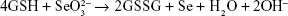Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels
Authors Wang Q, Larese-Casanova P, Webster T
Received 1 December 2014
Accepted for publication 5 March 2015
Published 13 April 2015 Volume 2015:10(1) Pages 2885—2894
DOI https://doi.org/10.2147/IJN.S78466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Carlos Rinaldi
Qi Wang,1 Philip Larese-Casanova,2 Thomas J Webster3,4
1Department of Bioengineering, College of Engineering, Northeastern University, Boston, MA, USA; 2Department of Civil and Environmental Engineering, College of Engineering, Northeastern University, Boston, MA, USA; 3Department of Chemical Engineering, College of Engineering, Northeastern University, Boston, MA, USA; 4Center of Excellence for Advanced Materials Research, King Abdulaziz University, Jeddah, Saudi Arabia
Abstract: There are wide spread bacterial contamination issues on various paper products, such as paper towels hanging in sink splash zones or those used to clean surfaces, filter papers used in water and air purifying systems, and wrappings used in the food industry; such contamination may lead to the potential spread of bacteria and consequent severe health concerns. In this study, selenium nanoparticles were coated on normal paper towel surfaces through a quick precipitation method, introducing antibacterial properties to the paper towels in a healthy way. Their effectiveness at preventing biofilm formation was tested in bacterial assays involving Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus epidermidis. The results showed significant and continuous bacteria inhibition with about a 90% reduction from 24 to 72 hours for gram-positive bacteria including S. aureus and S. epidermidis. The selenium coated paper towels also showed significant inhibition of gram-negative bacteria like P. aeruginosa and E. coli growth at about 57% and 84%, respectively, after 72 hours of treatment. Therefore, this study established a promising selenium-based antibacterial strategy to prevent bacterial growth on paper products, which may lead to the avoidance of bacteria spreading and consequent severe health concerns.
Keywords: selenium nanoparticles, paper towels, antibacterial
Introduction
Selenium and its compounds have been widely used in electronics, glasses, ceramics, steel, and pigment manufacturing.1 But due to toxicity concerns, not until recently has selenium been considered as a material for biomedical applications. Selenium is found naturally in humans and animals as a critical element in selenoproteins, which play an important role in antioxidant defense systems, thyroid hormone metabolism, and redox control of cell reactions.2 Because of this, selenium and selenium nanoparticles have been receiving dramatically increasing attention in several biomedical applications. For example, it has been shown that high levels of selenium in the blood (~154 μg/mL) correlated with a reduced number of cancer occurrence including pancreatic, gastric, lung, nasopharyngeal, breast, uterine, respiratory, digestive, and gynecological cancer3 and many in vitro and in vivo studies have demonstrated the inhibitory effects of selenium on the activity of various cancer cells.3–7 Selenium nanoparticles have also been considered as a biocompatible anticancer orthopedic implant material due to the increased adhesion of healthy osteoblasts (or bone forming cells) on nanostructured selenium.8
However, in terms of potential antibacterial applications, selenium is a novel material that has not been widely explored. There are a few studies concerning the antibacterial properties of selenium or its compounds. For example, selenium-enriched probiotics have been shown to strongly inhibit the growth of pathogenic Escherichia coli in vivo and in vitro.9 The synthesized organoselenium compounds were shown to be as effective as penicillin at inhibiting Staphylococcus aureus growth in solution in vitro.10 In addition, the growth of planktonic S. aureus was strongly inhibited in the presence of selenium nanoparticles.11
Many kinds of other nanomaterials, including silver nanoparticles, zinc oxide nanoparticles, copper nanoparticles, and so on, have been studied to provide a novel strategy to eradicate bacterial infections, especially for various medical device applications.12–15 But a major problem for these metal-based nanoparticles has been their toxicity to healthy mammalian cells due to their generation of reactive oxygen species,16–18 which could result in severe health problems when used as medical devices or clinical applications.
Compared with the above mentioned materials, selenium nanoparticles are expected to be healthier and less toxic to humans and the environment. For an adult, it is recommended by the US Food and Drug Administration (FDA) that selenium needs to be consumed at 53–60 μg per day, because it is the nutrition for 25 selenoproteins with selenocysteine at their active center.19 Moreover, studies have demonstrated that selenium and selenium nanoparticles can improve healthy human cell growth.8,20
Bacterial contaminations are frequently found on paper products worldwide, involving various strains from the genera Bacillus, Staphylococcus, Pseudomonas, and Enterobacter.21–23 These bacteria contribute to the formation of biofilm in machinery, resulting in contamination or even corrosion on paper products. In some circumstances, such as paper towels hanging in the splashing zone or those used for cleaning surfaces, they have been considered as potential sources of bacteria contaminations,24,25 especially in a hospital or a clinical environment. It has been shown by a previous study that hand washing with antibacterial soap followed by drying with unused paper towels results in more bacteria attached on hands than before washing.26 In addition, there are also concerns for bacterial contamination on numerous paper products, for example, filter paper in water and air purifying systems, and wrapping paper used in the food industry.27,28 Filter papers are commonly covered by a thick layer of bacterial biofilm and need frequent replacement. Microbial contamination is one of the major reasons causing food spoilage during food storage, especially when the foods are packaged and stored for a long period of time. Actually, developing effective antimicrobial packaging materials for controlling microbial growth in/on food is now an active area of research.29,30
Due to the porous structure of fibers in all paper products, such materials are prone to bacteria growth and, thus, are sources for continual contamination. Besides, this porous structure provides an environment that favors the attachment of bacteria and makes it more difficult to kill bacteria once a biofilm has been formed. One of the most promising approaches toward preventing infections is coating paper products with antimicrobial materials. Therefore, in this study, selenium nanoparticles were used as a coating material to introduce antibacterial properties to paper towels. Selenium nanoparticles were coated on paper towel surfaces through a quick precipitation reaction (happening within 60 seconds). In addition, their effectiveness at preventing biofilm formation was tested in bacterial assays involving four diverse types of bacteria. The results showed that the selenium coatings significantly inhibited all bacterial activities on paper towels which could decrease the presence and spreading of numerous human infections.
Materials and methods
Synthesis of selenium nanoparticle coatings
Selenium nanoparticles were synthesized and coated on paper towels (MB550A Hand Towel, Tork Advanced, cut into round chips, 7.00 mm in diameter) through a simple and quick precipitation reaction. The reaction of the synthesis was:
| (1) |
which involves glutathione (GSH) (reduced form) (97%, TCI America, Portland, OR, USA) and sodium selenite (99%, Alfa Aesar, Ward Hill, MA, USA) mixed at a 4:1 molar ratio. The paper towel samples were washed with 75% ethanol followed by rinsing with deionized (DI) water five times and then transferred into a flask containing a mixed solution of GSH and sodium selenite. Sodium hydroxide was added into the mixture of GSH and sodium selenite to bring the pH of the solution into the alkaline regimen to initiate the reaction at room temperature. Selenium nanoparticles were formed and precipitated on the surface of paper towels immediately following the addition of NaOH as visualized by a color change of the reactant solution from colorless to clear red. The paper towel samples were coated for 30 seconds under a 200 rpm agitation to ensure a uniform coating. The coated substrates were rinsed in DI water five times and soaked in water for 24 hours to remove the free, non-adherent, selenium nanoparticles, and remaining reactants. Then, the selenium coated paper towels were left in petri dishes for several days to completely dry before being used in any further experiments or tests. The uncoated samples that were used as a control group in the following experiments were prepared by treating paper towels with the same procedure for selenium coated paper towel samples described above except that sodium hydroxide was not added so that the reaction did not occur.
Characterization
Scanning electron microscopy (SEM; HITACHI S-4800, Hitachi Ltd., Tokyo, Japan) images of the paper towel surfaces were taken to determine the size, coverage, and distribution of selenium nanoparticles. Before scanning the surface of a paper towel under SEM, the samples were coated with a 5 nm platinum layer using a sputter coater (Cressington 208 HR, Cressington Scientific Instruments Ltd., Watford, Hertfordshire, UK) to make the samples conductive. The coverage of selenium nanoparticles on the paper towel surface was analyzed and calculated based on the SEM images using ImageJ (National Institutes of Health, Bethesda, MD, USA). Atomic absorption spectroscopy, (Furnace, AA600, Agilent Technologies, 710 Series, Santa Clara, CA, USA) was used to determine the amount of selenium on coated and uncoated samples. Measurements were completed in triplicate for both blank uncoated and selenium coated paper towel samples.
Mechanical tests
An MTESTQuattro materials testing system (ADMET, Norwood, MA, USA) was developed according to American Society for Testing and Materials (ASTM) and International Organization for Standardization (ISO) standards. The system was used to measure the mechanical properties of selenium coated and uncoated paper towels. The samples were prepared by cutting the paper towels into 18.00×40.00 mm rectangle pieces and then coating them with selenium nanoparticles using the procedure described above. Tension tests were performed by stretching the paper towel until complete breakage occurred. The size of tension loaded area was 18.00 mm wide by 30.00 mm long (the stretching direction). The tension tests were performed for both completely dry and wet (saturated by DI water) paper towels. Data were recorded automatically by the ADMET software. The values of maximum stress were calculated based on the data and the loaded area (18.00×30.00 mm) of the tested samples.
Bacterial inhibitory tests
Four bacterial cell lines were involved in the tests to evaluate the growth of various types of bacteria on selenium coated and uncoated paper towels. The bacterial cell lines, S. aureus (catalog number 25923), Pseudomonas aeruginosa (catalog number 27853), E. coli (catalog number 25922), and Staphylococcus epidermidis (catalog number 35984), were obtained in freeze-dried form from the American Type Culture Collection (Manassas, VA, USA). Each strain of bacteria was propagated in 30 mg/mL tryptic soy broth (TSB) for 20 hours. A bacteria solution was prepared using 0.3 mg/mL TSB at a concentration of 106 bacteria/mL, which was assessed by measuring the optical density of the bacterial solution using a standard curve correlating optical densities and bacterial concentrations. The optical densities were measured at 562 nm using a SpectraMax M5 plate reader (Molecular Devices LLC, Sunnyvale, CA, USA).
Selenium coated and uncoated paper towel samples were rinsed in separate petri dishes with 75% ethanol for 20 minutes for sterilization purposes and were left in the sterile petri dishes for 30 minutes to completely dry. Then, the samples were transferred into a 48-well plate and rinsed with sterile 0.3 mg/mL TSB twice to remove any possible ethanol residue. Each sample was treated separately with 1 mL of the prepared bacterial solutions (106 bacteria/mL) and cultured for either 24, 48, or 72 hours in an incubator (37°C, humidified, 5% CO2). For those samples that were cultured for 48 and 72 hours, the media was changed with 1 mL of sterile and fresh TSB every 24 hours. After the treatment, the samples were rinsed with a phosphate buffered saline (PBS) solution twice and placed into 1.5 mL microfuge tubes with 1 mL of PBS. These microfuge tubes were shaken at 3,000 rpm for 15 minutes on a vortex mixer to completely release the bacteria attached on the surface into the solution. Solutions with bacteria from each sample were spread on agar plates and bacteria colonies were counted after 20 hours of incubation. The bacterial inhibitory tests on the four types of bacteria strains were conducted individually and identically.
Protein adsorption assays
The total protein adsorption on the selenium coated and uncoated paper towel samples was measured using a Piece BCA Protein Assay Kit (catalog number 23227, Piece Biotechnology, Thermo Fisher Scientific, Waltham, MA, USA). The paper towel samples were rinsed with 75% ethanol for 20 minutes for sterilization purposes. After being left in sterile petri dishes for 30 minutes to completely dry, the samples were transferred into a 48-well plate and rinsed with sterile PBS twice. Each sample was treated separately with 1 mL of sterile TSB and then cultured for 24, 48, or 72 hours in an incubator (37°C, humidified, 5% CO2). For those samples that were cultured for 48 and 72 hours, the media was changed with 1 mL of sterile and fresh TSB every 24 hours. After the treatment, the samples were transferred to a new 48-well plate and washed with a cold (~4°C) PBS solution twice to remove unadsorbed proteins in the media. Then, each sample was treated with 0.5 mL radioimmunoprecipitation assay (RIPA) buffer (catalog number 89900, Piece Biotechnology, Thermo Fisher Scientific) for 10 minutes, swirling the plate occasionally at the same time. After the treatment, the solution in each well was transferred to a microcentrifuge tube and stored in a −80°C freezer for further analysis. The concentration of proteins in solution gathered after 24, 48, and 72 hours were measured following the manufacturer’s instructions for the Piece BCA Protein Assay Kit, in which the sample to work reagent ratio and incubation time was modified compared with the standard protocol to achieve a lower minimum detection level. The bovine serum albumin standards for plotting a standard curve were also treated in the same way as the samples. The concentration of proteins adsorbed on each sample was calculated based on the standard curve.
Release of selenium
The release of selenium from the surface of coated paper towels was measured using inductively coupled plasma mass spectrometry (ICP-MS, Bruker Aurora M90, Bruker Corporation, Billerica, MA, USA). To measure the total amount of Se on the surface of coated paper towels, 1 mL of 10 M NaOH was added to each sample (7 mm in diameter, as described in “Materials and Methods”) placed into a 10 mL vial. The vial was left standing for 3 days to allow all coated selenium to be completely dissolved by NaOH. Then, the solution was diluted 100 times using DI water before ICP-MS measurements. Paper towel samples without Se coatings were also treated with the same procedure and the solution was also collected for ICP-MS measurements. In order to measure the release of the selenium from the paper towel samples, both coated and uncoated samples were treated individually with 1 mL of sterile TSB in a 37°C incubator for 24, 48, and 72 hours, which are the same time scales as the bacterial assays. The media for each sample was collected every 24 hours and replaced with 1 mL of fresh TSB. The collected solutions were diluted ten times using DI water before ICP-MS measurements.
Statistics
All bacterial inhibitory tests and protein adsorption assays were conducted in triplicate and repeated three times. Data were collected and the significant differences were assessed with the probability associated with a one-tailed Student’s t-test. Statistical analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
Results and discussion
Synthesis and characterization of selenium nanoparticles
After coating with selenium nanoparticles, the color of the paper towel surfaces changed from white to light red (Figure 1). The red color indicated the formation of selenium nanoparticles as one of the selenium allotropes is red.31 The coated paper towels can maintain its red appearance for at least 12 months at room temperature, although the most stable form of selenium is considered to be the gray color crystal and this gray selenium can be formed by the mild heating of other allotropes. The nanosized red selenium has a high biological activity in terms of cell proliferation, enzyme induction, and protection of human cells against damage from free radicals. It also has a much lower acute toxicity compared with sodium selenite.32
The SEM images of the selenium coated paper towels (Figure 2A) and uncoated paper towels (Figure 2B) are shown in Figure 2. As shown in Figure 2A, the selenium nanoparticles were well distributed on the fibrous portions of the paper towels and covered almost all parts of the surface. Figure 2B shows a paper towel surface without a selenium coating, on which no particles were observed. From Figure 2C, which is a selenium coated sample observed under higher magnification, the diameters of the selenium particles were measured and the size distribution was found to be between 50 and 100 nm. The surface coverage of selenium nanoparticles was 4.36% according to measurements made by ImageJ. The concentration of the selenium nanoparticles on the coated paper towel surface was about 69.00 g/m2 based on our previous study.33 Compared with the flat surfaces of most other materials, the surface of paper towels with a fibrous structure had a larger surface area, resulting in increased deposition and adhesion of selenium nanoparticles. Overall, the selenium nanoparticles were successfully synthesized and coated on the paper towels using a simple quick reaction (within a minute), and the coatings are stable for a long time period (over a year) at room temperature.
Mechanical tests
In the mechanical tension tests, the maximum stress of the uncoated and selenium coated paper towels were 27.87 kN/m2 and 27.79 kN/m2 (or 2,046 g and 2,040 g stretching loads), respectively, as shown in Figure 3 and Table 1. After the samples were saturated with DI water, the maximum stresses are 2.969 kN/m2 and 2.901 kN/m2 (or 218 g and 213 g loads) for the wet paper towel samples with and without selenium coatings, respectively. Therefore, in both dry and wet conditions, there was no significant change in terms of the mechanical properties for the paper towels after coating with selenium nanoparticles.
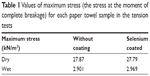  | Table 1 Values of maximum stress (the stress at the moment of complete breakage) for each paper towel sample in the tension tests |
Bacterial inhibitory tests
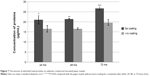
Most significantly, the results of the bacteria assays involving S. aureus, P. aeruginosa, E. coli, and S. epidermidis showed a high effectiveness for the selenium coated paper towels at inhibiting bacteria growth. The selenium coatings showed an 89% inhibition of the growth of S. aureus on the paper towel surface after 24 hours, 48 hours, and 72 hours, which means that the activity of S. aureus was significantly and continuously inhibited on selenium coated paper towels.33 A similar inhibitory activity was observed in the bacterial assays involving another gram-positive bacterium, S. epidermidis. As shown in Figure 4, the selenium coated paper towels had 90.66%, 92.50%, and 92.16% less bacteria attached compared with the uncoated paper towels after 24, 48, and 72 hours, respectively. From 24 hours to 48 hours, there was a large increase in the numbers of bacteria, which means the bacteria propagated quickly during this time period on paper towels without a selenium coating. However, on the surface of selenium coated paper towels, there was not an obvious increase in the bacteria numbers from 24 hours to 48 hours. In addition, after incubation for 48 hours and 72 hours, the uncoated paper towel was saturated by bacteria (S. epidermidis), indicating the formation of a matured biofilm, while the selenium coated paper towels still had a significantly reduced number of bacteria after 72 hours. Overall, the numbers of S. epidermidis on the selenium coated paper towels remained at a low level not increasing after 24, 48, or 72 hours, indicating successful inhibition of the growth and biofilm formation of S. epidermidis.
Results also showed a significant inhibition of P. aeruginosa on selenium coated paper towels. Specifically, the results in Figure 5 showed a successful inhibition of the growth of P. aeruginosa by 55% and 84% after 48 or 72 hours, respectively, on the surface of selenium coated paper towels. But after 24 hours of incubation, there was no significant decrease in bacteria numbers observed on selenium coated paper towels compared with uncoated paper towels. The reason may be that P. aeruginosa are gram-negative bacteria that have an extra bacteria outer membrane (made of lipopolysaccharides and proteins) as a part of their cell wall. Thus, compared with S. aureus and S. epidermidis, which are gram-positive bacteria that only have the plasma membrane as their cell wall but do not have the bacterial outer membrane, it took longer for selenium to penetrate into and interact with P. aeruginosa; thus, it may take a longer time for the gram-negative bacteria to be killed. In addition, the total amount of P. aeruginosa increased from 24 to 48 to 72 hours on paper towels without selenium coatings, while on the surface of selenium coated paper towels, the amount of P. aeruginosa decreased from 24 hours to 48 hours and further decreased dramatically from 48 hours to 72 hours. Therefore, the activity of P. aeruginosa was successfully inhibited on selenium coated paper towels and this inhibition increased as the time of treatment increased. Future studies will also investigate higher concentrations of selenium to decrease P. aeruginosa growth over long time periods.
The results of the bacterial inhibitory assays with E. coli are shown in Figure 6. The selenium coated paper towels inhibited the growth of E. coli by about 50%~60% compared with the uncoated paper towels after treatment for 24 hours, 48 hours, and 72 hours. As shown in Figure 3 and Figure 5, a saturation of S. epidermidis and P. aeruginosa on the uncoated paper towel surfaces was observed after incubation for 48 hours. But for E. coli, after 24 hours of incubation, the bacteria saturated the surface of the paper towels not coated with selenium and formed a mature biofilm. The reason for this could be that E. coli propagates much faster than the other two bacteria as the regeneration time of E. coli is only 15~20 minutes under optimal conditions in the laboratory. Moreover, although the growth of E. coli was significantly inhibited, the percentage of bacteria population reduction was not as much as the reduction for S. aureus and S. epidermidis. Because E.coli is also a gram-negative bacterium, its outer membrane can prevent the interaction between the selenium nanoparticles or the selenium element and the bacteria cells. Overall, compared with the uncoated paper towels, the selenium coated paper towels showed a significant inhibition to the growth of E. coli continuously from 24 to 72 hours.
In conclusion, the growth of S. aureus, S. epidermidis, P. aeruginosa, and E. coli on paper towels was successfully inhibited after coating with selenium nanoparticles. The selenium particles when coated on paper towels were extremely effective and continuously inhibited (about a 90% reduction) the growth of gram-positive bacteria including S. aureus and S. epidermidis after 24, 48, or 72 hours. From gram-negative bacteria like P. aeruginosa and E. coli, the selenium coated paper towels also significantly inhibited bacterial growth after either 48 or 72 hours of incubation. However, it took a longer time for P. aeruginosa and E. coli to be influenced by selenium nanoparticles and the overall reduction of the bacteria population was less than that observed for the gram-positive bacteria, which means that the activities of gram-negative bacteria were more difficult to slow by selenium nanoparticle coatings on the surface of paper towels. However, future studies will investigate higher concentrations or altered selenium nanoparticle sizes to maximally decrease gram-negative bacteria growth.
Protein adsorption assays
Based on the results from protein adsorption assays as shown in Figure 7, the selenium nanoparticle coated paper towels significantly increased protein adsorption over uncoated paper towels after TSB treatment for either 24, 48, or 72 hours. After coating the paper towels with selenium nanoparticles, there was an increase in the surface area and nanoscale roughness, which allowed more proteins in the media to adsorb to the surface of the paper towels. The increased protein adsorption might play an important role in inhibiting bacteria growth on the selenium coated paper towels, because those proteins could interact with bacteria cell membranes and prevent bacteria cells from attaching to the surface. This could eventually prevent bacteria from forming biofilms, thus, inhibiting bacteria growth on the surface.
Release of selenium
Table 2 shows the release of selenium from the selenium nanoparticle coated paper towels, which was measured by ICP-MS. About 0.94% of coated selenium was dissolved into media after the first 24 hours. Another 0.51% was dissolved in the following 24 hours and an even lower amount (0.45%) was released into the solution during the 3rd day of treatment. As observed from the antibacterial tests, the paper towels coated with selenium nanoparticles significantly inhibited bacteria growth from 24 to 72 hours (except the test with E. coli for 24 hours in which no significant difference of bacteria numbers was observed on selenium coated and uncoated paper towels). During this 3-day period, there was only 2% of selenium released from the surface, which means that low selenium concentrations between 15 ppb and 35 ppb were effective enough to inhibit bacterial growth. As less than 1% of selenium was released every 24 hours, potentially the selenium coatings could remain in an aqueous environment for more than 100 days. Thus, the selenium nanoparticle coatings on the paper towel surface could retain its potential antibacterial properties for several months and possibly even longer if coating conditions are optimized.
  | Table 2 The release of selenium from the coated paper towels |
Conclusion
Selenium nanoparticles were synthesized and coated on the surface of paper towels through a simple precipitation process which happened within 1 minute. Impressively, compared with the paper towels without selenium coatings, the selenium coated paper towels significantly inhibited the growth of S. aureus, S. epidermidis, P. aeruginosa, and E. coli after 24, 48, or 72 hours. There was significant and continuous bacteria inhibition with about a 90% reduction from 24 to 72 hours in the growth of gram-positive bacteria including S. aureus and S. epidermidis. The selenium coated paper towels showed a relatively lower effectiveness at inhibiting gram-negative bacteria like P. aeruginosa and E. coli with about a 57% and 84% reduction, respectively, after 72 hours of treatment. In addition, there were larger amounts of proteins adsorbed on the surface of selenium coated paper towels compared with uncoated paper towels, which might be an important reason why selenium nanoparticle coatings can inhibit diverse bacterial growth on the surface of paper towels. Therefore, this study suggested that coating paper products with selenium nanoparticles may be an effective way to decrease various gram-positive and gram-negative bacteria growth on paper products, which might be used for potentially important applications for antimicrobial purposes in the food packaging industry and in clinical environments.
Acknowledgments
The authors thank Mr William H Fowle (Northeastern University) for help with the SEM images. They also thank Northeastern University for funding.
Disclosure
The authors report no conflicts of interest in this work.
References
Barceloux DG. Selenium. J Toxicol Clin Toxicol. 1999;37(2): 145–172. | ||
McDowell LR. Minerals in animal and human nutrition. 2nd ed. Amsterdam: Elsevier; 2003. | ||
Navarro-Alarcon M, Lopez-Martinez MC. Essentiality of selenium in the human body: relationship with different diseases. Sci Total Environ. 2000;249(1–3):347–371. | ||
Redman C, Scott JA, Baines AT, et al. Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998;125(1–2):103–110. | ||
Kaeck M, Lu J, Strange R, Ip C, Ganther HE, Thompson HJ. Differential induction of growth arrest inducible genes by selenium compounds. Biochem Pharmacol. 1997;53(7):921–926. | ||
Huang Y, He L, Liu W, et al. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials. 2013;34(29):7106–7116. | ||
Kong L, Yuan Q, Zhu H, et al. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials. 2011;32(27):6515–6522. | ||
Perla V, Webster TJ. Better osteoblast adhesion on nanoparticulate selenium – A promising orthopedic implant material. J Biomed Mater Res A. 2005;75(2):356–364. | ||
Yang JJ, Huang KH, Qin SY, Wu XS, Zhao ZP, Chen F. Antibacterial Action of Selenium-Enriched Probiotics Against Pathogenic Escherichia coli. Dig Dis Sci. 2009;54(2):246–254. | ||
Pietka-Ottlik M, Wojtowicz-Mlociiowska H, Kolodziejczyk K, Piasecki E, Mlochowski J. New organoselenium compounds active against pathogenic bacteria, fungi and viruses. Chem Pharm Bull (Tokyo). 2008;56(10):1423–1427. | ||
Tran PA, Webster TJ. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int J Nanomedicine. 2011;6:1553–1558. | ||
Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. | ||
Ruparelia JP, Chatteriee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4(3):707–716. | ||
Zhang LL, Jiang YH, Ding YL, Povey M, York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanopart Res. 2007;9(3):479–489. | ||
Taylor EN, Kummer KM, Durmus NG, Leuba K, Tarquinio KM, Webster TJ. Superparamagnetic Iron Oxide Nanoparticles (SPION) for the Treatment of Antibiotic-Resistant Biofilms. Small. 2012;8(19):3016–3027. | ||
Xia T, Kovochich M, Liong M, et al. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano. 2008;2(10):2121–2134. | ||
AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano. 2009;3(2):279–290. | ||
Foldbjerg R, Olesen P, Hougaard M, Dang DA, Hoffmann HJ, Autrup H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 2009;190(2):156–162. | ||
Kryukov GV, Castellano S, Novoselov SV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300(5624):1439–1443. | ||
Tran P, Webster TJ. Enhanced osteoblast adhesion on nanostructured selenium compacts for anti-cancer orthopedic applications. Int J Nanomedicine. 2008;3(3):391–396. | ||
Desjardins E, Beaulieu C. Identification of bacteria contaminating pulp and a paper machine in a Canadian paper mill. J Ind Microbiol Biotechnol. 2003;30(3):141–145. | ||
Namjoshi K, Johnson S, Montello P, Pullman GS. Survey of bacterial populations present in US-produced linerboard with high recycle content. J Appl Microbiol. 2010;108(2):416–427. | ||
Oqvist CK, Kurola J, Pakarinen J, et al. Prokaryotic microbiota of recycled paper mills with low or zero effluent. J Ind Microbiol Biotechnol. 2008;35(10):1165–1173. | ||
Hattula JL, Stevens PE. A descriptive study of the handwashing environment in a long-term care facility. Clin Nurs Res. 1997;6(4):363–374. | ||
Harrison WA, Griffith CJ, Ayers T, Michaels B. Bacterial transfer and cross-contamination potential associated with paper-towel dispensing. Am J Infect Control. 2003;31(7):387–391. | ||
Gendron LM, Trudel L, Moineau S, Duchaine C. Evaluation of bacterial contaminants found on unused paper towels and possible postcontamination after handwashing: A pilot study. Am J Infect Control. 2012;40(2):E5–E9. | ||
Yokota H, Tanabe K, Sezaki M, et al. Arsenic contamination of ground and pond water and water purification system using pond water in Bangladesh. Eng Geol. 2001;60(1–4):323–331. | ||
Rodriguez A, Batlle R, Nerin C. The use of natural essential oils as antimicrobial solutions in paper packaging. Part II. Prog Org Coat. 2007;60(1):33–38. | ||
Balasubramanian A, Rosenberg LE, Yam K, Chikindas ML. Antimicrobial packaging: potential vs reality – a review. Journal of Applied Packaging Research. 2009;3(4):193–221. | ||
Garces LO, de la Puerta C. Inventors; Artibal S. A. assignee. Antimicrobial packaging based on the use of natural extracts and the process to obtain this packaging. Patent EP1657181 A1. 2006 May 17. | ||
Greenwood NN, Earnshaw A. Chemistry of the elements. 2nd ed. Oxford, Boston: Butterworth-Heinemann; 1997. | ||
Zhang JS, Gao XY, Zhang LD, Bao YP. Biological effects of a nano red elemental selenium. Biofactors. 2001;15(1):27–38. | ||
Wang Q, Webster TJ. Short communication: inhibiting biofilm formation on paper towels through the use of selenium nanoparticles coatings. Int J Nanomedicine. 2013;8:407–411. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.