Back to Journals » International Journal of General Medicine » Volume 10
Insidious onset of intermittent claudication as the primary manifestation of infective endocarditis
Authors Vasquez-Rios G , Gamero MT, De la Cruz J, Hernandez GA, Hernandez E, Dueñas R
Received 23 May 2016
Accepted for publication 29 September 2016
Published 9 January 2017 Volume 2017:10 Pages 11—14
DOI https://doi.org/10.2147/IJGM.S113385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
George Vasquez-Rios,1,2 Maria T Gamero,2 Jesus De la Cruz,3 Gabriel A Hernandez,4 Eduardo Hernandez,5 Roy Dueñas6
1Alexander Von Humboldt Tropical Medicine Institute, Cayetano Heredia University, Lima, Peru; 2Alberto Hurtado School of Medicine, Cayetano Heredia University, Lima, Peru; 3Internal Medicine Department, Cayetano Heredia Hospital, Lima, Peru; 4Department of Cardiology, Miller School of Medicine, University of Miami, Miami, FL, 5Department of Adult Cardiology, St. Luke’s Episcopal Hospital, Houston, TX, USA; 6Cardiology Department, Cayetano Heredia Hospital, Lima, Peru
Abstract: Musculoskeletal manifestations of infective endocarditis are well-described in the literature. However, insidious onset of localized calf pain is an uncommon presentation of embolization and may represent a diagnostic challenge owing to the nonspecific nature of the symptoms. This study reviewed the literature and reports a case of infective endocarditis in a patient who presented with bilateral calf pain as the primary complaint and reason for seeking medical attention.
Keywords: musculoskeletal pain, embolization, ischemia, endocarditis
Introduction
Infective endocarditis (IE) presents with variable symptoms, including systemic or cardiovascular manifestations.1 Some of these symptoms are related to either embolic seeding of the organism or an immunological phenomenon associated with it. Although musculoskeletal symptoms are described in the literature, these are frequently overlooked by physicians.2–3 In most reports, patients present with musculoskeletal manifestations resultant from acute limb ischemia,4–6 but more insidious presentations are rare. Failure to recognize this scenario may delay medical attention, contributing to a larger vegetation burden and increasing the mortality rates. Herein, a case is reported in which subacute bilateral calf pain was the primary manifestation of IE.
Case presentation
A 48-year-old man presented to the emergency department with 5 weeks of progressive intermittent claudication that was associated with low-grade fever and worsening dyspnea. His medical history was notable for tobacco use (five packs per day), heavy alcohol consumption, and cannabis use. He denied intravenous drug abuse and reported antibiotic use (amoxicillin) in the week prior to admission.
His vital signs were remarkable – a heart rate of 120 bpm, respiratory rate of 40 rpm, and oral temperature of 39°C. On physical examination, breath sounds were decreased bilaterally and a 3/6 holosystolic murmur was perceived in the apex. Along with this, he had erythematous and swollen gingiva. No erythema or swelling was noted in the lower limbs, but capillary filling time was bilaterally delayed. Complete blood count revealed a white blood cell count of 15.4×103 cells/µL (22% bands) and normocytic anemia with a hemoglobin level of 7.7 g/dL. Erythrocyte sedimentation rate and C-reactive protein were both increased at 94 mm/h and 185 mg/L, respectively.
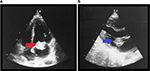
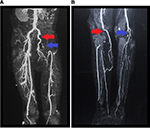
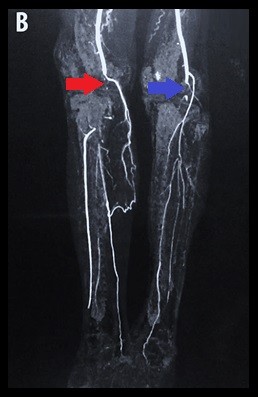
A chest X-ray showed bilateral pleural effusion, and the remaining biochemistry panel, urinalysis, and coagulation tests were within normal limits. Furthermore, serologic tests for HIV, viral hepatitis, and syphilis, in addition to immunologic studies (including antinuclear antibodies and anti-dsDNA) were negative. During evaluation, transthoracic echocardiography revealed a 2.1×2 cm hyperechoic lesion on the anterior leaflet of the mitral valve (Figure 1A: a red arrow shows a mass in the apical four-chamber view. Figure 1B: a blue arrow shows the mass in the long axis view), which was associated with severe mitral regurgitation (regurgitant volume of 73 mL and vena contracta of 19 mm) and an eccentrically directed posterior jet (despite jet angle, the effective regurgitation orifice was >0.4 cm2). In addition, the ejection fraction was preserved. Similar findings were confirmed by transesophageal echocardiography. Furthermore, Doppler ultrasound of both inferior limbs showed bilateral arterial thrombosis in the right popliteal artery and in the left common femoral artery, with patency of collateral circulation. A subsequent computed tomography (CT) angiography of the lower extremities showed intraluminal filling defects in left common femoral and iliac artery (Figure 2A) and in the distal popliteal artery of both limbs (Figure 2B). The patient was started empirically on penicillin and gentamicin for possible infectious endocarditis. Blood culture results demonstrated no growth on the day of admission or during the follow-up (day 1, 3, and 5).
Despite the early use of broad-spectrum antibiotics for 4 days, he remained febrile and developed amaurosis fugax in the right eye, but CT scan of the head did not show evidence of an acute cerebrovascular event. His renal function declined, and the patient showed signs of hemodynamic instability with a mean arterial pressure of less than 60 mmHg. Thus, he was taken to the operating room on day 5 of hospitalization for mitral valve debridement and valve replacement with a 27 mm size Carbomedics Orbis metallic valve (Sorin Group, Milan, Italy). After surgery, his fever subsided and clinical condition improved gradually. Histopathological and microbiologic studies (gram stain, bacterial and fungal cultures [Chocolate Agar, Mac Conkey agar, Heart–Brain Infusion agar, Czapek-Dox agar, and CHROMagar]) failed to find any organism. Considering his previous exposure to β-lactam medications and given the absence of etiologic risk factors other than his extensive gingival inflammation, he continued empiric treatment with ceftriaxone for a total of 4 weeks to cover Streptococci viridans, Staphylococcus aureus, and HACEK group organisms. Peripheral vascular procedures were not pursued due to the improvement of his bilateral calf pain and capillary filling time. Finally, the patient was placed on anticoagulation for his metallic valve and was enrolled in physical therapy. During follow-up (1, 3, and 6 months), he showed mild improvement in his bilateral claudication, and his overall status was stable.
Discussion
Musculoskeletal manifestations of IE, such as intermittent claudication, are described in the literature but are still poorly defined in medical practice. In a retrospective analysis including 192 cases of IE, 44% of them presented one or more types of musculoskeletal manifestations (mainly arthralgia and low back pain);3 however, only 5.7% of the patients presented myalgia localized to the thigh or calf. In addition, 27% of the patients reported musculoskeletal complaints as one of the first symptoms of this disease. Furthermore, myalgia may be the sole manifestation of IE and can precede the diagnosis by as much as 6 weeks.2 No association has been noted between the severity of the rheumatic symptoms and laboratory test abnormalities.3
A number of embolic complications in IE have been reported in the literature. Previous reports have addressed acute embolic effects that may compromise limbs,5–7 spleen, or the central nervous system.8 Nonetheless, a subtle onset of musculoskeletal complaints, such as in this case, is rare and may lead to an adverse outcome if left unrecognized. The delayed clinical presentation of the patient may be partially explained by the compromise of small and medium-sized arteries as well as the progressive development of collateral circulation. In addition, it is possible that a certain degree of vascular damage was already present given his long-standing history of smoking.
Despite performing several blood cultures (6), no organism was isolated, possibly due to previous antibiotic use, which is the main cause of culture-negative endocarditis.9 Clinical guidelines recommend treatment with broad-spectrum antibiotic coverage.9 However, on the basis of the local epidemiology,10 international data of IE,11 and taking into consideration the patient’s risk factors (eg, extensive gingival inflammation and the lack of intravenous drug use), it was decided to prescribe ceftriaxone to treat Streptococci viridans. Appropriate antibiotic coverage is pivotal to prevent severe embolic events that may occur particularly during the first 2 weeks of the disease,12 especially if organisms such as Staphylococcus aureus,13 Streptococcus bovis,14 or fungi are involved. Additional factors such as vegetation characteristics (mobile vegetation length >10 mm, determined by transesophageal echocardiography)9,15 may also be useful to estimate the real risk of further embolism, as in the patient (Box 1). Taking into account the aforementioned factors, a careful evaluation is needed when estimating the real embolic burden as early surgical intervention may be warranted (recently acknowledged in the 2015 European guidelines for IE).16
  | Box 1 High risk for embolic events Note: Data from Baddour et al9 and Habib et al.16 Abbreviation: IE, infective endocarditis. |
Musculoskeletal manifestations of IE may appear early in the course of disease and may be a subtle clue to diagnosis. This case emphasizes the necessity of an early comprehensive examination and a broad differential diagnosis in patients with subacute vascular ischemic symptoms.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author contributions
George Vasquez-Rios and Maria T Gamero wrote the manuscript and conducted the literature search. George Vasquez-Rios, Maria T Gamero, Jesus de la Cruz, and Roy Dueñas were part of the medical team taking care of the patient. Gabriel A Hernandez and Eduardo Hernandez were consulted for expert opinion. George Vasquez-Rios, Maria T Gamero, Jesus de la Cruz, Gabriel A Hernandez, Eduardo Hernandez, and Roy Dueñas critically revised the manuscript for important intellectual consent. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspect of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Hoen B, Duval X. Infective endocarditis. N Engl J Med. 2013;368(15):1425–1433. | ||
Meyers OL, Commerford PJ. Musculoskeletal manifestations of bacterial endocarditis. Ann Rheum Dis. 1977;36(6):517–519. | ||
Churchill MA Jr, Geraci JE, Hunder GG. Musculoskeletal manifestations of bacterial endocarditis. Ann Intern Med. 1977;87(6):754–759. | ||
Pecoraro R, Tuttolomondo A, Parrinello G, Pinto A, Licata G. Staphylococcus lugdunensis endocarditis complicated by embolism in an 18-year-old woman with mitral valve prolapse. Case Rep Infect Dis. 2013;2013:4. | ||
Lozano P, Flores D, Blanes I, et al. Acute lower limb ischemia complicating endocarditis due to Candida parapsilosis in a drug abuser. Ann Vasc Surg. 1994;8(6):591–594. | ||
Freischlag JA, Asbun HA, Sedwitz MM, Hye RJ, Sise M, Stabile BE. Septic peripheral embolization from bacterial and fungal endocarditis. Ann Vasc Surg. 1989;3(4):318–323. | ||
Card L, Lofland D. Candidal endocarditis presenting with bilateral lower limb. Clin Lab Sci. 2012;25(3):130–134. | ||
Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography : a prospective multicenter study. Circulation. 2005;112(1):69–75. | ||
Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111(23):e394–434. | ||
Romane F, Cuadra J, Atencia F, Vargas F, Canelo C. Endocarditis infecciosa: análisis retrospectivo en el Hospital Nacional Arzobispo Loayza, 2002–2007. Rev Peru Epidemiol. 2009;13(2). Available from: http://sisbib.unmsm.edu.pe/bvrevistas/epidemiologia/v13_n2/pdf/a04v13n2.pdf. Accessed April 3, 2015. | ||
Slipczuk L, Codolosa JN, Davila CD, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One. 2013;8(12):e82665. | ||
Vuielle C, Nirdof M, Weyman AE, Picard MH. Natural history of vegetation during successful medical treatment of endocarditis. Am Heart J. 1994;128(6):1200–1209. | ||
Erbel R, Liu F, Ge J, Rohmann S, Kupferwasser I. Identification of high-risk sub-groups in infective endocarditis and the role of echocardiography. Eur Heart J. 1995;16(5):588–602. | ||
Pergola V, Di Salvo G, Habib G, et al. Comparison of clinical and echocardiographic characteristics of Streptococcus bovis endocarditis with that caused by other pathogens. Am J Cardiol. 2001;88(8):871–875. | ||
Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91(5):571–575. | ||
Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.