Back to Journals » International Journal of Women's Health » Volume 14
Inoperable Bowel Obstruction in Ovarian Cancer: Prevalence, Impact and Management Challenges
Authors Gonzalez-Ochoa E, Alqaisi HA, Bhat G, Jivraj N, Lheureux S
Received 17 September 2022
Accepted for publication 17 December 2022
Published 28 December 2022 Volume 2022:14 Pages 1849—1862
DOI https://doi.org/10.2147/IJWH.S366680
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Eduardo Gonzalez-Ochoa,1 Husam A Alqaisi,1 Gita Bhat,1 Nazlin Jivraj,1 Stephanie Lheureux1,2
1Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada; 2Department of Medicine, University of Toronto, Toronto, Ontario, Canada
Correspondence: Stephanie Lheureux, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, 610 University Avenue, Toronto, Ontario, M5G 2M9, Canada, Tel +1 416-946-2818, Email [email protected]
Abstract: Malignant bowel obstruction (MBO) is one of the most severe complications in patients with advanced ovarian cancer, with an estimated incidence up to 50%. Its presence is related to poor prognosis and a life expectancy measured in weeks for inoperable cases. Symptoms are usually difficult to manage and often require hospitalization, which carries a high burden on patients, caregivers and the healthcare system. Management is complex and requires a multidisciplinary approach to improve clinical outcomes. Patients with inoperable MBO are treated medically with analgesics, antiemetics, steroids and antisecretory agents. Parenteral nutrition and gut decompression with nasogastric tube, venting gastrostomy or stenting may be used as supportive therapy. Treatment decision-making is challenging and often based on clinical expertise and local policies, with lack of high-quality evidence to optimally standardize management. The present review summarizes current literature on inoperable bowel obstruction in ovarian cancer, focusing on epidemiology, prognostic factors, clinical outcomes, medical management, multidisciplinary interventions and quality of life.
Keywords: bowel obstruction, inoperable, ovarian cancer, medical management, multidisciplinary
Introduction
Malignant bowel obstruction (MBO) represents one of the most severe complications of advanced intraabdominal and intraperitoneal malignancies. It was defined in the International Conference on MBO and Clinical Protocol Committee as (a) clinical evidence of bowel obstruction via history, physical and radiographic examination, (b) bowel obstruction beyond the ligament of Treitz, (c) intraabdominal primary cancer with incurable disease, and (d) non-intraabdominal primary cancer with clear intraperitoneal disease.1
Methods
We conducted a narrative literature review on inoperable malignant bowel obstruction in ovarian cancer, including epidemiology, clinical outcomes, complications, medical management, multidisciplinary interventions, quality of life and healthcare cost.
Search was performed through MEDLINE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews and Embase. The search was limited to human studies and English publications, with a timeframe of 20 years (from 2002 to July 2022). Conferences, case reports, comments, editorials, letters and personal narratives were excluded. A PubMed targeted search using same criteria was conducted by one author and reviewed by another author for articles between 1980 and 2002.
Prevalence
Epidemiology of malignant bowel obstruction is not well established; retrospective and autopsy studies have reported an incidence up to 50% in ovarian cancer and between 10 and 28% in gastrointestinal cancers.2 It is associated with a poor prognosis, with survival estimates of 3–8 months for those with operable disease and 4–5 weeks in inoperable cases.3,4
Classification and Etiology
Obstruction may originate in the small bowel, large bowel, or in both simultaneously. MBO can be classified as partial or complete, according to the presence or absence of residual air and fluid in the collapsed distal bowel segments.5 A closed-loop obstruction indicates a complete blockage distally and proximally in a given segment of the intestine.6
The etiology is multifactorial and can be divided into two main groups: mechanical and functional. Mechanical causes include extrinsic obstruction due to mesenteric/omental masses or malignant adhesions, intramural, and intraluminal occlusions. Functional obstruction includes motility disorders caused by tumor infiltration of the mesentery, bowel muscle or enteric plexuses.2
Here, we focus on malignant bowel obstruction due to ovarian cancer, where peritoneal disease frequently leads to extrinsic compression of the bowel.
Pathophysiology
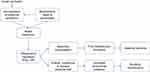
Pathophysiology of MBO is multifactorial. An occlusion of the lumen prevents the propulsion of the intestinal contents, causing accumulation of intestinal secretions and malabsorption of water and electrolytes in the obstructed segment. Bowel distension stimulates intestinal fluid secretion, creating a vicious cycle7 (Figure 1).
Damage in the bowel triggers an inflammatory response, with release of potent secretagogues such as prostaglandins (PGs) and vasoactive intestinal peptide (VIP). This leads to local and systemic alterations such as hyperemia, edema of the intestinal wall and accumulation of fluid in the lumen.4,8
High portal levels of VIP cause splanchnic vasodilatation leading to blood flow redistribution and fluid sequestration in the third space, which can cause hypotension and multiple organ failure. Increased endoluminal pressure, stasis and ischemia promote bacterial translocation from the intestinal wall into the lymphatic and systemic circulation, which may result in sepsis, gangrene and perforation.8,9
Diagnosis and Prognostic Factors
MBO manifests insidiously and results in variety of symptoms such as nausea, vomiting, abdominal cramping, distension, bloating, and decreased passage of flatus or stool elimination.4,8,9
MBO is diagnosed clinically and confirmed radiologically. Typically, erect abdominal X-rays demonstrate bowel-loop distention with air-fluid levels proximal to the point of obstruction and potentially diminution of gas and fecal material distally.10–12 Such signs are detected in around 40–80% of cases and, therefore, absence of those signs with high clinical index of suspicion should prompt further investigation. Small bowel follow-through (SBFT) has proven useful to confirm suspected small-bowel obstruction in 68% to 100% of cases;13 however, the ability to diagnose complications is limited. Computed tomography (CT) scan is the most sensitive study (100%), with a predictive value of 83–94%; it provides detailed information on obstruction level, tumor extension and potential complications such as superimposed ischemia and intestinal perforation.6,14,15
Many clinical, radiologic, and treatment factors contribute to prognosis of ovarian cancer patients with MBO. Several studies showed that presence of poor performance status, pain, exertional shortness of breath, cognitive impairment, ascites, palpable masses in abdomen and pelvis, poor nutritional status, low albumin, liver failure, multifocal obstruction, and proximal MBO correlated with dismal survival.16–23 Moreover, a recent retrospective review identified CT findings of ascites, mesenteric disease, and dilated bowel loop wall thinning as poor prognostic indicators.24
Retrospective analyses have shown that systemic chemotherapy after the development of MBO is associated with superior survival, irrespective of whether patients were treated medically or surgically for MBO.25–27 In advanced ovarian cancer, disease status is an important prognostic factor of treatment response and thus MBO outcome. For example, patients newly diagnosed or with platinum-sensitive recurrence have a high likelihood of response to platinum-based chemotherapy (around 80 and 50% respectively), whereas those with platinum-resistant disease have a lower response rate of 10–15%.28 Selection is key, and chemotherapy may be useful to improve MBO in a subset of patients.
Studies comparing surgical interventions and medical management have reported mixed results; this can be explained at least partially by the diverse definitions of clinical outcome, heterogeneous clinical practice, and selection bias within these studies.6,27 Daniele et al demonstrated that surgical palliation was associated with longer survival (p=0.025), when compared to medical management.23 Nonetheless, Yu et al showed no significant difference in survival between surgical and non-surgical patients, albeit their cohort included a minority of ovarian cancer patients;27 this was consistent with a population-based analysis that showed similar overall survival outcome between surgical and medical patients (median 6.5 vs 6.4 months).25
General Management
Patients with MBO should be managed by a multi-disciplinary team, and several approaches can be considered. The focus of this review is on medical management of inoperable bowel obstruction; key publications are summarized in Table 1.
 |
Table 1 Summary of Key Publications in the Management of Inoperable MBO |
Diet
To alleviate symptoms like nausea and vomiting, patients admitted with acute MBO are put on a strict nil-by-mouth (NPO) and subjected to decompressive procedures.29,30 Due to a lack of consensus and few published evidence, the management of the nutritional needs of patients presenting with advanced malignancies and MBO is controversial and ethically demanding.30–32
The Multinational Association of Supportive Care in Cancer (MASCC) recommends that patients are initially kept NPO and, with guidance from a dietitian, gradually transitioned in stages of oral diets if their MBO symptoms are resolving: from 1) clear fluids to, 2) full fluids to, 3) minced/pureed low fiber diet and then, 4) low fiber diet.29
The terms low residue/fiber are used interchangeably, and neither term has a clear definition. Essentially, residue is undigested food in the intestinal tract that adds bulk to the stool, and 10 grams of fiber a day equates to a low fiber diet.33 The purpose of a low residue/fiber diet is to decrease the size/bulk of the stool to help ease passage as patients can have tumors and/or adhesions that interrupt normal bowel flow. A low residue/fiber diet assists to alleviate abdominal discomfort, such as bloating, pain, and nausea/vomiting.23,29,33,34 It is recommended that patients and their caregivers receive nutrition instructions to help them adjust their diet in response to their symptoms.32
Hydration
Parenteral hydration (PH) is the administration of fluids via the parenteral route, alternative to oral or enteral administration (ie, intravenously or subcutaneously).35 PH is a component of palliative care and should adhere to predetermined, realistic therapeutic objectives. A small prospective randomized trial evaluating inoperable MBO patients, the amount of parenterally administered fluids did not correlate with thirst, dry mouth intensity, or abdominal distention; however, a volume of more than 500 mL/day may improve nausea and drowsiness.37 PH may seem effective in management of dehydration, a common cause of delirium; yet, in the dying phase, even moderate quantities of parenteral hydration may be hazardous. Fluid overload, peripheral swelling, ascites, and pulmonary edema are all conditions that could be exacerbated by PH in the terminal phase of life.38 Subcutaneous infusion of 1000 mL daily did not alleviate dehydration symptoms (fatigue, myoclonus, drowsiness, and hallucinations) in a multicenter, double-blind, randomized study with a placebo control group.39
Clinically assisted hydration at end of life is controversial; a feasibility study showed that a definitive trial to address its utility can be done but will require standardization of the administration methods.40 In general, parenteral hydration is an important component of acute management of patients with MBO; however, it is not recommended at the end of life.29,41
Total Parenteral Nutrition (TPN)
Along with pharmacological management of MBO, care must be taken to ensure that hydration and nutritional needs are met, as oral intake is significantly impaired in patients with MBO. Based on the goals of care, this may range from comfort feeding alone to TPN to maintain adequate calorie intake.29 While deciding upon TPN, it is of prime importance to understand its role, cost, logistics and potential complications and also to discuss in detail goals of care and disease prognosis.29
The various terms used to describe parenteral nutrition include “total parenteral nutrition” (TPN) which is nutrition administered exclusively intravenously and “supplemental parenteral nutrition” (SPN) in which the patient receives nutrition via a combination of intravenous and enteral routes. However, the enteral route is not used in inoperable MBO due to ovarian cancer.29 Any type of parenteral nutrition administered at home is defined as “home parenteral nutrition” (HPN).29 Here, we focus on TPN. Central venous access devices such as a chemoport or a peripherally inserted central catheter are the main modes of TPN administration, as the use of peripheral veins is associated with phlebitis.
Before recommending TPN, risks and benefits must be carefully measured, including the predicted cancer-related survival.42–44 As our understanding of the evolution and biology of MBO evolves, various ways of patient selection for TPN have been devised.43,45 TPN can be considered in a selective group of patients, including ECOG performance status 0 to 1, extensive peritoneal carcinomatosis in the absence of distant metastases (such as lung and liver metastases), patients not amenable for diverting stoma and interventional radiological procedures such as insertion of self-expanding metal stents and those with certain histology such as low grade serous ovarian carcinoma.6 Patients should be reassessed periodically based on clinical assessment and radiology as we know that ultimately, most patients may not benefit due to functional decline resulting from cancer progression, chronic cancer treatment,46 latent infections,47 depression,48,49 and suboptimal nutritional treatment.50
TPN is associated with metabolic and catheter-related complications.29 In the study by Sowerbutts et al, 6 to 21% of patients with MBO developed a central venous catheter infection or were hospitalized because of complications related to parenteral nutrition.51 The risk of potential complications can be minimized with the help of nutrition support teams.52
TPN is also initiated on an inpatient basis given that refeeding syndrome, which can be seen in severely malnourished patients, usually occurs within the first few days after initiation of TPN.52,53 Refeeding syndrome includes acute beriberi due to Vitamin B1 deficiency, acute volume overload leading to anasarca and pulmonary edema, dyselectrolytemia including hypophosphatemia, hypokalemia and hypomagnesemia, arrhythmias including bradycardia and ventricular arrhythmia, hyperglycemia which if left untreated can lead to hyperosmolar coma.52
Malnutrition and cachexia are usually irreversible and treatment-resistant,54 especially in those patients who are heavily pre-treated and have platinum-resistant disease. The European Society for Clinical Nutrition and Metabolism (ESPEN) practical guidelines for clinical nutrition reported that parenteral hydration and nutrition are unlikely to provide any benefit for most patients at the end of life.31 A randomized Phase II clinical trial by Oh et al also showed that though patients and families are concerned about starvation at the end of life, parenteral nutrition fails to add meaningful survival benefit.55
Surgical Management/Inoperability
A surgical consultation should be obtained at the time of MBO diagnosis; surgery can be beneficial in restoring bowel function in a subset of MBO patients with a good performance status and viable cancer therapy choices.23,56 Obstruction of the large bowel carries a high risk of morbidity, perforation, and mortality, and expectant management is typically inappropriate in this scenario. Rather than initial resection and anastomosis or bypass, stoma diversion is the most common surgical treatment for large intestinal blockage.57,58 Unstrangulated small intestinal obstruction is closely linked to widespread peritoneal carcinomatosis and therefore is generally treated conservatively.57–59 Small bowel resection with anastomosis or internal bypass is reserved for a minority of patients.
Furnes et al reviewed 868 patients with MBO and showed that surgery might ameliorate obstructive symptoms, allow the reintroduction of diet and promote earlier patient discharge to home.57 Helyer et al found that surgical intervention in MBO patients may facilitate palliative chemotherapy and this was correlated with better survival.26
Furthermore, a prospective study including 26 with MBO due to ovarian cancer evaluated outcomes of palliative surgical (bowel diversion or bypass/resection) and endoscopic interventions. The majority (88%) of patients reported symptomatic improvement or MBO resolution by 30 days, and 16 patients achieved continued symptom control at 60 days.60
These studies support the idea of palliative surgery in patients with specific clinical criteria, including excellent performance capacity, longer treatment-free interval, utter lack of ascites, disease uni-focality, and adequate albumin levels.61,62 This also reflects on results of a recent bicentric analysis of 87 patients with ovarian cancer and MBO, where regression analysis revealed that better Eastern Cooperative Oncology Group (ECOG) status, platinum sensitivity, and no or volume ascites of <500 mL were associated with a better prognosis.63
At this juncture, there is a well-recognized correlation between MBO palliative surgery and higher morbidity and mortality rates, thus, it should be considered with caution in this cohort of patients.64,65 De Boer et al found that 58.1% of the patients suffered postoperative complications, and 8.8% of the patients died in the hospital.66 Olson’s systematic review revealed that fatalities are rather high (6–32%) and that significant complications are frequent (7–44%).58 Counter-intuitively, Yu et al showed no significant difference in terms of survival between surgical and non-surgical management in patients with MBO, albeit the cohort of the study included a minority of ovarian cancer patients.27 A population-based analysis also demonstrated a similar outcome as overall survival was similar between surgical and medical patients (median 6.5 vs 6.4 months).25 Therefore, selection of patients for surgery requires detailed consideration of overall prognosis, performance status, as well as the risk of perioperative complications.
Non-Surgical Interventions
Temporary decompression via nasogastric tube is often used to evacuate a large amount of pooled gastric secretions to improve symptoms during acute MBO episodes.67 Nasogastric tubes are not well tolerated for a prolonged time and are associated with complications such as occlusion and displacement, nasal cartilage erosions, aspiration pneumoniae, esophagitis and bleeding.68
Percutaneous transesophageal gastro-tubing (PTEG) has shown to be superior to nasogastric tube in terms of symptomatic improvement and quality of life, with no differences in survival between both interventions.69
Percutaneous gastrostomy tube, also called venting gastrostomy, can also be used as a more permanent measure for gastric decompression and are associated with reduction of nausea and vomiting.29 Complications include wound infection or leakage of fluid around the tube. In a systematic review that included 25 studies and 1194 patients, Thampy et al summarized a variety of outcomes related to venting gastrostomy insertion, including successful insertion at first attempt in 91%, major complication in 2% and minor complication in 20%.70 Patients with malignant ascites, frequently associated with advanced gynecologic cancers, have an increased likelihood of complications or unsuccessful gastrostomy tube insertion, although this is not considered a contraindication.71
Endoscopic stents have been used to improve symptoms from MBO related to a single point of obstruction mainly in patients with colorectal cancer, but few data support its use in extracolonic malignancies. Small studies evaluated the use of stents in extraluminal narrowing secondary to genitourinary, gynecologic and non-colorectal GI malignancies, with technical success ranging from 20 to 87%.72,73 Extrinsic compression is associated with higher likelihood of technical and clinical failure, and higher likelihood of requiring surgical diversion for persistent obstructive symptoms despite stent insertion. Risk of stent insertion includes bowel perforation, migration and re-obstruction.74
Patient selection is critical when choosing the most appropriate intervention. Patients with single-level obstruction may benefit from local treatment such as stenting or surgery.75 However, most patients with ovarian cancer and MBO have multifocal sites of obstruction and are not considered good candidates for the aforementioned procedures, thus managed conservatively.
Pharmacologic Management
In MBO, medical treatment aims to relieve pain and uncomfortable symptoms by lowering inflammation, luminal pressure, and secretions.29 Effective symptomatic management for MBO can be achieved with medical therapy that combines steroids, opioids, antiemetics, and antisecretory medications in addition to intravenous (IV) hydration and bowel decompression (if required).29,65,76,77 As MBO patients tend not to tolerate medications orally, alternative routes of administration can be pursued (eg, intravenous, subcutaneous, sublingual, or transdermal).65,77 Periodically adjusting the pharmacological regimen based on the course of MBO and treatment response is of paramount importance.29
Corticosteroids can relieve acute symptoms of MBO, likely due to an anti-inflammatory and anti-secretory effect, which decrease intestinal wall edema and promote salt and water absorption.78
In a small clinical trial, 35 patients with MBO mostly due to ovarian cancer were randomized to dexamethasone 4 mg every 6 hours or placebo for 5 days; all patients received standardized medical management with fluids, analgesia antiemetics and laxatives. In the dexamethasone group, 62% of patients showed resolution of obstruction at day 5, compared to 57% in the placebo group; numbers were too small to allow a formal statistical analysis.79 In separate study including 52 patients, the administration of methylprednisolone (240 mg or 40 mg versus placebo for 3 days) was associated with a tendency toward symptom improvement in the methylprednisolone group (59% versus 33.5%, p = 0.08).80 Finally, in a recent retrospective analysis evaluating 91 ovarian cancer patients with small bowel MBO who received dexamethasone during 154 hospitalizations, 89% of women had initial partial or total symptom control; platinum-sensitive patients were more likely to respond to dexamethasone than platinum-resistant patients (p = 0.04).81 Duration of treatment is not well established; doses ranging from 4 to 16 mg equivalent of dexamethasone daily for 3 to 5 days are usually used.29 It is recommended to use the lowest effective dose for the shortest period of time to avoid long-term toxicity days.82 Concerns about extended glucocorticoid therapy include increased susceptibility to infections, peptic ulcers, impaired mood, and insomnia; consequently, if limited response is noted, glucocorticoids should be discontinued.83
Somatostatin analogues reduce intestinal and pancreatic secretion, modulate gastrointestinal motility, decrease biliary contraction and intestinal edema.37 Although a systematic review of these agents in the treatment of MBO revealed no observable benefit based on the broadly diverse main outcomes defined by the seven eligible trials (427 patients in total) included,84 a recent multicenter prospective phase II study of Lanreotide proved benefit in patient with MBO secondary to ovarian and GI malignancies.85 Another randomized Phase III study confirmed effectiveness of octreotide and dexamethasone over traditional medical treatment in patients with inoperable MBO.86 Moreover, as described in the randomized controlled trials (RCTs), somatostatin analogues proved to be well tolerated, with no withdrawals owing to toxicity.86,87
The anticholinergic drug hyoscine (scopolamine) butylbromide can be used to alleviate spasmodic abdominal discomfort. In addition to decreasing gastrointestinal secretions, it slows propulsive peristalsis and relaxes the smooth muscles of the gastrointestinal tract. Hyoscine butylbromide may be used for the treatment of inoperable MBO, but there is less evidence to support its use.9 Obita et al concluded in their systematic review that somatostatin analogues (eg, octreotide) are more effective than hyoscine butylbromide in decreasing continuous pain in 2 studies with high Cochrane risk of bias.37,84,87 Another study, however, did not show the same findings.88 In contrast, colicky discomfort was not significantly different between octreotide and hyoscine butylbromide in all three studies.37,84,87,88
Laxatives are not recommended for complete MBO. Patients with incomplete MBO may utilize oral osmotic laxatives (eg, polyethylene glycol 3350) with caution. Osmotic laxatives pull water into the lumen of the intestine to soften feces and increase peristalsis. Bulk-forming laxatives (eg, psyllium) are contraindicated because they increase stool consistency and may aggravate MBO.29 If a digital rectal examination reveals a full rectum or fecal impaction, suppositories and fecal disimpaction may be explored for partial MBO.89 Due to the danger of intestinal perforation, enemas are typically contraindicated in complete MBO. It is possible to employ stool softeners (eg, docusate), although their effect on bowel movements is poorly studied.29
As recognized by the WHO recommendations, opioid analgesia is a frequent and effective method for pain control in advanced malignancies.90 Pain associated with MBO may be colicky (cramping and intermittent) or persistent. Experts recommend opioid analgesia because it may be supplied bypassing the oral route (intravenous, subcutaneous, sublingual, or trans dermal) and because the depressed impact on intestinal motility may decrease colicky pain.1,29,64 Evidence supporting its use in MBO is lacking and further investigation is warranted, given that opioids are known to impair gastrointestinal motility and can cause nausea, vomiting and constipation.29
Prokinetics (eg, metoclopramide) boost gastric and small bowel movement, enhance gastric peristalsis and emptying, and promote the transit of intestinal contents through the digestive tract. Metoclopramide is generally regarded as a potential option for first-line therapy in patients with partial MBO (ie, intermittent vomiting as opposed to continuous vomiting), and it mediates myenteric cholinergic action91,92 However, it is contraindicated for complete MBO as it might exacerbate abdominal cramping, nausea, and vomiting.93,94 A small study by Mercadante et al analyzed the effects of combining metoclopramide, octreotide, dexamethasone, and diatrizoate in 15 consecutive advanced cancer patients with MBO and found that the therapeutic effect of the combination was better than that of any single drug; additionally, the patients who persisted on this treatment did not have recurrent symptoms until death.93
Role Chemotherapy
The underlying cause of bowel obstruction in patients with advanced ovarian cancer is peritoneal carcinomatosis involving the mesentery and the various folds of peritoneum. This can be seen at the time of presentation in patients with newly diagnosed ovarian cancer as well as at the time of recurrence. The main aim of administering chemotherapy is to treat the underlying peritoneal carcinomatosis. Careful attention must be paid while choosing the drug, keeping in mind the nature and extent of disease, anticipated response, for example, newly diagnosed or platinum-sensitive versus bulky, platinum-resistant disease and tolerability.6
Evidence of chemotherapy in MBO is scarce as these patients are generally not included in clinical trials. Most of the patients with MBO have already received multiple lines of treatment, are chemo-resistant and may not have a response that is significant enough to result in resolution of obstruction.95,96 Yet, chemotherapy with or without surgery may improve survival in ovarian cancer.25 A retrospective study included 2983 patients with advanced cancer from several disease subsites with MBO, of whom 1472 were medically managed. In the entire study population, there were 406 patients (13.6%) with ovarian cancer of whom 244 (60%) were medically managed. More than half of these patients received chemotherapy (with or without surgery) after the diagnosis of MBO; however, there are no details regarding number of lines of treatment or platinum sensitivity.25 The single center, retrospective study by Chouhan et al,97 evaluated 82 patients with malignant small bowel obstruction (16 patients with ovarian cancer) who were managed with a combination of systemic chemotherapy and total parenteral nutrition. In the ovarian cancer cohort, 4 patients (25%) had resolution of the small bowel obstruction; however, details on number of lines and chemotherapy protocols for these patients specifically were not reported.97
Patients with MBO are often malnourished and may be unable to tolerate standard chemotherapy protocols; hence, dose-modification and weekly protocols can be considered. Based on where the patients with MBO are in their disease trajectory and clinical assessment, including ECOG performance status, tempo of disease progression and their bone marrow reserve, weekly carboplatin-paclitaxel or weekly paclitaxel are some of the suggested regimens.97
Treatment needs to be tailored based on goals of care on a case-by-case basis. While patients who are heavily pre-treated and have bulky chemo-resistant peritoneal deposits would benefit from a conservative approach focused on de-escalation of care and symptom management to avoid therapeutic morbidity, it is important to avoid under-treatment of carefully selected patients who are newly diagnosed, healthier or have a good performance status.98,99 Overall, the evidence to support the use of chemotherapy in patients with advanced gynecologic cancers who developed MBO is still limited, and decisions must be tailored on a case-by-case basis, preferable after multi-disciplinary discussion.
Role of Multi-Disciplinary Management
Every patient with a diagnosis of MBO should be assessed by the palliative care team. Multi-disciplinary team approach for the management of patients with MBO has shown to be associated with improved outcomes such as shorter hospital stays and better survival.98 The team generally includes the expertise of surgeons specializing in gastro-intestinal and gynecologic oncology, medical oncologists, specialists in TPN, nurses or nurse-practitioners with special interest in MBO, interventional radiologists, palliative care, and wherever needed, radiation oncologists and infectious disease specialists.
A retrospective analysis from a tertiary care center in Toronto, Canada, which included 107 patients with gynecologic cancer admitted for malignant bowel obstruction, showed that patients who received active management with a dedicated team had improved outcome.100 A well-planned and coordinated inter-professional team approach ideally includes nurse-led outpatient management, a well devised, easily accessible and implementable inpatient treatment algorithm, patient-directed bowel management educational material and multi-disciplinary case conferences (MCCs).101
Supported Self-Management
Given the complexity in managing MBO, several studies assert the importance of a multidisciplinary approach to care and engaging patients on self-management strategies.23,29,34,100–102 Castro et al conducted a retrospective cohort study of ovarian cancer patients with MBO using a protocol management tool developed by their multidisciplinary team, to ascertain patient’s clinical progression. They found patients can experience recurrent episodes of MBO prior to death and suggest engaging in a multidisciplinary team approach using evidence-based care pathways to improve the management of patients with MBO.102
The development of an outpatient interprofessional MBO program for gynecologic patients is feasible and was established in a tertiary cancer centre by some authors of this review.101 Patients enrolled in the program receive teaching and educational materials from the nurse on how to (1) recognize MBO symptoms; (2) manage with diet and laxatives; (3) and when to seek support and receive nursing proactive bowel management calls. Lee et al conducted a retrospective analysis pre/post MBO program implementation and found patients had a decrease in length of stay and increase in overall survival validating an improvement in clinical outcomes using this model of care (MOC).101
To evaluate the patient’s perspective on the impact of this outpatient MBO MOC program in their ability to self-manage bowel function and the physical and psychological supports received, Cusimano et al conducted a qualitative descriptive study using semi-structured interviews patients enrolled in the study. The results showed that patients felt empowered to self-manage their bowel function through the education they received on nutritional and laxative management, in addition to understanding symptoms and causes of MBO.34 Most importantly, patients with MBO expressed fear and anxiety related to worsening symptoms but felt that the proactive management with nursing calls and physician interventions decreased their degree of distress and provided a sense of security when seeking support.34
These data highlight the importance of pro-active management of MBO and patient education regarding bowel management and risk of MBO.
Impact
Patient and Family: Quality of Life (QOL)
As discussed, MBO can involve surgical and/or medical management and is considered a pre-terminal event.23,34,100 The physical burden compounded by the psychological distress that both patients and their caregivers endure impacts their QOL. Considering the severity of this disease and complication with MBO, it is imperative that clinicians establish goals of care with their patients to optimize QOL.6,29,34,103,104 Sun et al and Lowe et al refer to QOL as a personal evaluation of one’s life’s challenges and achievements and their psychological, physical, and social well-being following treatment of a medical condition.105,106 A few studies report several quantitative QOL measurement tools such as the European Organization for Research Quality of Life Core Questionnaire 30 (EORTC-QLQ-C30); the Functional Assessment of Cancer Therapy (FACT-G), which also has an ovarian cancer subscale (FACT-O).105–107 According to Shariff et al, research on the impact of MBO using these QOL tools is lacking.107
The qualitative studies reviewed found that despite physical symptom improvement through surgical and/or medical management, the psychological burden remains high.34,103,104,106 Patients with MBO reported significant anxiety, fear and powerlessness during and after an MBO episode.36,105,106
Patients also reported a sense of social and emotional loss/isolation among family and friends with regard to nutritional intake. Food can impact an individual’s QOL as it serves as a source of socialization bringing family and friends together in daily living. A restricted diet, particularly for those with a venting gastrostomy tube or TPN, contributed to disengagement in mealtimes leading to isolation and loss.34,103,104 Although some patients reported TPN improved their QOL as they were able to spend more time at home with their families, they still faced challenges with body image and felt they were a burden to their families.6,29,34,103,104
The American Society of Clinical Oncology (ASCO) supports introducing palliative care early on with guidelines for patients with advanced metastatic cancer.108,109 Miller et al reviewed initiation of palliative care in ovarian cancer patients and found a need to standardize screening to optimize care as research indicates that most patients are diagnosed at an advanced stage and have a high recurrence rate.110
Establishing goals of care and timely discussion of palliative care has been the consensus among several authors.6,29,74,105,111–113 Communication with patients that an MBO diagnosis is considered a pre-terminal event, particularly at the time of platinum-resistant ovarian cancer, is imperative to alleviate any misperceptions. For instance, Shariff et al conducted a literature review on the management of MBO and found that patients who received interventions improving their symptoms perceived they had a better overall survival rate.107 In addition, Frey et al found that patients with recurrent disease prioritize survival before QOL and 27% would accept an MBO episode if it meant they would eventually be cured.111 These misperceptions may be attributed to a gap in communication.
One qualitative study specifically assessing patient-physician communication found that patients admitted with MBO were frustrated at the paucity of information provided on their prognosis and management and that physicians were too negative in their communication. Interestingly, physicians felt they could not communicate an effective response to the patient’s prognosis given the complexity in management with MBO and conveying realistic expectations without being negative.113 Clinicians must be transparent that treatment in this setting is not curative, and the importance of balancing treatment-related toxicities and the associated risks with interventions must be considered and discussed to optimize their QOL.111,112,114
Palliative care is a sensitive discussion, and patients have attested that it is considered a death sentence.34 Communicating with patients that a referral to palliative care in advance will aid in prioritizing goals of care, improve symptom relief, QOL and provide support for caregivers. Furthermore, patients should be made aware that palliative care provides a holistic approach, encompassing all aspects of the patient not just physical and psychosocial but spiritual and cultural as well.29,110–112,114 Patients with an MBO diagnosis would greatly benefit from the support, knowledge and expertise that palliative care teams can provide through their disease trajectory.
Impact of MBO on Health Care Expenditure
A retrospective analysis by Lee et al showed that implementation of systematic interprofessional management of MBO and patient education significantly reduced the duration of inpatient admission, thereby reducing the expenditure per hospital admission as well as the cost per patient (including multiple hospital admissions).101,115
Conclusion
While MBO is a common and significant complication for patients with gynecological cancers, especially advanced and recurrent ovarian cancer, there is a lack of evidence-based management protocols, which stresses upon the need for clinical research initiatives.
Patients with inoperable MBO are treated medically with analgesics, antiemetics, steroids and antisecretory agents. Gut decompression with nasogastric tube, venting gastrostomy or stenting are used as supportive therapy. Intravenous hydration, parenteral nutrition and palliative chemotherapy may derive benefit in a subgroup of patients; careful selection is critical to ensure the best possible clinical outcome.
Timely, systematic multidisciplinary and pro-active approach alongside patient education have been shown to add clarity to the management of complex cases. Efforts are needed to help optimize outcomes in this unique patient population.
Disclosure
Dr Stephanie Lheureux reports grants, personal fees from Astra-Zeneca, grants, personal fees from GSK, grants from Roche, personal fees from Eisai, grants, personal fees from Merck, personal fees from Novartis, outside the submitted work.
The authors report no other conflicts of interest in this work.
References
1. Anthony T, Baron T, Mercadante S., et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34(1):S49–59. doi:10.1016/j.jpainsymman.2007.04.011
2. Cousins SE, Tempest E, Feuer DJ. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev. 2016;3:1.
3. Shariat-Madar B, Jayakrishnan TT, Gamblin TC, Turaga KK. Surgical management of bowel obstruction in patients with peritoneal carcinomatosis. J Surg Oncol. 2014;110(6):666–669. doi:10.1002/jso.23707
4. Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4(1):159–169. doi:10.2147/CMAR.S29297
5. Torreggiani WC, Harris AC, Lyburn ID, et al. Computed tomography of acute small bowel obstruction: pictorial essay. Can Assoc Radiol J. 2003;54(2):93–99.
6. Lee YC, Jivraj N, O’Brien C, et al. Malignant Bowel Obstruction in Advanced Gynecologic Cancers: an Updated Review from a Multidisciplinary Perspective. Obstet Gynecol Int. 2018;2018:1867238. doi:10.1155/2018/1867238
7. Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer. 2002;12(2):135–143.
8. Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44(8):1105–1115. doi:10.1016/j.ejca.2008.02.028
9. Huang X, Xue J, Gao M, et al. Medical management of inoperable malignant bowel obstruction. Ann Pharmacother. 2021;55(9):1134–1145. doi:10.1177/1060028020979773
10. Gore RM, Silvers RI, Thakrar KH, et al. Bowel Obstruction. Radiol Clin North Am. 2015;53(6):1225–1240. doi:10.1016/j.rcl.2015.06.008
11. Paulson EK, Thompson WM. Review of small-bowel obstruction: the diagnosis and when to worry. Radiology. 2015;275(2):332–342. doi:10.1148/radiol.15131519
12. Jaffe T, Thompson WM. Large-Bowel Obstruction in the Adult: classic Radiographic and CT Findings, Etiology, and Mimics. Radiology. 2015;275(3):651–663. doi:10.1148/radiol.2015140916
13. Chang KJ, Marin D, Kim DH, et al. ACR Appropriateness Criteria® Suspected Small-Bowel Obstruction. J Am Coll Radiol. 2020;17(5):S305–s314. doi:10.1016/j.jacr.2020.01.025
14. Suri S, Gupta S, Sudhakar PJ, Venkataramu NK, Sood B, Wig JD. Comparative evaluation of plain films, ultrasound and CT in the diagnosis of intestinal obstruction. Acta Radiol. 1999;40(4):422–428. doi:10.3109/02841859909177758
15. Thompson WM, Kilani RK, Smith BB, et al. Accuracy of abdominal radiography in acute small-bowel obstruction: does reviewer experience matter? AJR Am J Roentgenol. Mar. 2007;188(3):W233–8. doi:10.2214/ajr.06.0817
16. Pothuri B, Vaidya A, Aghajanian C, Venkatraman E, Barakat RR, Chi DS. Palliative surgery for bowel obstruction in recurrent ovarian cancer: an updated series. Gynecol Oncol. 2003;89(2):306–313. doi:10.1016/s0090-8258(03)00073-8
17. Badgwell BD, Contreras C, Askew R, Krouse R, Feig B, Cormier JN. Radiographic and clinical factors associated with improved outcomes in advanced cancer patients with bowel obstruction. J Palliat Med. 2011;14(9):990–996. doi:10.1089/jpm.2011.0083
18. Dalal KM, Gollub MJ, Miner TJ, et al. Management of patients with malignant bowel obstruction and stage IV colorectal cancer. J Palliat Med. 2011;14(7):822–828. doi:10.1089/jpm.2010.0506
19. Henry JC, Pouly S, Sullivan R, et al. A scoring system for the prognosis and treatment of malignant bowel obstruction. Surgery. 2012;152(4):747–756. doi:10.1016/j.surg.2012.07.009
20. Kolomainen DF, Daponte A, Barton DP, et al. Outcomes of surgical management of bowel obstruction in relapsed epithelial ovarian cancer (EOC). Gynecol Oncol. 2012;125(1):31–36. doi:10.1016/j.ygyno.2011.11.007
21. Lodoli C, Covino M. Prognostic factors for surgical failure in malignant bowel obstruction and peritoneal carcinomatosis. Front Surg. 2021;8:769658. doi:10.3389/fsurg.2021.769658
22. Perri T, Korach J, Ben-Baruch G, et al. Bowel obstruction in recurrent gynecologic malignancies: defining who will benefit from surgical intervention. Eur J Surg Oncol. 2014;40(7):899–904. doi:10.1016/j.ejso.2013.10.025
23. Daniele A, Ferrero A, Fuso L, et al. Palliative care in patients with ovarian cancer and bowel obstruction. Support Care Cancer. 2015;23(11):3157–3163. doi:10.1007/s00520-015-2694-9
24. Micco M, Sbarra M, Gui B, Bianco NC, Rodolfino E, Manfredi R. Prognostic CT findings of malignant bowel obstruction in patients with advanced ovarian cancer. Tumori. 2020;106(2):149–154. doi:10.1177/0300891619886657
25. Bateni SB, Gingrich AA, Kirane AR, et al. Chemotherapy After Diagnosis of Malignant Bowel Obstruction is Associated with Superior Survival for Medicare Patients with Advanced Malignancy. Ann Surg Oncol. 2021;28(12):7555–7563. doi:10.1245/s10434-021-09831-0
26. Helyer LK, Law CH, Butler M, Last LD, Smith AJ, Wright FC. Surgery as a bridge to palliative chemotherapy in patients with malignant bowel obstruction from colorectal cancer. Ann Surg Oncol. 2007;14(4):1264–1271. doi:10.1245/s10434-006-9303-6
27. Yu K, Liu L, Zhang X, et al. Surgical and Conservative Management of Malignant Bowel Obstruction: outcome and Prognostic Factors. Cancer Manag Res. 2020;12:7797–7803. doi:10.2147/cmar.S256219
28. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–32. doi:10.1093/annonc/mdt333
29. Madariaga A, Lau J, Ghoshal A, et al. MASCC multidisciplinary evidence-based recommendations for the management of malignant bowel obstruction in advanced cancer. Support Care Cancer. 2022;30(6):4711–4728. doi:10.1007/s00520-022-06889-8
30. Bozzetti F. The role of parenteral nutrition in patients with malignant bowel obstruction. Support Care Cancer. 2019;27(12):4393–4399. doi:10.1007/s00520-019-04948-1
31. Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: clinical Nutrition in cancer. Clin Nutr. 2021;40(5):2898–2913. doi:10.1016/j.clnu.2021.02.005
32. Scotland N Scottish palliative care guidelines. Bowel Obstruction. NHS Scotland; 2022. Available from: https://www.palliativecareguidelines.scot.nhs.uk/.guidelines/symptom-control/bowel-obstruction.aspx.
33. Vanhauwaert E, Matthys C, Verdonck L, De Preter V. Low-residue and low-fiber diets in gastrointestinal disease management. Adv Nutr. 2015;6(6):820–827. doi:10.3945/an.115.009688
34. Cusimano MC, Sajewycz K, Nelson M, et al. Supported self-management as a model for end-of-life care in the setting of malignant bowel obstruction: a qualitative study. Research Support, Non-U.S. Gov’t. Gynecol Oncol. 2020;157(3):745–753. doi:10.1016/j.ygyno.2020.03.009
35. Druml C, Ballmer PE, Druml W, et al. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr. 2016;35(3):545–556. doi:10.1016/j.clnu.2016.02.006
36. Coelho TA, Wainstein AJA, Drummond-Lage AP. Hypodermoclysis as a Strategy for Patients With End-of-Life Cancer in Home Care Settings. Am J Hosp Palliat Care. 2020;37(9):675–682. doi:10.1177/1049909119897401
37. Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage. 2000;19(1):23–34. doi:10.1016/s0885-3924(99)00147-5
38. Lokker ME, van der Heide A, Oldenmenger WH, van der Rijt CCD, van Zuylen L. Hydration and symptoms in the last days of life. BMJ Support Palliat Care. 2021;11(3):335–343. doi:10.1136/bmjspcare-2018-001729
39. Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31(1):111–118. doi:10.1200/jco.2012.44.6518
40. Davies AN, Waghorn M, Webber K, Johnsen S, Mendis J, Boyle J. A cluster randomised feasibility trial of clinically assisted hydration in cancer patients in the last days of life. Palliat Med. 2018;32(4):733–743. doi:10.1177/0269216317741572
41. Crawford GB, Dzierżanowski T, Hauser K, et al. Care of the adult cancer patient at the end of life: ESMO Clinical Practice Guidelines. ESMO Open. 2021;6(4):100225. doi:10.1016/j.esmoop.2021.100225
42. Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34(5):825–837. doi:10.1016/j.clnu.2014.09.010
43. Bozzetti F, Cotogni P, Lo Vullo S, Pironi L, Giardiello D, Mariani L. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann Oncol. 2015;26(11):2335–2340. doi:10.1093/annonc/mdv365
44. Messing B, Lémann M, Landais P, et al. Prognosis of patients with nonmalignant chronic intestinal failure receiving long-term home parenteral nutrition. Gastroenterology. 1995;108(4):1005–1010. doi:10.1016/0016-5085(95)
45. Bozzetti F, Santarpia L, Pironi L, et al. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: a multi-centre observational study with prospective follow-up of 414 patients. Ann Oncol. 2014;25(2):487–493. doi:10.1093/annonc/mdt549
46. Jairam V, Lee V, Park HS, et al. Treatment-Related Complications of Systemic Therapy and Radiotherapy. JAMA Oncol. 2019;5(7):1028–1035. doi:10.1001/jamaoncol.2019.0086
47. Shim J, Seo TS, Song MG, et al. Incidence and risk factors of infectious complications related to implantable venous-access ports. Korean J Radiol. 2014;15(4):494–500. doi:10.3348/kjr.2014.15.4.494
48. Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–130. doi:10.1002/pon.3409
49. Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer. 2019;19(1):943. doi:10.1186/s12885-019-6181-4
50. Caillet P, Liuu E, Raynaud Simon A, et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin Nutr. 2017;36(6):1473–1482. doi:10.1016/j.clnu.2016.12.003
51. Sowerbutts AM, Lal S, Sremanakova J, et al. Palliative home parenteral nutrition in patients with ovarian cancer and malignant bowel obstruction: experiences of women and family caregivers. BMC Palliat Care. 2019;18(1):120. doi:10.1186/s12904-019-0507-5
52. Hartl WH, Jauch KW, Parhofer K, Rittler P. Complications and monitoring - Guidelines on Parenteral Nutrition, Chapter 11. Ger Med Sci. 2009;7:Doc17. doi:10.3205/000076
53. Klein S. A primer of nutritional support for gastroenterologists. Gastroenterology. 2002;122(6):1677–1687. doi:10.1053/gast.2002.33574
54. Ni J, Zhang L. Cancer Cachexia: definition, Staging, and Emerging Treatments. Cancer Manag Res. 2020;12:5597–5605. doi:10.2147/cmar.S261585
55. Oh SY, Jun HJ, Park SJ, et al. A randomized phase II study to assess the effectiveness of fluid therapy or intensive nutritional support on survival in patients with advanced cancer who cannot be nourished via enteral route. J Palliat Med. 2014;17(11):1266–1270. doi:10.1089/jpm.2014.0082
56. Pothuri B, Meyer L, Gerardi M, Barakat RR, Chi DS. Reoperation for palliation of recurrent malignant bowel obstruction in ovarian carcinoma. Gynecol Oncol. 2004;95(1):193–195. doi:10.1016/j.ygyno.2004.07.028
57. Furnes B, Svensen R, Helland H, Ovrebo K. Challenges and outcome of surgery for bowel obstruction in women with gynaecologic cancer. Int J Surg. 2016;27:158–164. doi:10.1016/j.ijsu.2016.02.002
58. Paul Olson TJ, Pinkerton C, Brasel KJ, Schwarze ML. Palliative surgery for malignant bowel obstruction from carcinomatosis: a systematic review. JAMA Surg. 2014;149(4):383–392. doi:10.1001/jamasurg.2013.4059
59. Smith BP. Surgery Improves Survival Among Patients with Intestinal Obstruction. Temple University; 2010.
60. Chi DS, Phaeton R, Miner TJ, et al. A prospective outcomes analysis of palliative procedures performed for malignant intestinal obstruction due to recurrent ovarian cancer. Research Support, Non-U.S. Gov’t. Oncologist. 2009;14(8):835–839. doi:10.1634/theoncologist.2009-0057
61. Bryan DN, Radbod R, Berek JS. An analysis of surgical versus chemotherapeutic intervention for the management of intestinal obstruction in advanced ovarian cancer. Int J Gynecol Cancer. 2006;16(1):125–134. doi:10.1111/j.1525-1438.2006.00283.x
62. Kolomainen DF, Barton DP. Surgical management of bowel obstruction in gynaecological malignancies. Curr Opin Support Palliat Care. 2011;5(1):55–59. doi:10.1097/SPC.0b013e3283436d1b
63. Armbrust R, Chekerov R, Sander S, et al. Surgery due to mechanical bowel obstruction in relapsed ovarian cancer: clinical and surgical results of a bicentric analysis of 87 patients. Arch Gynecol Obstet. 2022;305(4):963–968. doi:10.1007/s00404-021-06237-x
64. Krebs HB, Goplerud DR. Surgical management of bowel obstruction in advanced ovarian carcinoma. Obstet Gynecol. 1983;61(3):327–330.
65. Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48(1):75–91. doi:10.1016/j.jpainsymman.2013.08.022
66. de Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017;31(4):499–504. doi:10.1016/j.bpa.2017.07.002
67. Dolan EA. Malignant bowel obstruction: a review of current treatment strategies. Am J Hosp Palliat Care. 2011;28(8):576–582. doi:10.1177/1049909111406706
68. Prabhakaran S, Doraiswamy VA, Nagaraja V, et al. Nasoenteric tube complications. Scand J Surg. 2012;101(3):147–155. doi:10.1177/145749691210100302
69. Aramaki T, Arai Y, Takeuchi Y, et al. A randomized, controlled trial of the efficacy of percutaneous transesophageal gastro-tubing (PTEG) as palliative care for patients with malignant bowel obstruction: the JIVROSG0805 trial. Support Care Cancer. 2020;28(6):2563–2569. doi:10.1007/s00520-019-05066-8
70. Thampy S, Najran P, Mullan D, Laasch HU. Safety and Efficacy of Venting Gastrostomy in Malignant Bowel Obstruction: a Systematic Review. J Palliat Care. 2020;35(2):93–102. doi:10.1177/0825859719864915
71. Shaw C, Bassett RL, Fox PS, et al. Palliative venting gastrostomy in patients with malignant bowel obstruction and ascites. Ann Surg Oncol. 2013;20(2):497–505. doi:10.1245/s10434-012-2643-5
72. Keswani RN, Azar RR, Edmundowicz SA, et al. Stenting for malignant colonic obstruction: a comparison of efficacy and complications in colonic versus extracolonic malignancy. Gastrointest Endosc. 2009;69(3 Pt 2):675–680. doi:10.1016/j.gie.2008.09.009
73. Shin SJ, Kim TI, Kim BC, Lee YC, Song SY, Kim WH. Clinical application of self-expandable metallic stent for treatment of colorectal obstruction caused by extrinsic invasive tumors. Dis Colon Rectum. 2008;51(5):578–583. doi:10.1007/s10350-008-9207-6
74. Yeo CT, Merchant SJ. Considerations in the Management of Malignant Bowel Obstruction. Surg Oncol Clin N Am. 2021;30(3):461–474. doi:10.1016/j.soc.2021.02.003
75. Ferguson HJ, Ferguson CI, Speakman J, Ismail T. Management of intestinal obstruction in advanced malignancy. Ann Med Surg. 2015;4(3):264–270. doi:10.1016/j.amsu.2015.07.018
76. Davis MP, Hallerberg G. A systematic review of the treatment of nausea and/or vomiting in cancer unrelated to chemotherapy or radiation. J Pain Symptom Manage. 2010;39(4):756–767. doi:10.1016/j.jpainsymman.2009.08.010
77. Landrum LM, Blank S, Chen LM, et al. Comprehensive care in gynecologic oncology: the importance of palliative care. Gynecol Oncol. 2015;137(2):193–202. doi:10.1016/j.ygyno.2015.02.026
78. Hsu K, Prommer E, Murphy MC, Lankarani-Fard A. Pharmacologic management of malignant bowel obstruction: when surgery is not an option. J Hosp Med. 2019;14(6):367–373. doi:10.12788/jhm.3187
79. Hardy J, Ling J, Mansi J, et al. Pitfalls in placebo-controlled trials in palliative care: dexamethasone for the palliation of malignant bowel obstruction. Palliat Med. 1998;12(6):437–442. doi:10.1191/026921698666334766
80. Laval G, Girardier J, Lassaunière JM, Leduc B, Haond C, Schaerer R. The use of steroids in the management of inoperable intestinal obstruction in terminal cancer patients: do they remove the obstruction? Palliat Med. Jan. 2000;14(1):3–10. doi:10.1191/026921600669298725
81. Jones APM, McGauran MFG, Jagasia N, Hiscock RJ, Hyde S, Grant P. Efficacy of dexamethasone in the management of malignant small bowel obstruction in advanced epithelial ovarian cancer. Support Care Cancer. 2022;30(3):2821–2827. doi:10.1007/s00520-021-06694-9
82. Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev. 2017;6:548.
83. Cape JD, Beca JM, Hoch JS. Introduction to Cost-Effectiveness Analysis for Clinicians. Univ Toronto Med J. 2013;90:3.
84. Obita GP, Boland EG, Currow DC, Johnson MJ, Boland JW. Somatostatin Analogues Compared With Placebo and Other Pharmacologic Agents in the Management of Symptoms of Inoperable Malignant Bowel Obstruction: a Systematic Review. J Pain Symptom Manage. 2016;52(6):901–919.e1. doi:10.1016/j.jpainsymman.2016.05.032
85. Duck L, Demolin G, D’Hondt L, et al. Efficacy and Safety of Lanreotide Autogel in the Treatment of Clinical Symptoms Associated With Inoperable Malignant Intestinal Obstruction: a Prospective Phase II Study. Clin Ther. 2021;43(12):2136–2145.e2. doi:10.1016/j.clinthera.2021.10.014
86. Laval G, Rousselot H, Toussaint-Martel S, et al. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer. 2012;99(2):E1–9. doi:10.1684/bdc.2011.1535
87. Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double- blind, controlled clinical trial. Anticancer Res. 2002;22(2b):1187–1192.
88. De Conno F, Caraceni A, Zecca E, Spoldi E, Ventafridda V. Continuous subcutaneous infusion of hyoscine butylbromide reduces secretions in patients with gastrointestinal obstruction. J Pain Symptom Manage. 1991;6(8):484–486. doi:10.1016/0885-3924(91)
89. Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(Suppl4):iv111–iv125. doi:10.1093/annonc/mdy148
90. Carlson CL. Effectiveness of the World Health Organization cancer pain relief guidelines: an integrative review. J Pain Res. 2016;9:515–534. doi:10.2147/jpr.S97759
91. Stiefel F, Die Trill M, Berney A, Olarte JM, Razavi A. Depression in palliative care: a pragmatic report from the Expert Working Group of the European Association for Palliative Care. Support Care Cancer. 2001;9(7):477–488. doi:10.1007/s005200100244
92. Wickham RJ. Nausea and Vomiting: a Palliative Care Imperative. Curr Oncol Rep. 2020;22(1):1. doi:10.1007/s11912-020-0871-6
93. Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage. 2004;28(4):412–416. doi:10.1016/j.jpainsymman.2004.01.007
94. Hisanaga T, Shinjo T, Imai K, et al. Clinical Guidelines for Management of Gastrointestinal Symptoms in Cancer Patients: the Japanese Society of Palliative Medicine Recommendations. J Palliat Med. 2019;22(8):986–997. doi:10.1089/jpm.2018.0595
95. Diver E, O’Connor O, Garrett L, et al. Modest benefit of total parenteral nutrition and chemotherapy after venting gastrostomy tube placement. Gynecol Oncol. 2013;129(2):332–335. doi:10.1016/j.ygyno.2013.02.002
96. Brard L, Weitzen S, Strubel-Lagan SL, et al. The effect of total parenteral nutrition on the survival of terminally ill ovarian cancer patients. Gynecol Oncol. 2006;103(1):176–180. doi:10.1016/j.ygyno.2006.02.013
97. Chouhan J, Gupta R, Ensor J, et al. Retrospective analysis of systemic chemotherapy and total parenteral nutrition for the treatment of malignant small bowel obstruction. Cancer Med. 2016;5(2):239–247. doi:10.1002/cam4.587
98. Bateni SB, Gingrich AA, Stewart SL, Meyers FJ, Bold RJ, Canter RJ. Hospital utilization and disposition among patients with malignant bowel obstruction: a population-based comparison of surgical to medical management. BMC Cancer. 2018;18(1):1166. doi:10.1186/s12885-018-5108-9
99. Pujara D, Chiang YJ, Cormier JN, Bruera E, Badgwell B. Selective approach for patients with advanced malignancy and gastrointestinal obstruction. J Am Coll Surg. 2017;225(1):53–59. doi:10.1016/j.jamcollsurg.2017.04.033
100. Tigert M, Lau C, Mackay H, L’Heureux S, Gien LT. Factors impacting length of stay and survival in patients with advanced gynecologic malignancies and malignant bowel obstruction. Research Support, Non-U.S. Gov’t. Int J Gynecol Cancer. 2021;31(5):727–732. doi:10.1136/ijgc-2020-002133
101. Lee YC, Jivraj N, Wang L, et al. Optimizing the Care of Malignant Bowel Obstruction in Patients With Advanced Gynecologic Cancer. Research Support, Non-U.S. Gov’t. J Oncol Practice. 2019;15(12):e1066–e1075. doi:10.1200/JOP.18.00793
102. Martinez Castro P, Vargas L, Mancheno A, et al. Malignant bowel obstruction in relapsed ovarian cancer with peritoneal carcinomatosis: an occlusive state. Int J Gynecol Cancer. 2017;27(7):1367–1372. doi:10.1097/IGC.0000000000001049
103. Baddeley E, Mann M, Bravington A, et al. Symptom burden and lived experiences of patients, caregivers and healthcare professionals on the management of malignant bowel obstruction: a qualitative systematic review. Palliat Med. 2022:2692163221081331. doi:10.1177/02692163221081331
104. Sowerbutts AM, Lal S, Sremanakova J, et al. Discharging women with advanced ovarian cancer on home parenteral nutrition: making and implementing the decision. Nutrients. 2020;12(1):07. doi:10.3390/nu12010166
105. Sun CC, Ramirez PT, Bodurka DC. Quality of life for patients with epithelial ovarian cancer. Nat Clin Pract Oncol. 2007;4(1):18–29. doi:10.1038/ncponc0693
106. Lowe T, Ferrell B, Leong L. Quality-of-life issues in the management of epithelial ovarian cancer. Curr Treat Options Oncol. 2007;8(6):402–416. doi:10.1007/s11864-007-0049-6
107. Shariff F, Bogach J, Guidolin K, Nadler A. Malignant Bowel Obstruction Management Over Time: are We Doing Anything New? A Current Narrative Review. Ann Surg Oncol. 2022;29(3):1995–2005.
108. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880–887. doi:10.1200/jco.2011.38.5161
109. Ferrell BR, Temel JS, Temin S, Smith TJ. Integration of Palliative Care Into Standard Oncology Care: ASCO Clinical Practice Guideline Update Summary. J Oncol Pract. 2017;13(2):119–121. doi:10.1200/jop.2016.017897
110. Miller D, Nevadunsky N. Palliative care and symptom management for women with advanced ovarian cancer. Hematology. 2018;32(6):1087–1102. doi:10.1016/j.hoc.2018.07.012
111. Frey MK, Ellis AE, Koontz LM, et al. Ovarian cancer survivors’ acceptance of treatment side effects evolves as goals of care change over the cancer continuum. Gynecol Oncol. 2017;146(2):386–391. doi:10.1016/j.ygyno.2017.05.029
112. Gabriel E, Kukar M, Groman A, et al. Care Service Improves the Quality of Care in Patients with Stage IV Cancer and Bowel Obstruction. Am J Hosp Palliat Care. 2017;34(1):20–25. doi:10.1177/1049909115603960
113. Hoppenot C, Hlubocky FJ, Chor J, Yamada SD, Lee NK. Gaps in patient-physician communication at the time of malignant bowel obstruction from recurrent gynecologic cancer: a qualitative study. Support Care Cancer. 2022;30(1):367–376. doi:10.1007/s00520-021-06441-0
114. Holmes C, Mitchell A, Downham E. Palliative care in gynaecological oncology. Obstetrics Gynaecol Reproductive Med. 2021;31(3):77–83. doi:10.1016/j.ogrm.2021.01.002
115. Centre PMC. Bowel obstruction for women with gynecological cancer; 2022. Available from: https://www.youtube.com/watch?v=4iYs14htuxg&list=PLaLgrtXadEF8JI-TtwYcaaYM1DnxksUI8.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.