pilot study to investigate the feasibility and safety of insula dTMS in subjects with SE-AN.
Results: We found that dTMS is a safe and well-tolerated treatment. We also found a reduction in AN-related obsessions and compulsions, as well as depression and anxiety scores from baseline to the end of the trial. Due to small sample size, the results of this study should be interpreted with great caution.
Discussion: The results suggest that dTMS is safe and well tolerated and may be of some clinical interest in patients with SE-AN. However, to determine the true efficacy of dTMS for SE-AN, there is a need to conduct a randomized controlled trial comparing real versus sham dTMS in a larger number of AN subjects.
Keywords: severe and enduring anorexia nervosa, deep transcranial magnetic stimulation, repetitive transcranial magnetic stimulation, insula
Introduction
Anorexia nervosa (AN) is a complex disorder of unknown etiology that is characterized by severely low weight and significant obsessions and compulsions in relation to body shape, weight, and calorie intake. It has the highest mortality rate of all psychiatric disorders, with exceptionally high relapse rates, where only 10–30% recover with the best available psychotherapies, as pharmacological interventions are largely ineffective and have low acceptability.1,2
Amongst multiple etiology models, recent advances in understanding the neurobiology of AN point toward neurobiological functioning as a potential explanatory model in AN psychopathology.3–5 Brain areas, such as the dorsomedial and the dorsolateral prefrontal cortex have been shown to play an important role in the regulation of mood and affect in people with AN.4,6 Current research also points to deeper prefrontal and subcortical areas as being implicated in eating disorders.7 Neuroimaging studies have identified deeper brain structures and limbic regions as critical in the modulation of eating behavior and self-awareness.7,8 Current studies demonstrate that deep prefrontal (subcallosal and cingulate gyrus) and subcortical areas (thalamus and insula) played a role in the pathophysiology of AN.3–8 Specifically, functional MRI studies have shown that when emaciated and malnourished AN individuals are shown pictures of food they display abnormal activity in the insula.7–9 The insula has also been identified as a potentially important region in AN pathophysiology due to its role in gustatory modulation and feeding behavior, as well as in the processing of interoceptive stimuli and self-awareness.8,9 According to Nunn et al (2008), the insula plays a predominant role in orchestrating the balance between areas of the brain responsible for adaptation to the environment and those responsible for internal homeostasis.8 The authors proposed that the clinical phenomenon of AN may be explained by insula dysfunction, specifically the restricted capacity to operate effectively in the integration of visual and body perceptions with feelings/emotions and the inhibition of higher cognitive processes. The experience of disgust has also been described as a dominant trait in the aberrant eating behaviors of AN.9 A number of investigations show that, among other structures, the insula and the amygdala are activated when subjects are exposed to disgusting odors or tastes.10 It has been also demonstrated that insular cortex is active in a wide variety of tasks involving subjective awareness of both positive and negative feelings and is believed to play a domain-general role in identifying the most salient among several internal and extrapersonal stimuli in guiding eating behaviors.11
Achieving recovery in AN can be difficult. AN continues to be among the most pernicious of psychiatric disorders, demonstrating a mortality rate 6 times greater than the general population and a crude mortality rate of 5–6%.12 At a 10-year follow-up, more than 30% of the patients were found to have AN that was refractory to treatment (SE-AN), while 10% of the patients died from disease.13,14 Even though there is evidence to support the effectiveness of family based treatment for young adolescents with a short duration of illness, no evidence-based treatments for adults with SE-AN are currently available, and therefore new approaches are constantly sought.15 Recent research points out that treatment for this population should be aimed toward a better understanding of the underlying neurobiological mechanisms, along with the development of interventions able to mitigate and address these abnormalities.15
Repetitive transcranial magnetic brain stimulation (rTMS) is of particular interest as a treatment. It is a non-invasive intervention method that modulates brain activity through magnetic fields applied to different parts of the brain. rTMS has demonstrated its efficacy in a range of psychiatric conditions, such as unipolar depression16,17 including treatment-resistant cases,18–20 anxiety conditions,21,22 psychosis,23 and addictions.24 The overall symptom reduction following rTMS is reported to be approximately 40%, with studies demonstrating its effectiveness in treatment-resistant depression.25 New studies on rTMS in people with AN are promising and report some clinical response, specifically a decrease in preoccupations around weight, shape, and calorie intake.26,27 However, these studies have employed conventional rTMS where its functional capacity is limited to the stimulation of only the superficial cortical regions of the brain,28 such as the dorsolateral prefrontal cortex (DLPFC) and some subcortical areas. This conventional rTMS does not reach deeper areas of the brain, such as the insula, as the intensity of the electric field decreases rapidly as a function of tissue depth.28–31
In this study, we used a Hesed coil (H-coil), a novel transcranial magnetic stimulation (TMS) device, to target the insula in patients with SE-AN, referred to as deep transcranial magnetic stimulation (dTMS). Compared to a regular rTMS coil, the H-coil is capable of inducing a magnetic field with a deeper and wider distribution and can reach the insula bitemporally to a depth of 5–6 cm.30,31 However, even though it is difficult to conclude that we are actually stimulating the insula cortex with the H-coil and not the surrounding tissue and/or structures, it is generally accepted that TMS-induced excitation of one region will likely influence other areas.32
Objectives
Here we report the results of a pilot study investigating the feasibility, safety, tolerability, and clinical effects of the H-coil in targeting the insula in patients with SE-AN. We hypothesized that 42 sessions of dTMS to the insula would be both safe and well tolerated, as measured by the absence of adverse events such as seizures and drop in weight (eg, BMI).
Design
Patient and public involvement
Patients and/or public were not involved in the development of the research question and/or outcome measures.
Participants
Eight females with a diagnosis of AN (restricting and binge-purging type), confirmed by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), were recruited from the community from November 2012 to September 2016. The diagnosis was established with the eating disorder module for the Structured Clinical Interview for DSM-IV-axis I Disorders (SCID), performed by a trained clinician. Patients with a lifetime diagnosis of schizophrenia or bipolar disorders, unstable medical conditions, or active substance use disorder in past 3 months were all excluded. Contraindications to TMS were checked with the adult safety screen questionnaire. Patients with a personal/family history of epilepsy, neurodevelopmental disorders, and ongoing pregnancy were excluded.
Ethics
This study was approved by the research ethics board at Centre for Addiction and Mental Health, and informed consent was obtained from each participant. Participant consent was written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Intervention
The treatment involved a total of 42 sessions applied daily for 20 mins, 5 days per week over the first 6 weeks, followed by twice per week for 6 more weeks. Multiple assessments were performed bi-weekly, followed by 1- and 6-month follow-ups to ensure frequent and regular check-in points were in place, given the high risk of this population and the novelty of the procedure.
Each treatment session was composed of two steps: cue provocation and dTMS. Participants in this study received no concurrent treatments and did not to initiate any new treatments during both the acute and maintenance phases.
Cue provocation
We used a cue provocation technique before administering dTMS treatment. The main purpose was to activate relevant brain circuitries, a technique previously used in different studies and referred to as priming.33,34 The stimuli material was developed by the first author through adopting the technique from Brooks et al35 and consisted of 32 professional color images of high calorie, sweet and savory food presented in one block on a computer screen against a neutral background. Each image was shown for 5 s followed by a blank screen for 1 s.
dTMS procedure
The treatment trial included two stages: (1) an acute and (2) a maintenance course, for a total of 42 dTMS sessions per subject applied using a Brainsway H-Coil device. Each dTMS session included stimulation with 18 Hz, 2 s on, 20 s off, number of pulses 36, number of trains 80, over 20 mins; the protocol was developed in collaboration with the study’s co-investigators, who are experts in brain stimulation interventions. Stimulation intensity was determined relative to the patient’s resting motor threshold according to previously published protocols.36 The acute course included daily sessions, 5 days per week for 6 weeks, for a total of 30 sessions. The maintenance course included 2 sessions per week for 6 weeks, for a total of 12 sessions.
Primary and secondary outcome measures
The primary outcomes were safety and feasibility. Safety was assessed by the (1) absence of any adverse events, especially seizures; and (2) the subject’s medical stability, as measured by the patient’s body mass index (eg, no decrease in BMI), and cognition (eg, no decrease in scores as measured by Montreal Cognitive Assessment Scale (MOCA)). Feasibility was assessed by the subject’s adherence to the research protocol and attrition rate from the trial.
The secondary outcomes were the clinical responses to the treatment evaluated by the symptomatic improvement in AN-specific psychopathology, specifically (1) AN-related compulsions and obsessions as measured by Yale-Brown-Cornell Eating Disorder Scale (YBC-EDS) mean score; followed by the improvement in (2) symptoms of depression and anxiety as measured by Hamilton Depression Rating Scale (HDRS) and Montgomery–Asberg Depression Rating Scale (MADRS), administered by a trained clinician; and (3) Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI), collected through each patient’s self-reports.
Statistical analyses
We used descriptive statistics calculated at each time point, specifically distributions of continuous variables described using means (M) and standard deviations (SD); categorical variables were described by the use of percentages. We used non-parametric Mann–Whitney U t-test to assess whether within-group differences in key parameters were statistically significant (see Table 1). Given the very small sample size and to understand the clinical significance of the observed clinical responses, we calculated Cohen’s d – a measure of the effect size as the difference in the two groups’ means divided by the average of their SDs (Becker, 2000).
 |
Table 1 Baseline, 6-week, 12-week, and 6-month scores for the ED-related measures |
Results
Demographic and clinical variables
Eight subjects diagnosed with SE-AN were enrolled and received dTMS treatment with the same protocol. All subjects were women with a mean age of 33±11.56 years, mean years of education of 16.9±2.2 years, and a mean BMI of 16.6±0.9. All were unemployed at the time of assessment; the majority identified as Caucasian and one identified as a person of color. Six patients met the criteria for Anorexia restrictive type and two for binge-purging type. Even though no criteria for judging refractoriness to treatment or defined gradations of medical seriousness or psychological morbidity in anorexia nervosa exist,28 we based our definition of treatment resistance on both duration and the number of failed treatment trials. All subjects met the criteria for treatment resistance, which was defined in this study as the persistence of anorexia symptoms for over 5 years with failure to respond to at least 2 trials of intensive treatment interventions.
All 8 participants completed daily treatments of dTMS for the first 6 weeks as a part of an acute course. Four participants completed the remaining 6 weeks of twice weekly, followed by 2 follow-up assessments a month, and 6 months after the completion of this trial.
Tolerability and safety
All participants tolerated treatments well. Apart from the occasional mild headache/discomfort associated with dTMS (which we did not calculate due to low occurrence rates), there were no significant adverse events reported. For the duration of this trial, the BMI remained stable, as evident from no statistically significant change in BMI score (see Table 1 for details), and there was no change in cognition, as measured by MOCA. The study demonstrated that dTMS was safe and well tolerated.
Eating disorder-related symptoms
The results in this pilot study demonstrated changes in scores in AN-related obsessions and compulsions, depression, and anxiety from baseline to 6-week (eg, completion of the acute course), 12-week (completion of the full trial, including maintenance course), and a 6-month follow-up (see Table 1). Due to the limited number of subjects included in this trial, results were not statistically significant (see Table 1 for details).
Our findings indicate that the severity of AN-related obsessions and compulsions around food, shape, and weight as measured by YBC-EDS decreased by 0.6 following the first 6 weeks of treatment with dTMS as compared to pre-treatment (M=23.4; SD=3.4; Cohen’s d=0.21). This decrease in severity continued and was sustained after the completion of a full trial of 12 weeks (M=18.8; SD=5.9, Cohen’s d=0.96) and was further sustained over the period of 6 months post-treatment (M=19.75; SD=3.76; Cohen’s d=1.02) (see Figure 1 for details).
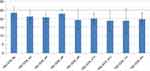 |
Figure 1 Yale-Brown-Cornell Eating Disorder Scale (YBC-EDS). |
Next, we found a reduction in depressive scores as measured by the HDRS (HDRS-17) (see Figure 2) following the completion of the first 6 weeks (acute phase) of the dTMS course (M=6.8; SD=5.4) compared to the baseline (M=12.2; SD=5.05) (Cohen’s d=1.99). A reduction in depressive and anxiety symptoms was also reflected in a reduction in depression and anxiety scores as measured by MADRS after 6 weeks of dTMS treatment compared to the baseline (Cohen’s d=0.85) (see Figure 3). These improvements sustained at 12 weeks (completion of the full trial) as measured by HDRS (M=5.5; SD=4.7; Cohen’s d=1.31) and MADRS (M=11; SD=7.9; Cohen’s d=1.02) compared to baseline. Interestingly enough, depressive scores increased over the next 6 months as measured by HDRS (M=10.25; SD=5.11; Cohen’s d=0.37) and MDRS (M=15; SD=5; Cohen’s d=0.56) compared to baseline; however, they did not return to their pre-treatment levels.
 |
Figure 2 Hamilton Rating Scale for Depression Scores. |
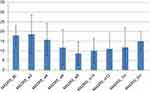 |
Figure 3 Montgomery-Asberg Depression Rating Scale Scores (MADRS). |
These results mirrored those analyzed from self-reports, indicating a decrease in depression scores as measured by BDI (see Figure 4). Specifically, there was a decrease in depression scores in the post-acute course of the first 6 weeks (M=12.8; SD=12.74; Cohen’s d=1.036), and in the post-maintenance course of 12 weeks (M=10; SD=11.29; Cohen’s d=1.37), with a slight increase toward the 6-month mark (M=13.25; SD=13.27; Cohen’s d=0.97), as compared to baseline pre-treatment (M=24.66; SD=9.97). In addition to this, these findings demonstrate a decrease in anxiety as measured by self-reports administered by the BAI scale (see Figure 5) following an acute course of 6 weeks (M=11.83; SD=10.31; Cohen’s d=0.4) and post-trial after 12 weeks (M=7; SD=2.73; Cohen’s d=1.68). These gains were sustained over the next 6 months, demonstrating a decrease in scores (M=6.25; SD=3.76; Cohen’s d=1.63) as compared to pre-treatment (M=15.44; SD=7).
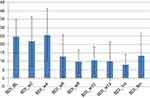 |
Figure 4 Beck Depression Inventory (BDI). |
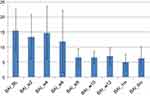 |
Figure 5 Beck Anxiety Inventory (BAI). |
We are unable to conclude that differences in AN-related symptoms of obsessions, compulsions, depression, and anxiety are due to dTMS due to the limited statistical power. However, a potentially moderate-to-large effect size (Cohen’s d=0.5–1.65) suggests that the difference between these indicators may be large enough to be considered as clinically significant and grants further investigation.
Strengths and limitations
This study failed to demonstrate statistically significant results for clear clinical benefits due to limited statistical power. However, the study demonstrated some encouraging results, such as a clinical improvement in AN-related psychopathology, specifically in the decrease of the severity of obsessions and compulsions as measured by YBC-EDS change in total scores, and concerns around shape, weight, and calorie intake as measured by EDE. These findings are consistent with others reported in the use of TMS in AN,30 suggesting that the use of neuromodulation treatment modalities in those with SE-AN may be a promising direction to pursue, as it can help patients with a severe and enduring AN to make some changes and has the potential to improve their overall quality of life. The effect on more complex behaviors, such as self-perceptions, urge to restrict, and urge to engage in compensatory behaviors is less clear. Overall, dTMS was safe and well tolerated, providing preliminary evidence toward its potential use in this severe treatment-resistant population.
Limitations of the research study
We planned on enrolling 12 subjects. However, recruitment proved to be difficult due to a low number of referrals, constrained resources to advertise widely, and a high attrition rate due to an extensive time commitment, considering the treatment protocol was 12 weeks, with multiple assessments on a bi-weekly basis, which included clinical assessments, physical, and laboratory testing. Even though all participants were able to complete daily treatments for the first 6 weeks, only 4 participants continued with the remaining 6 weeks of the maintenance course and follow-up sessions. Given that all subjects were able to complete the acute phase, which included treatments 5 days per week for 6 weeks, we believe that tolerability was less of an issue in this trial. Participants consistently reported the time commitment, along with extensive assessments, including psychological scales and blood work every 2 weeks, as major limitations in continuing this trial. These limitations should definitely be considered in further trials.
Next, while promising, our findings were not statistically significant and that was largely due to the small number of subjects (ie, eight) that were included in this trial. Future clinical trials in this population should aim to be multicenter given the recruitment challenges seen in the early stages and development of this trial and should be randomized controlled trial (RCT) comparing sham versus real dTMS. Also, given the high attrition rate, future protocols should be limited to 4–6 weeks and assessments performed pre- and post-treatment to avoid burdening patients.
The findings of this study contribute to a field of neurotherapeutics and treatment of severe and hard-to-treat AN. There are few recent studies on the therapeutics of brain stimulation modalities in AN,26,27 with none exploring dTMS targeting the insula, as was done in this study. This study provided preliminary evidence for the therapeutic potential of dTMS targeting the insula in TrAN. In line with other literature on the effects of rTMS on affective symptoms,19,21 the results also indicate a reduction in affective symptoms as measured by HDRS, MADRS, BDI, and BAI.
Strengths of the research study
The findings are of clinical interest given the severity the participants’ illness and treatment history, even though the small sample size does not allow us to make any statistical comparisons. All eight participants in this study were middle-aged women first diagnosed with anorexia in their early or mid-teens, with an average duration of anorexia for 10–15 years and with a history of many treatments, including intensive re-feeding, with little long-term impact. Thus, we would posit that given the severity of their AN and their history of treatment resistance, any reported improvement in symptoms following an experimental treatment such as dTMS merits a properly conducted RCT in a larger group of subjects comparing active treatment with sham TMS.
Conclusion
This study was exploratory in nature and primarily conducted to test safety and feasibility, which it successfully demonstrated in small number of subjects. The findings of this study have potential clinical value in this difficult to treat, very ill group of patients and merit follow-up with a larger sample in a RCT that also measures biological target engagement.
Summary
Strengths and limitations of this study include next:
- This is a first study testing safety and tolerability of deep H-coil TMS in people with severe and enduring anorexia nervosa.
- We demonstrated deep TMS is safe and well tolerated and may be beneficial to improve symptoms in those with SE-AN.
- Small sample size does not allow to generalize findings to a larger population.
- More research, specifically a RCT comparing real versus sham dTMS, is needed to be able to demonstrate the potential clinical utility of this treatment modality in this population.
Data statement
Dataset is stored at the Centre for Addiction and Mental Health, Toronto repository.
Acknowledgments
We would like to thank our participants who agreed to be a part of this study. Our thanks also to the personnel of the Temerty Brain Stimulation Center at the Center for Addiction and Mental Health, who provided technical assistance for this trial. We would also like to acknowledge the important contribution of Brainsway Inc., the company that developed and supplied H-coil equipment for this study. The abstract for this paper was presented at the XXIVth Annual Meeting of the Eating Disorders Research Society in Sydney, October 25–27, 2018 as an oral presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” http://edresearchsociety.org/web/printable-program-abstracts.php Sydney. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Brainsway Inc provided in-kind contribution by supplying H-coil for no charge.
Author contributions
Dr Allan S Kaplan is the principle investigator on this study. Dr Yuliya O Knyahnytska is the study MD, study coordinator, and first author on this manuscript. Dr Zafiris J Daskalakis is the brain stimulation supervisor and the main developer of the research protocol. Dr Daniel M Blumberger is the Medical Head of Brainstimulation Services with clinical responsibilities and provided overseeing on H-coil functioning. Dr Reza Zomorrodi is the Project Scientist who provided supervision with statistical analysis, and manuscript preparation. All authors contributed to data analysis, drafting and revising the article, gave final approaval, for the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Kaplan is the chairman of Shire Binge Eating Disorder Advisory Board for Canada for which he receives support. Dr Daniel M Blumberger reports in-kind equipment support as an investigator of initiated trials from Magventure, and as a site principle investigator of three sponsored trials from Brainsway, and attending one advisory board meeting from Janssen, during the conduct of the study. Dr Zafiris J Daskalakis reports industry sponsored clinical trial from Brainsway Inc, and equipment support from Magventure Inc, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Gorwood P, Blanchet-Collet C, Chartrel N, et al. New insights in anorexia nervosa. Front Neurosci. 2016;10:256.
2. McClelland J, Kekic M, Campbell IC, Schmidt U. Repetitive transcranial magnetic stimulation (rTMS) treatment in enduring anorexia nervosa: a case series. Eur Eat Disord Rev. 2016;24(2):157–163. doi:10.1002/erv.2414
3. Brewerton TD, Frampton I, Lask B. The neurobiology of anorexia nervosa. Eur Rev Psychiatry. 2009;1:59–64.
4. Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36(2):110–120. doi:10.1016/j.tins.2013.01.003
5. Pietrini F, Castellini G, Ricca V, Polito C, Pupi A, Faravelli C. Functional neuroimaging in anorexia nervosa: a clinical approach. Eur Psychiatry. 2011;26:176–182. doi:10.1016/j.eurpsy.2010.07.011
6. Phillipou A, Rossell SL, Castle DJ. (2014). The neurobiology of anorexia nervosa: a systematic review. Aust N Z J Psychiatry. 48(2):128–152. doi:10.1177/0004867413509693
7. Nunn K, Frampton I, Fuglset TS, Torzsok-Sonnevend M, Lask B. Anorexia nervosa and the insula. Med Hypotheses. 2011;76:353–357. doi:10.1016/j.mehy.2010.10.038
8. Nunn K, Frampton I, Gordon I, Lask B. The fault is not in her parents but in her insula—a neurobiological hypothesis of anorexia nervosa. Eur Eat Disord Rev. 2008;16(5):355–360. doi:10.1002/erv.890
9. Monteleone AM, Monteleone P, Esposito F, et al. Altered processing of rewarding and aversive basic taste stimuli in symptomatic women with anorexia nervosa and bulimia nervosa: an fMRI study. J Psychiatr Res. 2017;90:94–101. doi:10.1016/j.jpsychires.2017.02.013
10. Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664.
11. Vicario CM, Rafal RD, Martino D, Avenanti A. Core, social and moral disgust are bounded: a review on behavioral and neural bases of repugnance in clinical disorders. Neurosci Biobehav Rev. 2017;80:185–200. doi:10.1016/j.neubiorev.2017.05.008
12. Murray SB, Loeb KL, Le Grange D. Treatment outcome reporting in anorexia nervosa: time for a paradigm shift? J Eat Disord. 2018;6(1):10. doi:10.1186/s40337-018-0195-1
13. Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159:1284–1293. doi:10.1176/appi.ajp.159.8.1284
14. Ratnasuriya RH, Eisler I, Szmukler GI, Russell GF. Anorexia nervosa: outcome and prognostic factors after 20 years. Br J Psychiatry. 1991;158:495–502. doi:10.1192/bjp.158.4.495
15. Bamford B, Barras C, Sly R, et al. Eating disorder symptoms and quality of life: where should clinicians place their focus in severe and enduring anorexia nervosa? Int J Eat Disord. 2015;48(1):133–138. doi:10.1002/eat.22327
16. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587–596. doi:10.1002/da.21969
17. Ciobanu C, Girard M, Marin B, Labrunie A, Malauzat D. rTMS for pharmacoresistant major depression in the clinical setting of a psychiatric hospital: effectiveness and effects of age. J Affect Disord. 2013;150(2):677–681. doi:10.1016/j.jad.2013.03.024
18. Lee J, M Blumberger D, B Fitzgerald P, J Daskalakis Z, J Levinson A. The role of transcranial magnetic stimulation in treatment-resistant depression: a review. Curr Pharm Des. 2012;18(36):5846–5852.
19. Connolly KR, Helmer A, Cristancho MA, Cristancho P, O’Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567–e573. doi:10.4088/JCP.11m07413
20. Fitzgerald PB, Hoy K, Gunewardene R, et al. A randomized trial of unilateral and bilateral prefrontal cortex transcranial magnetic stimulation in treatment-resistant major depression. Psychol Med. 2011;41(6):1187–1196. doi:10.1017/S0033291710001923
21. Vicario CM, Salehinejad MA, Felmingham K, Martino G, Nitsche MA. A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosci Biobehav Rev. 2018;96:219–231. doi:10.1016/j.neubiorev.2018.12.012
22. Li H, Wang J, Li C, Xiao Z. Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults. Cochrane Database Syst Revies. 2014;9(9):CD009083.
23. Hovington CL, McGirr A, Lepage M, Berlim MT. Repetitive transcranial magnetic stimulation (rTMS) for treating major depression and schizophrenia: a systematic review of recent meta-analyses. Ann Med. 2013;45(4):308–321. doi:10.3109/07853890.2013.783993
24. Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327(1):79–93. doi:10.1111/nyas.12479
25. Dell’Osso B, Camuri G, Castellano F, et al. Meta-review of metanalytic studies with repetitive transcranial magnetic stimulation (rTMS) for the treatment of major depression. Clin Pract Epidemiol Mental Health. 2011;7(167):e77. doi:10.2174/1745017901107010074
26. Van den Eynde F, Guillaume S, Broadbent H, Campbell IC, Schmidt U. Repetitive transcranial magnetic stimulation in anorexia nervosa: a pilot study. Eur Psychiatry. 2013;28(2):98–101. doi:10.1016/j.eurpsy.2011.06.002
27. Dalton B, Bartholdy S, McClelland J, et al. Randomised controlled feasibility trial of real versus sham repetitive transcranial magnetic stimulation treatment in adults with severe and enduring anorexia nervosa: the TIARA study. Br Med J Open Access. 2018;8(7):e021531.
28. Eaton H, Electric field induced in a spherical volume conductor from arbitrary coils: application to magnetic stimulation and MEG. Med Biol Eng Comput. 1992;30:433–440. doi:10.1007/BF02446182
29. Tofts PS, Branston NM. The measurement of electric field, and the influence of surface charge, in magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:238–239. doi:10.1016/0168-5597(91)90077-B
30. Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. 2002;19(4):361–370.
31. Malik S, Jacobs M, Cho SS, et al. Deep TMS of the insula using the H-coil modulates dopamine release: a crossover [11C] PHNO-PET pilot trial in healthy humans. Brain Imaging Behav. 2017;12(5):1306–1317. doi:10.1007/s11682-017-9800-1
32. Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy og the H-coil. Clin Neurophysiol. 2005;116(4):775–779. doi:10.1016/j.clinph.2004.11.008
33. Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett. 2009;463(1):82–86. doi:10.1016/j.neulet.2009.07.041
34. Fitzgerald PB, Hoy K, McQueen S, et al. Priming stimulation enhances the effectiveness of low-frequency right prefrontal cortex transcranial magnetic stimulation in major depression. J Clin Psychopharmacol. 2008;28(1):52–58. doi:10.1097/jcp.0b013e3181603f7c
35. Brooks SJ, Prince A, Stahl D, Campbell IC, Treasure J. 2011. A systematic review and meta-analysis of cognitive bias to food stimuli in people with disordered eating behavior. Clin Psychol Rev. (IF. 4.901). Available from: http://www.ncbi.nlm.nih.gov/pubmed/21130935. Accessed May 30, 2012.
36. Varnava A, Stokes MG, Chambers CD. Reliability of the ‘observation of movement’method for determining motor threshold using transcranial magnetic stimulation. J Neurosci Methods. 2011;201(2):327–332. doi:10.1016/j.jneumeth.2011.08.016
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
