Back to Journals » Journal of Pain Research » Volume 13
Injection of Bupivacaine into the Pleural and Mediastinal Drains: A Novel Approach for Decreasing Incident Pain After Cardiac Surgery – Montreal Heart Institute Experience
Authors Cogan J, André M, Ariano-Lortie G, Nozza A , Raymond M, Rochon A, Vargas-Shaffer G
Received 27 August 2020
Accepted for publication 17 November 2020
Published 16 December 2020 Volume 2020:13 Pages 3409—3413
DOI https://doi.org/10.2147/JPR.S279071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Jennifer Cogan,1 Maud André,2 Gabrielle Ariano-Lortie,2 Anna Nozza,3 Meggie Raymond,1 Antoine Rochon,1 Grisell Vargas-Shaffer4
1Department of Anesthesiology, Montreal Heart Institute, Université de Montréal, Montreal, QC H1T 1C8, Canada; 2Department of Nursing, Montreal Heart Institute, Montreal, QC H1T 1C8, Canada; 3Montreal Health Innovations Coordinating Center, Montreal Heart Institute, Montreal, QC H1Y 3N1, Canada; 4Department of Anesthesiology, Centre Hospitalier de l’Université de Montréal, Montreal, QC H2X 3E4, Canada
Correspondence: Jennifer Cogan
Montreal Heart Institute, 5000 Belanger, Montreal, Quebec H1T 1C8, Canada
Tel +1 514-376-3330
Fax +1 514-376-8784
Email [email protected]
Background: We conducted a chart review of prospectively collected data in order to demonstrate the safety and efficacy of an innovative technique of pleural and mediastinal drain injections.
Methods: Patients who had undergone cardiac surgery and who continued to have pain despite the use of a multimodal pain protocol received injections of 20 mL of 0.25% bupivacaine in pleural and/or mediastinal chest drainage tubes.
Results: Patients were evaluated for the incidence mediastinitis, osteitis, and deep sternal wound infection as well as the speed and intensity of pain relief. The odds ratio of infection in the infused group was 0.955 (CI = 0.4705, 1.9384). The adjusted mean “decrease in pain” was 4.01 (SEM = 0.15 and 95% CI = 3.78, 4.38), using the 11-point Likert Numerical Rating Scale. The mean adjusted “time to maximum pain relief” was 8.33 minutes (SEM = 0.42 and 95% CI = 7.50, 9.15).
Conclusion: This technique is a powerful, safe, and efficient tool in the armamentarium of pain management and its growing use within our institution has provided a substantial benefit in the treatment of early post-operative pain.
Keywords: acute pain service, bupivacaine, cardiac surgery, pain management, pleural drain, safety considerations
Plain Language Summary
After innovating a new technique of post-operative pain management in cardiac surgery, we undertook to evaluate our results using data that we had collected prospectively on a daily basis. After cardiac surgery, pleural drains are left in place for 2–4 days to evacuate excess fluid. However, for some of our patients, the drains are often the source of severe discomfort even though all our patients receive an individually tailored multimodal pain regimen of acetaminophen, NSAID’s, and self-administered opioids. Faced with this problem we decided to use the drains to inject a local anesthetic to numb the pleural area in contact with the drains. We used 20 mL of 0.25 bupivacaine. Our team found that this technique offered rapid and significant pain relief in under 10 minutes and, contrary to the surgeon’s worries, did not contribute to increased infection at the wound site. In fact, it was so rapid, efficient, and safe that we adopted it without reservation for all patients who continue to have unrelieved pain thought to be caused by pleural drains.
Introduction
The intensity of pain immediately after cardiac surgery varies significantly between patients.1,2 While for some the experience is not painful for others it is impossible to achieve a reasonable level of comfort. This is important as studies have shown that high levels of acute post-operative pain are correlated with a high prevalence of chronic post-operative pain.3
For many patients, one of the main sources of post-operative cardiac pain is the presence of pleural and mediastinal drains inserted by the surgeon at the end of surgery and maintained for 2 −3 days. This pain is often described as sharp, piercing, radiating to the back or shoulder, and occurring upon breathing, movement, or coughing. It generally remains unrelieved despite the administration of medications.
Intrapleural and chest tube-delivered bupivacaine, both infusion and bolus dosing, were first described by Reiestad et al4 in 1986 and have been used in many circumstances since. It has been used after various procedures such as post-thoracoscopy,5 post-thoracotomy,6–8 after hepatic resection,9 for upper abdominal surgery,10 and as analgesia during chest drainage treatment for pneumothorax.5 In most of these procedures, the catheters are placed under sterile conditions and left in place for several days allowing for repeated use. Occasionally, bolus doses are injected through the pleural infusion channel of a specially designed silicone chest tube.5 The literature has shown that, overall, this technique is successful in decreasing pain4–8,11–14 and has a low rate of complications such as pneumothorax, hemothorax, empyema, systemic analgesic toxicity, infection or Horner’s syndrome,10 although there is one case report of ventricular standstill with intrapleural bupivacaine.15 Despite the variety of uses of bupivacaine that have been described no publication has discussed the injection of bupivacaine directly into a regular drainage chest tube, in the setting of cardiac surgery, for the treatment of pain caused by the chest tube. There is one report on the use of bilateral intrapleural lidocaine and fentanyl after cardiac surgery.16
Faced with uncontrollable/unrelieved pain in some patients, despite the application of a full multimodal analgesia protocol, the acute pain service team embarked on an unexplored avenue to pain management. We hypothesized that the instillation of local anesthetic into pleural and mediastinal drains would simultaneously decrease pain and opioid requirements. We report here on the technique, its safety, and efficacy.
Methods
This paper presents a chart review of prospectively collected data using acute pain and hospital infection records by the hospital Infection Control Service for all patients between May 9, 2016, and September 5, 2018. Ethics permission from the Montreal Heart Institute was obtained for the review of hospital charts. The identification of patients who had received injections was possible due to the computerized pain database in operation since 2009. The study sample included 100% of the patients undergoing surgery during this time who continued to complain of uncontrolled pain despite the use of the institutional multimodal pain protocol which included, but was not limited to, acetaminophen, NSAIDs, and self-administered opioids, and who received an injection of bupivacaine. The sample can therefore be described as a convenience sample. The patients who received pleural injections all underwent cardiac surgery using a sternotomy approach. Pain was assessed using an 11-point (0 to 10) Likert Numerical Rating Scale at regular 4-hour intervals; however, the pain scores that were recorded in the database were all taken between 08:00 and 10:00 every morning by the same 3 nurses of the acute pain service. Additionally, the charts of 171 patients who had surgery between December 2018 and September 2019 were reviewed to systematically document the rapidity and intensity of the decrease in pain using this technique. These pain scores were recorded at four time points: before injection and at 5, 10, and 15 minutes after the injection. Our institution does not require patient consent for chart review but does require that the author obtain permission for review of the charts from the Medical Director of Professional Services. This was obtained for all sections of the review. All researchers followed the Good Clinical Practice (GCP) guidelines and patient data confidentiality and compliance with the Declaration of Helsinki were respected.
Statistical Analysis
Data were analyzed by a statistician at the coordinating center. The odds ratio and its 95% confidence interval (CI) were used to measure the strength of the association between injection (exposure) and infection (outcome). A proc mixed model was used to analyze the intensity and pain relief. To take into account the correlation within the subjects (ie, since a subject can have multiple injections), a random intercept was included in the model. The assumption of normality was also tested.
Injection Technique and Safeguards
Beginning in 2014 the acute pain service began to explore the potential benefit of this novel technique for patients. By 2016 the protocol for injecting the drains had achieved success and buy-in from medical and nursing staff. The applicability and safety were evaluated by the Montreal Heart Institute Protocol and Orders Committee and formal training and documentation sessions were created and disseminated. The protocol had been in use for 2 years by the nurses of the acute pain service at the time of this evaluation. The injection technique used was the following: 1) the patient was identified as having pain generated by the pleural or mediastinal drain, 2) the soft portion of the connecting drain was cleansed with alcohol, 3) the drain was clamped distal to the injection site, 4) the patient was placed in a slightly lateral decubitus position, toward the intended drain, 5) bupivacaine 0.25% 20 mL was injected rapidly using a 25G needle through the soft portion of the drain, 6) the drain was left clamped for 15 minutes, 7) after this time the patient was returned to a more comfortable position. The same technique was used when injecting the mediastinal drains with two exceptions: the patient remained in the dorsal position and the drain was not clamped.
Of importance to this technique is that the nurses were trained to recognize and treat local anesthetic toxicity, a physician was always present in the ICU at the time of injection, Intralipids were immediately available and protocols for their use were well known by the hospital staff.
Results
Safety
A total of 4392 patients had surgery between May 2016 and September 2018. Of these, 1348 patients received pleural or mediastinal drain injections. Over the same time period, a total of 37 patients were identified as having an episode of mediastinitis, osteitis, or a deep sternal wound infection (Table 1). The odds of infection in the exposed group was 0.82% (11/1337) while the odds in the non-exposed group was 0.86% (26/3018). The odds ratio is 0.955 (95% CI = 0.4705, 1.9384). No adverse cardiac event, such as arrest, hypotension, or bradycardia was reported over this time period related to the injection of drains. Table 2 presents the same data for the period between December 2018 and September 2019. The odds of infection in the exposed group was 0.58% (1/170) while the odds in the non-exposed group was 0.96% (12/1250). The odds ratio is 0.6127 (95% CI = 0.0792, 4.7421).
 |
Table 1 Odds of Infection in the Exposed and Non-Exposed Groups (May 2016 to September 2018) |
 |
Table 2 Odds of Infection in the Exposed and Non-Exposed Groups (December 2018 to September 2019) |
Efficacy
The evaluation of the speed and intensity of pain relief was carried out in 171 patients who received 250 injections. The analysis of this data showed that the adjusted mean “decrease in pain” was 4.08 (SEM = 0.15 and 95% CI = 3.78, 4.38), using the 11-point (0 to 10) Likert Numerical Rating Scale. The mean adjusted “time to maximum pain relief” of 8.33 minutes (SEM = 0.42 and 95% CI = 7.50, 9.15). The effect of type of drain was not significant in either of these analyses, p=0.5171 and p=0.1049, respectively.
Comparability of Groups
Table 3 presents data for absolute pain scores at rest and upon movement for both groups which shows both very low scores and comparability of the groups. Additionally, we calculated the average consumption of morphine equivalents to be 15 to meq equivalents on day one. This is indeed extremely low for post-operative surgery of this type. Finally, because this study straddles two time periods we provide demographic data in Table 4 which shows the comparability of these two groups in terms of age, sex, and type of surgery carried out at these two time points.
 |
Table 3 Absolute Pain Scores on Day 1 |
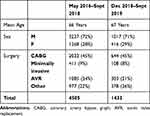 |
Table 4 Demographics at 2 Time Points |
Discussion
Our institution has a well-established acute post-operative pain service with 24/7 patient coverage. The nurse-run and anesthesia-supervised service implements a standardized multimodal pain protocol which is then tailored for each individual on daily basis. All patients who have surgery are followed for a minimum of 4 days and their pain scores are recorded daily in an electronic database.17
As a consequence of following the patients closely after surgery, it was noted that opioids were not effective in decreasing pain that was described as “piercing through to the back, side or shoulder” and that it would “prevent them from taking a deep breath”. Although patients do not always describe a pleuritic pain, they have a general discomfort upon breathing even when at rest.
In our institution, the drug that is used in local anesthetic infusions for the purpose of pain management is bupivacaine. Bupivacaine was chosen for its long duration and the dosage was selected after review of the literature which describes the use of both 0.5% bupivacaine12,14,18 and 0.25% bupivacaine.6–8,19 In view of the fact that we use continuous infusions of 0.125% bupivacaine in our infusion pumps and that the original testing and positive patient response to our technique was accomplished with 0.25% bupivacaine, we decided to maintain the lower dose of 0.25%. Additionally, this concentration provides for a larger margin of safety with respect to local anesthetic toxicity. Early studies indicated that mean peak plasma concentrations after injection of 20 mL of 0.5% bupivacaine ranged from 1.28 mcg/mL ± 0.48 mcg/mL,12 to 2.04 mcg/mL (range 1.6 to 3.26)4 and that 30 to 40% of the dose of bupivacaine was lost via thoracostomy tube over a 4-hour period.14 The volume of 20 mL was chosen to ensure rapid adequate spread of the local anesthetic in the pleural space.
The strength of this paper is that it presents information from a very large number of cases. Over a period of 17 months, 4392 patients had surgery and 1348 received injections in their pleural or mediastinal drains. The information regarding risk of infection is robust and demonstrates not only that the overall risk is very low, but there is no difference between the injected and non-injected groups. Although the information regarding the decrease in pain is calculated using a smaller number of different patients (n=171) the results, which were adjusted for repeated measures, show a dramatic decrease in pain over a time period of 15 minutes: a decrease of an average of 4 points on the Likert scale in 8 minutes. No previous study has shown such an impressive decrease in pain with the administration of any drug or combination of drugs in such a short period of time. One weakness of this paper stems from the use of two separate groups of patients; however, the authors have evaluated the demographic characteristics of both groups in order to demonstrate their similarity. A second weakness of this paper stems from its design as there is no way to account for the potential of a placebo effect. However, the authors concur that it is extremely unlikely that a decrease in pain of this magnitude can be attributed to a placebo effect especially since a full multimodal pain protocol was deployed for all patients prior to resorting to injection in the drains for any patient.
In conclusion, this technique is a powerful, safe, and efficient tool in the armamentarium of pain management and its growing use within our institution has provided a substantial benefit in the treatment of early post-operative pain. The technique has been taught to all the ICU nurses and has proven especially empowering to nurses on the night shift who are often at a disadvantage caring for a patient at a time when post-operative pain is difficult to manage and medical personnel are scarce. Within our institution, the injection of both pleural and mediastinal drains is considered safe and highly effective.
Ethics
IRB Number: ICM No. MAR-THO 2018.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Montreal Heart Institute Research Foundation. The sponsor had no involvement in the study.
Disclosure
The authors report no conflicts of interest for this work and declare that they have no conflict of interest with respect to this research related to this article.
References
1. Choiniere M, Watt-Watson J, Victor JC, et al. Prevalence of and risk factors for persistent postoperative nonanginal pain after cardiac surgery: a 2-year prospective multicentre study. CMAJ. 2014;186(7):E213–E223.
2. Guimaraes-Pereira L, Reis P, Abelha F, Azevedo LF, Castro-Lopes JM. Persistent postoperative pain after cardiac surgery: a systematic review with meta-analysis regarding incidence and pain intensity. Pain. 2017;158(10):1869–1885. doi:10.1097/j.pain.0000000000000997
3. Bjornnes AK, Parry M, Lie I, et al. The impact of an educational pain management booklet intervention on postoperative pain control after cardiac surgery. Eur J Cardiovasc Nurs. 2017;16(1):18–27. doi:10.1177/1474515116631680
4. Reiestad F, Stromskag KE. Interpleural catheter in the management of postoperative pain, a preliminary report. Reg Anesth Pain Med. 1986;11:89–91.
5. Demmy TL, Nwogu C, Solan P, Yendamuri S, Wilding G, DeLeon O. Chest tube-delivered bupivacaine improves pain and decreases opioid use after thoracoscopy. Ann Thorac Surg. 2009;87(4):
6. Mann LJ, Young GR, Williams JK, Dent OF, McCaughan BC. Intrapleural bupivacaine in the control of postthoracotomy pain. Ann Thorac Surg. 1992;53(3):449. doi:10.1016/0003-4975(92)90267-8
7. Tetik O, Islamoglu F, Ayan E, Duran M, Buket S, Cekirdekci A. Intermittent infusion of 0.25% bupivacaine through an intrapleural catheter for post-thoracotomy pain relief. Ann Thorac Surg. 2004;77(1):284–288. doi:10.1016/S0003-4975(03)01338-9
8. McIlvaine WB, Chang JH, Jones M. The effective use of intrapleural bupivacaine for analgesia after thoracic and subcostal incision in children. J Pediatr Surg. 1988;23(12):1184–1187. doi:10.1016/S0022-3468(88)80339-7
9. Weinberg L, Scurrah N, Parker F, Story D, McNicol L. Interpleural analgesia for attenuation of postoperative pain after hepatic resection. Anaesthesia. 2010;65(7):721–728. doi:10.1111/j.1365-2044.2010.06384.x
10. Al-Kayat HS, Parwari A, El-Khatib MS, Osman H, Naguib K. Intrapleural bupivacaine analgesia: bolus versus continuous infusion technique for postoperative pain relief in children. Indian J Anaesth. 2008;52(4):404–406.
11. Dhanjal S, Shannon C. Interpleural Analgesia. StatPearls. Treasure Island (FL): StatPearls Publishing; January, 2020.
12. Kambam JR, Hammon J, Winston CV, Parris F, Lupinetti M. Intrapleural analgesia for post-thoracotomy pain and blood levels of bupivacaine following intrapleural injection. Can J Anaesth. 1989;36(2):106–109. doi:10.1007/BF03011428
13. Broome IJ, Sherry KM, Reilly CS. A combined chest drain and intrapleural catheter for post-thoracotomy pain relief. Anaesthesia. 1993;48(8):724–726. doi:10.1111/j.1365-2044.1993.tb07190.x
14. Ferrante FM, Chan VW, Arthur GR, Rocco AG. Interpleural analgesia after thoracotomy. Anesth Analg. 1991;72(1):105–109. doi:10.1213/00000539-199101000-00019
15. Jagadeesan J, Kannan R, Dujon D. Ventricular standstill: a complication of intrapleural anesthesia using bupivacaine in a patient with free transverse rectus abdominus myocutaneous flap breast reconstruction. Ann Plast Surg. 2007;59(4):445–446. doi:10.1097/SAP.0b013e31802fa932
16. Shadvar K, Sanaie S, Mahmoodpoor A, Safarpoor M, Nagipour B. The effect of bilateral intrapleural infusion of lidocaine with fentanyl versus only lidocaine in relieving pain after coronary artery bypass surgery. Pak J Med Sci. 2017;33(1):177–181. doi:10.12669/pjms.331.10847
17. Cogan J, Schaffer GV, Ouimette MF, Yegin Z, Ferland V. Transforming the concept of “state of the art” into “real pain relief” for patients after cardiac surgery – a combined nursing-anesthesia initiative. J Pain Relief. 2014;3(4):152.
18. Graziotti PJ, Smith GB. Multiple rib fractures and head injury – an indication for intercostal catheterisation and infusion of local anaesthetics. Anaesthesia. 1988;43(11):964–966. doi:10.1111/j.1365-2044.1988.tb05664.x
19. Pennefather SH, Akrofi ME, Kendall JB, Russel GN, Scawn NDA. Double-blind comparison of intrapleural saline and 0.25% bupivacaine for ipsilateral shoulder pain after thoracotomy in patients receiving thoracic epidural analgesia. Br J Anaesth. 2005;94(2):234–238. doi:10.1093/bja/aei030
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
