Back to Journals » OncoTargets and Therapy » Volume 8
Inhibitive effects of 15-deoxy-Δ12,14-prostaglandin J2 on hepatoma-cell proliferation through reactive oxygen species-mediated apoptosis
Authors Chen K , Dai W, Wang F, Xia Y, Li J, Li S, Liu T, Zhang R, Wang J, Lu W, Zhou Y, Yin Q, Zheng Y, Abudumijiti H, Chen R, Lu J, Zhou Y, Guo C
Received 21 July 2015
Accepted for publication 30 October 2015
Published 1 December 2015 Volume 2015:8 Pages 3585—3593
DOI https://doi.org/10.2147/OTT.S92832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Faris Farassati
Kan Chen,1 Weiqi Dai,1 Fan Wang,1 Yujing Xia,1 Jingjing Li,1 Sainan Li,1 Tong Liu,1 Rong Zhang,2 Jianrong Wang,2 Wenxia Lu,2 Yuqing Zhou,3 Qin Yin,3 Yuanyuan Zheng,1 Huerxidan Abudumijiti,1 Rongxia Chen,1 Jie Lu,1 Yingqun Zhou,1 Chuanyong Guo1
1Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, 2Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, First Clinical Medical College of Nanjing Medical University, Nanjing, 3Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China
Objective: 15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) induces reactive oxygen species (ROS)-mediated apoptosis in many malignant cells, which has not been studied in hepatoma cells. In this study, we investigated whether 15d-PGJ2 induced apoptosis in hepatocellular carcinoma (HCC) associated with ROS.
Materials and methods: The LM3, SMMC-7721, and Huh-7 HCC cell lines were treated with 15d-PGJ2 (5–40 µM) for 24, 48, and 72 hours. Cholecystokinin 8 was used to detect the cytotoxicity of 15d-PGJ2. Flow cytometry, Hoechst staining, and Western blotting were used to analyze apoptosis. ROS were combined with the fluorescent probe dihydroethidium and then observed by fluorescence microscopy and flow cytometry. Activation of JNK and expression of Akt were detected by Western blotting.
Results: 15d-PGJ2 inhibited HCC cell proliferation and induced apoptosis in a dose- and time-dependent manner. Apoptosis was mainly induced via an intrinsic pathway and was ROS-dependent, and was alleviated by ROS scavengers. ROS induced JNK activation and Akt downregulation in HCC cells.
Conclusion: 15d-PGJ2 induced ROS in HCC cell lines, and inhibition of cell growth and apoptosis were partly ROS-dependent.
Keywords: 15-deoxy-Δ12,14-prostaglandin J2, hepatoma cell, ROS, apoptosis, JNK
Introduction
On account of its gradually increased incidence and high recurrence rate, hepatocellular carcinoma (HCC) has become the third-most frequent cancer and leading cause of cancer death worldwide.1–3 Surgery is currently the preferred and most effective treatment; however, the prognosis remains poor.4,5 Chemotherapeutic agents that are active against liver cancer are constantly being developed and tested in order to improve the survival rate of patients with HCC, but their effect is still unsatisfactory.6,7
15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) is a dehydrated product of prostaglandin D2. It was originally discovered as an endogenous lipid mediator and ligand for the nuclear receptor PPARγ, which regulates lipid and carbohydrate metabolism, adipocyte differentiation, and inflammation.8,9 Early studies indicated that 15d-PGJ2 can inhibit cancer cell proliferation and induce apoptosis through a PPARγ-dependent pathway.10–12 However, recent evidence indicates that the induction of apoptosis and inhibition of cancer cell proliferation by 15d-PGJ2 are attributable to generation of reactive oxygen species (ROS) rather than activation of PPARγ.13–15 15d-PGJ2 contains an α,β-unsaturated carbonyl group that acts as an electrophilic center, and thus it can react with and modify nucleophiles, such as glutathione and thioredoxin, resulting in alteration of the cellular redox status.16–18 The accumulation of oxidized proteins induced by 15d-PGJ2 also increases cellular oxidative stress.19,20
ROS, including superoxide anion, hydrogen peroxide, and hydroxyl radical, are produced from normal oxygen metabolism. In normal cells, excessive ROS can be eliminated by a series of potent antioxidants, thus ROS can be maintained at low concentrations, which exerts their biological activities.21 By activating several pathways, such as p53 proapoptotic signaling and c-Jun N-terminal kinase (JNK), and regulating phosphorylation and ubiquitination of Bcl-2 family members, ROS induced apoptosis.22–24
In the present study, we investigated whether 15d-PGJ2 induced ROS and ROS-mediated apoptosis in the LM3, Huh-7, and SMMC-7721 HCC cell lines. We confirmed that 15d-PGJ2-induced ROS in HCC cell lines and inhibition of cell growth, as well as apoptosis, were partly ROS-dependent.
Materials and methods
Cell lines and culture
All experiments were performed in accordance with ethical standards and in compliance with the Declaration of Helsinki, as per national and international guidelines. This study was approved by the Ethics Committee of Tongji University. Three HCC cell lines – LM3, Huh-7, and SMMC-7721 – and normal hepatocytes (LO2) were purchased from the Cell Bank of the Chinese Academy of Sciences Committee Type Culture Collection (Shanghai, People’s Republic of China [PRC]). All four cell lines were cultured in high-glucose Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (GE Healthcare, Little Chalfont, UK), 100 U/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific) in a 5% CO2 and 95% air incubator at 37°C.
Reagents
15d-PGJ2 (dissolved in methyl acetate) and N-acetylcysteine (NAC) were purchased from Sigma-Aldrich (St Louis, MO, USA). The cell-counting kit (CCK-8) used was produced by Dojindo (Dojindo Laboratories, Kumamoto, Japan). The antibodies used in this study included those directed against proliferating cell nuclear antigen (PCNA; Proteintech, Chicago, IL, USA), cytochrome C (Cell Signaling Technology, Danvers, MA, USA), Bax (Cell Signaling Technology), caspase 3 (Cell Signaling Technology), caspase 9 (Proteintech), caspase 8 (Proteintech), PARP1 (Proteintech), Akt (Proteintech), p-Akt (Cell Signaling Technology), JNK (Proteintech), p-JNK (Cell Signaling Technology), and β-actin (Proteintech). An annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) apoptosis-detection kit was purchased from BD Biosciences (San Jose, CA, USA). The ROS fluorescent probe dihydroethidium (DHE) was purchased from Beyotime Biotechnology (Shanghai, People’s Republic of China).
Cell proliferation and viability
The LM3, SMMC-7721, and Huh-7 HCC cell lines were plated at 3×104 cells/mL in 96-well plates (100 μL medium per well). After being seeded for 24 hours, all wells were treated with 15d-PGJ2 (diluted with phosphate-buffered saline [PBS] to final concentrations of 5, 10, 20, 30, and 40 μM); each concentration was incubated with five replicate samples. Cell proliferation and viability were measured using the CCK-8 assay and a microplate reader (Synergy H4; BioTek Instruments Inc, Winooski, VT, USA) at a wavelength of 450 nm after treatment for 24, 48, and 72 hours. Half-maximal inhibitory concentration (IC50) values were calculated by using CalcuSyn Version 2.0 software (Biosoft, Cambridge, UK).
Apoptosis analyses
The HCC cells were first plated into six-well plates at 106 cells/mL, exposed to 10, 20, and 30 μM 15d-PGJ2 for 48 hours, and analyzed with flow cytometry and Hoechst staining.
Flow cytometry
The cells were collected after trypsin treatment for digestion and washed twice with PBS. The processed cells were added to Falcon tubes at 106 cells/mL containing 100 μL 1× binding buffer and incubated for 20 minutes at room temperature with annexin-V/FITC supplemented with PI. Cells were considered to be apoptotic if they were either annexin V+/PI− (early apoptotic) or annexin V+/PI+ (late apoptotic). The rate of apoptosis was measured by flow cytometry within 1 hour.
Hoechst 33342 staining
The cells in six-well plates treated with 15d-PGJ2 at designed concentrations for 48 hours were washed with PBS and fixed in 4% paraformaldehyde for 10 minutes. The fixed cells were then washed with PBS and perfused with Hoechst 33342 stain (1 μL added to 200 μL PBS). The filled six-well plates were placed at 4°C in the dark for 20 minutes. Fluorescence microscopy (Leica Microsystems, Wetzlar, Germany) was used to examine the blue fluorescent cells.
Western blotting
Cells treated with 15d-PGJ2 (10, 20, and 30 μM) for 48 hours were collected after trypsin digestion and washed twice with PBS. Radioimmunoprecipitation-assay lysis buffer with protease inhibitors and phenylmethanesulfonyl fluoride were added to the cell suspensions to lyse the cells and collect cellular extracts. The concentration of the prepared protein was determined by the bicinchoninic acid protein assay (Thermo Fisher Scientific). The cellular proteins were then mixed with 5× sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample-loading buffer and boiled for 10 minutes. The treated samples were subjected to SDS-PAGE following standard protocols. Nonspecific binding was blocked with 5% nonfat powdered milk (dissolved in PBS) for 2 hours, and blots were incubated overnight at 4°C with the following antibodies: PCNA (1:500), cytochrome C (1:500), Bax (1:1,000), caspase 3 (1:200), caspase 9 (1:500), caspase 8 (1:500), Akt (1:1,000), JNK (1:500), p-JNK (1:500), p-Akt (1:500), and β-actin (1:1,000). The next day, all the membranes were washed three times with PBS containing 0.1% Tween 20 before incubation with the secondary antibody (antirabbit or antimouse IgG [1:2,000]) for 1 hour at room temperature. Finally, membranes were washed with PBS containing 0.1% Tween 20 three times for 5 minutes each, and proteins were detected by fluorescence using the Odyssey two-color infrared laser imaging system (Li-Cor Biosciences, Lincoln, NE, USA).
Measurement of ROS
The HCC cells were first plated into six-well plates at 106 cells/mL, exposed to 10, 20, and 30 μM 15d-PGJ2 for 4 hours, or pretreated with NAC for 1 hour. The fluorescent probe DHE (dissolved in dimethyl sulfoxide) was added to the medium at a final concentration of 50 μM. The processed cells were incubated at 37°C for 1 hour, avoiding light, and washed twice with PBS before observation under fluorescence microscopy. For flow-cytometry analysis, the processed cells were collected for suspension in PBS and washed with 1 mL cold PBS. ROS were detected at a wavelength of 590–610 nm, and the cells were divided into two subsets.
Statistical analysis
All experimental data were evaluated by calculating means ± standard deviation analyzed by SPSS 20.0 software (IBM, Armonk, NY, USA). Student’s t-test and one-way analysis of variance, followed by Tukey’s test when F was significant, were performed to compare the results of the CCK-8 assay. In all comparisons, P<0.05 was considered statistically significant. The bar charts were obtained using GraphPad Prism for Windows version 5.0 (GraphPad Software Inc, La Jolla, CA, USA).
Results
15d-PGJ2 inhibits HCC cell growth
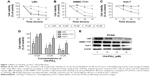
Proliferation of all three cell lines (LM3, MMC-7721, and Huh-7) after treatment with 15d-PGJ2 (5, 10, 20, 30, and 40 μM) for 24, 48, and 72 hours was examined using the CCK-8 assay. The cell-viability curve was constructed according to OD, as shown in Figure 1A–D. 15d-PGJ2 inhibited HCC cell growth in a dose- and time-dependent manner. IC50 values were calculated according to the results of CCK8 assays. IC50 values for these cell lines were 15.5 (LM3), 18.7 (SMMC-7721), and 36.7 μM (Huh-7). PCNA was detected by Western blotting (Figure 1E).
15d-PGJ2 induces apoptosis in HCC cell lines
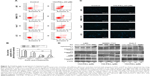
Flow cytometry, Hoechst staining, and Western blotting were used to investigate whether 15d-PGJ2 induced apoptosis in HCC cell lines. Flow cytometry showed that the percentage of early and late apoptotic cells was significantly higher after treatment with 15d-PGJ2 for 48 hours than in the normal culture-group cells (Figure 2A). Besides, it also showed the difference between the HCC cell lines and normal hepatocytes (LO2), which served as a control for the apoptosis assays (Figure 2B). After 15d-PGJ2 treatment, all cells were stained with Hoechst 33342, which revealed that the DNA differed in shape and showed high intensity in fluorescence compared with the normal group (Figure 2C). Western blotting demonstrated apoptosis-related protein expression, including cytochrome C, Bax, caspase 3, caspase 9, caspase 8, and PARP1, in all HCC cell lines. Figure 2D shows the significant change in all detected proteins except caspase 8, which indicated that the apoptosis induced by 15d-PGJ2 was mainly dependent on the intrinsic apoptosis pathway.
15d-PGJ2 induces ROS generation in HCC cell lines
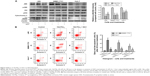
ROS generated after 15d-PGJ2 treatment were observed and measured using fluorescence microscopy and flow cytometry, respectively. DHE-probe staining of ROS-positive cells showed increased fluorescence intensity (Figure 3A). The ratio of ROS-positive to normal cells was analyzed by flow cytometry. ROS generation was shown to be dependent on treatment time with 15d-PGJ2 (Figure 3B).
15d-PGJ2 induces ROS-mediated JNK activation and downregulation of Akt pathway
ROS are suggested to be strong activators of JNK, leading to mitochondrial cytochrome C release, caspase 9 activation, and initiation of the intrinsic apoptosis pathway. JNK activation was detected by Western blotting. Figure 4A shows that phosphorylation of JNK was increased in all three cell lines after 15d-PGJ2 treatment for 48 hours when compared to the normal group. Expression of phosphorylated Akt was decreased by 15d-PGJ2. ROS-mediated JNK activation, cytochrome C release, and apoptosis induction, as well as downregulation, were alleviated by the appearance of the ROS scavenger NAC.
Discussion
The present study confirmed ROS generation by 15d-PGJ2 in HCC cell lines. The apoptosis induced by 15d-PGJ2 in HCC cells was partly dependent on ROS, and was alleviated by the ROS scavenger NAC. These results are in accordance with previous studies of 15d-PGJ2 in other cell lines, from which we determined the dose of drug to use.13,25
We showed that 15d-PGJ2 exerted cytotoxic activity and inhibited proliferation in all HCC cell lines. According to the CCK-8 assay, 15d-PGJ2 inhibited proliferation of LM3 and SMMC-7721 cells in a time- and dose-dependent manner, and to a lesser extent in Huh-7 cells. After 48 hours’ treatment with 20 μM 15d-PGJ2, cell viability was reduced to approximately 25%, 50%, and 75% in LM3, SMMC-7721, and Huh-7, respectively, and we chose the three effective concentrations (10, 20, and 30 μM) for our continuing experiments (Figure 1D). PCNA is an auxiliary protein of DNA polymerase, which is related to DNA synthesis in eukaryotic cell DNA and reflects the state of cell proliferation. It was detected by Western blotting to corroborate the growth inhibition induced by 15d-PGJ2, and the result was consistent with the CCK-8 assay.26
15d-PGJ2 has been reported to induce apoptosis in many malignant cells, including hepatoma cells.10,13,14,25 In the present study, flow cytometry and Hoechst 33342 staining were used to detect and analyze the number and state of apoptotic cells. The increase in the proportion of early and late apoptotic cells and metamorphic DNA confirmed that the HCC cell death induced by 15d-PGJ2 was mainly via apoptosis, in accordance with previous studies.13,25 Apoptosis-related proteins were investigated using Western blotting. The increased expression of cleaved caspase 3 and cleaved PARP1 indicated apoptosis activation and DNA damage in 15d-PGJ2-treated HCC cell lines. The expression of proteins of the intrinsic apoptosis pathway, including cytochrome C, Bax, and caspase 9, was also increased relevantly to the concentration of 15d-PGJ2. Caspase 8, the key enzyme activated in the extrinsic apoptosis pathway, was not significantly affected. These data indicate that apoptosis induced by 15d-PGJ2 is mainly dependent on the intrinsic but not extrinsic apoptosis pathway, which is consistent with Date et al, who used the caspase 3 inhibitor Z-DEVD-FMK to block apoptosis of human hepatoma cells that had been treated with 15-d-PGJ2.10
The ROS concentration in cancer cells is higher than in normal cells, due to their unique increased metabolic properties.27,28 Elevated levels of ROS in cancer cells promote carcinogenesis and cancer progression by angiogenesis.29,30 However, it has been shown that excess ROS beyond the antioxidant capacity of the cells can induce apoptosis.31,32 The present study revealed that the level of ROS generation is related to the treatment time of 15d-PGJ2. ROS were detected at 2 hours after 15d-PGJ2 treatment; the level increased up to 6 hours, and was still detectable after 24 hours by flow cytometry. NAC pretreatment increased the cell viability and decreased expression of cytochrome C and cleaved PARP, indicating that ROS generation is critical in 15d-PGJ2-induced HCC cell apoptosis (Figure 4). Although both the result of CCK-8 assays and flow cytometry showed that 15d-PGJ2 showed an obvious effect on cell-proliferation inhibition and cell-apoptosis promotion in all three cell lines, the difference of the effect among the cell lines was also big. According to our results, 15d-PGJ2 also had a different effect on ROS production in these cell lines, which is consistent with the trend of the apoptosis and cell viability. Therefore, we speculate that the different sensitivity to ROS of different hepatoma cell lines may count for the different effect under the 15d-PGJ2 treatments.
The activation of JNK, a potent proapoptotic kinase, is thought to be one the main factors induced by excess ROS.13,23 Phosphorylation of JNK was detected by Western blotting in 15d-PGJ2-treated cells compared with normal cells, and decreased when NAC was present (Figure 4A). These data suggest that ROS-mediated JNK activation contributes to apoptosis induced by 15d-PGJ2. We also observed downregulation of the Akt pathway, which is one of the most effective antiapoptotic pathways.33 The decreased expression of phosphorylated Akt signifies the weakened antiapoptotic pathway and reduces inhibition of JNK, which in turn increases apoptosis (Figure 4B).
As mentioned earlier, 15d-PGJ2 contains an α,β-unsaturated carbonyl group that can act as an electrophilic center and allow itself to exert biological actions beyond some of the receptor-dependent pathways, such as inhibition of the NFκB pathway, disruption of the Keap1–Nrf2 complex, and alteration of cellular redox status.34 Some studies have shown the other possible pathways by which 15d-PGJ2 can exert anticancer activity.35–37
Conclusion
In summary, this study verifies that 15d-PGJ2 can inhibit HCC cell proliferation and induce apoptosis in a dose- and time-dependent manner. We also demonstrated that 15d-PGJ2 induces ROS production and ROS-mediated apoptosis, as well as JNK activation and Akt downregulation in hepatoma cells.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81270515).
Disclosure
The authors report no conflicts of interest in this work.
References
Cheng P, Dai W, Wang F, et al. Ethyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the HMGB1-RAGE and AKT pathways. Biochem Biophys Res Commun. 2014;443(4):1162–1168. | ||
Yang J, Li J, Dai W, et al. Golgi protein 73 as a biomarker for hepatocellular carcinoma: a diagnostic meta-analysis. Exp Ther Med. 2015;9(4):1413–1420. | ||
Dai W, Wang C, Wang F, et al. Anti-miR-197 inhibits migration in HCC cells by targeting KAI 1/CD82. Biochem Biophys Res Commun. 2014;446(2):541–548. | ||
Jie L, Fan W, Weiqi D, et al. The hippo-yes association protein pathway in liver cancer. Gastroenterol Res Pract. 2013;2013:187070. | ||
De Giorgi V, Buonaguro L, Worschech A, et al. Molecular signatures associated with HCV-induced hepatocellular carcinoma and liver metastasis. PLoS One. 2013;8(2):e56153. | ||
Gerardi AM, Stoppino LP, Liso A, Macarini L, Landriscina M. Rapid long-lasting biochemical and radiological response to sorafenib in a case of advanced hepatocellular carcinoma. Oncol Lett. 2013;5(3):975–977. | ||
Wang F, Dai W, Wang Y, et al. The synergistic in vitro and in vivo antitumor effect of combination therapy with salinomycin and 5-fluorouracil against hepatocellular carcinoma. PLoS One. 2014;9(5):e97414. | ||
Li CC, Yang HT, Hou YC, Chiu YS, Chiu WC. Dietary fish oil reduces systemic inflammation and ameliorates sepsis-induced liver injury by up-regulating the peroxisome proliferator-activated receptor gamma-mediated pathway in septic mice. J Nutr Biochem. 2014;25(1):19–25. | ||
Chen K, Li J, Wang J, et al. 15-Deoxy-γ12,14-prostaglandin J2 reduces liver impairment in a model of ConA-induced acute hepatic inflammation by activation of PPARγ and reduction in NF-κB activity. PPAR Res. 2014;2014:215631. | ||
Date M, Fukuchi K, Morita S, Takahashi H, Ohura K. 15-Deoxy-Δ12,14-prostaglandin J2, a ligand for peroxisome proliferators-activated receptor-γ, induces apoptosis in human hepatoma cells. Liver Int. 2003;23(6):460–466. | ||
Li MY, Deng H, Zhao JM, Dai D, Tan XY. Peroxisome proliferator-activated receptor gamma ligands inhibit cell growth and induce apoptosis in human liver cancer BEL-7402 cells. World J Gastroenterol. 2003;9(8):1683–1688. | ||
Brockman JA, Gupta RA, Dubois RN. Activation of PPARγ leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115(5):1049–1055. | ||
Yen CC, Hsiao CD, Chen WM, et al. Cytotoxic effects of 15d-PGJ2 against osteosarcoma through ROS-mediated AKT and cell cycle inhibition. Oncotarget. 2014;5(3):716–725. | ||
Lee SJ, Kim MS, Park JY, Woo JS, Kim YK. 15-Deoxy-Δ12,14-prostaglandin J2 induces apoptosis via JNK-mediated mitochondrial pathway in osteoblastic cells. Toxicology. 2008;248(2–3):121–129. | ||
Kitz K, Windischhofer W, Leis HJ, Huber E, Kollroser M, Malle E. 15-Deoxy-Δ12,14-prostaglandin J2 induces Cox-2 expression in human osteosarcoma cells through MAPK and EGFR activation involving reactive oxygen species. Free Radic Biol Med. 2011;50(7):854–865. | ||
Kondo M, Oya-Ito T, Kumagai T, Osawa T, Uchida K. Cyclopentenone prostaglandins as potential inducers of intracellular oxidative stress. J Biol Chem. 2001;276(15):12076–12083. | ||
Fukushima M. Biological activities and mechanisms of action of PGJ2 and related compounds: an update. Prostaglandins Leukot Essent Fatty Acids. 1992;47(1):1–12. | ||
Shibata T, Yamada T, Ishii T, et al. Thioredoxin as a molecular target of cyclopentenone prostaglandins. J Biol Chem. 2003;278(28):26046–26054. | ||
Ishii T, Uchida K. Induction of reversible cysteine-targeted protein oxidation by an endogenous electrophile 15-deoxy-Δ12,14-prostaglandin J2. Chem Res Toxicol. 2004;17(10):1313–1322. | ||
Ishii T, Sakurai T, Usami H, Uchida K. Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 S proteasome. Biochemistry. 2005;44(42):13893–13901. | ||
D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. | ||
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. | ||
Davis W Jr, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. Biochem Pharmacol. 1995;50(7):1021–1029. | ||
Li D, Ueta E, Kimura T, Yamamoto T, Osaki T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004;95(8):644–650. | ||
Shin SW, Seo CY, Han H, et al. 15d-PGJ2 induces apoptosis by reactive oxygen species-mediated inactivation of Akt in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin Cancer Res. 2009;15(17):5414–5425. | ||
Maga G, Hübscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116(Pt 15):3051–3060. | ||
Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–798. | ||
Kuntz S, Mazerbourg S, Boisbrun M, et al. Energy restriction mimetic agents to target cancer cells: comparison between 2-deoxyglucose and thiazolidinediones. Biochem Pharmacol. 2014;92(1):102–111. | ||
Petros JA, Baumann AK, Ruiz-Pesini E, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci U S A. 2005;102(3):719–724. | ||
Gao N, Ding M, Zheng JZ, et al. Vanadate-induced expression of hypoxia-inducible factor 1α and vascular endothelial growth factor through phosphatidylinositol 3-kinase/Akt pathway and reactive oxygen species. J Biol Chem. 2002;277(35):31963–31971. | ||
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407(6802):390–395. | ||
Pelicano H, Feng L, Zhou Y, et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278(39):37832–37839. | ||
Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26(11):657–664. | ||
Uchida K, Shibata T. 15-Deoxy-Δ12,14-prostaglandin J2: an electrophilic trigger of cellular responses. Chem Res Toxicol. 2008;21(1):138–144. | ||
Park SW, Cho C, Cho BN, Kim Y, Goo TW, Kim YI. 15-Deoxy-Δ12,14-prostaglandin J2 down-regulates activin-induced activin receptor, Smad, and cytokines expression via suppression of NF-κB and MAPK signaling in HepG2 cells. PPAR Res. 2013;2013:751261. | ||
Han H, Shin SW, Seo CY, et al. 15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) sensitizes human leukemic HL-60 cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through Akt downregulation. Apoptosis. 2007;12(11):2101–2114. | ||
Diers AR, Dranka BP, Ricart KC, et al. Modulation of mammary cancer cell migration by 15-deoxy-Δ(12,14)-prostaglandin J2: implications for anti-metastatic therapy. Biochem J. 2010;430(1):69–78. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.