Back to Journals » Journal of Experimental Pharmacology » Volume 11
Inhibition of Trypanosoma evansi Protein-Tyrosine Phosphatase by Myristic Acid Analogues Isolated from Khaya senegalensis and Tamarindus indica
Authors Dingwoke EJ , Adamude FA, Chukwuocha CE, Ambi AA , Nwobodo NN, Sallau AB, Nzelibe HC
Received 13 August 2019
Accepted for publication 8 November 2019
Published 18 December 2019 Volume 2019:11 Pages 135—148
DOI https://doi.org/10.2147/JEP.S226632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Bal Lokeshwar
Emeka John Dingwoke,1 Fatima Amin Adamude,2 Chimee Ethel Chukwuocha,1 Ahmed Adamu Ambi,1 Nwobodo Ndubuisi Nwobodo,3,4 Abdullahi Balarabe Sallau,1 Humphrey Chukwuemeka Nzelibe1
1Department of Biochemistry, Faculty of Life Sciences, Ahmadu Bello University, Zaria, Kaduna State, Nigeria; 2Department of Biochemistry, Faculty of Medical Sciences, Federal University Lafia, Nasarawa State, Nigeria; 3Department of Pharmacology and Therapeutics, College of Medicine, Enugu State University of Science and Technology, Enugu, Enugu State, Nigeria; 4Department of Pharmacology and Therapeutics, College of Health Sciences, Nile University of Nigeria, Abuja, Nigeria
Correspondence: Emeka John Dingwoke
Department of Biochemistry, Faculty of Life Sciences, Ahmadu Bello University, Zaria, Kaduna State, Nigeria
Tel +234 7030665559
Email [email protected]
Background: Trypanosome infections still pose severe health and economic consequences, especially in the endemic regions of Sub-Saharan Africa. Trypanosome differentiation to the procyclic forms which lack the immune evasion mechanisms for survival in the bloodstream is prevented by tyrosine dephosphorylation which is catalyzed by protein-tyrosine phosphatase; thereby promoting survival of the parasites in the host. Inhibition of Protein-tyrosine phosphatase is a strategic therapeutic target that could attenuate trypanosomiasis. This study investigated the in vitro inhibitory effect of stem bark extracts of Khaya senegalensis and Tamarindus indica on the enzymatic activity of protein-tyrosine phosphatase.
Methods: All determinations were carried out following standard procedures for analytical experiments. The analogues of myristic acid that inhibited the enzymatic activity of protein-tyrosine phosphatase were isolated by bioassay-guided fractionation of stem bark extracts of Khaya senegalensis and Tamarindus indica.
Results: Analogues of myristic acid proved to be potent inhibitors of protein-tyrosine phosphatase. Double reciprocal (Lineweaver–Burk) plots of the initial velocity data indicated non-competitive inhibition with Ki of 0.67 mg/mL for Khaya senegalensis and 2.17 mg/mL for Tamarindus indica. The kinetic parameters for the cleavage of para-nitrophenylphosphate by the enzyme showed a KM of 3.44 mM and Vmax of 0.19 μmol/min. Sodium orthovanadate, the enzymes’ specific inhibitor, inhibited the enzyme competitively with Ki of 0.20 mg/mL. Gas chromatography-mass spectrometry analysis of the stem bark bioactive fractions of Khaya senegalensis and Tamarindus indica revealed the presence of myristic acid analogues.
Conclusion: Analogues of myristic acid are potent inhibitors of protein-tyrosine phosphatase that could be developed as trypanocide to inhibit the enzymatic activity of protein-tyrosine phosphatase in order to prevent transmission of trypanosomes.
Keywords: Trypanosoma evansi, protein-tyrosine phosphatase, signal transduction, regulation, inhibition, myristic acid analogues, Khaya senegalensis; Tamarindus indica
Introduction
African Animal Trypanosomiasis (AAT) is a parasitic disease that poses serious threat to livestock production and food security, especially in sub-Saharan Africa. Generally, the disease infects humans, livestock and wild animal reservoirs. Compared to other pathogenic Trypanosoma species; including its ancestral Trypanosoma brucei, Trypanosoma evansi (T. evansi) is known to have the widest range of hosts and geographical distribution worldwide,1 implying that livestock are susceptible to infection by Trypanosoma evansi. Trypanosoma evansi is now considered as an emerging zoonotic parasite.2 The evolution of Trypanosoma evansi as having the widest range of host distribution is largely attributed to the new modes of transmission acquired by the parasite, due to the loss of its genetic material (kinetoplast DNA) that aids the cyclical transmission in tsetse flies.1 Trypanosoma evansi is one of the subgenus Trypanozoon known as the Brucei group. It exhibits variable clinical effects (such as anaemia and infertility) depending on the host and the geographical area.1,2 This characteristic makes the disease not only multispecies but also a polymorphic disease.1 T. evansi is transmitted mechanically from infected host to healthy animals through the haematophagous Dipteran flies belonging to the genera Tabanus, Stomoxys, Haematopota, Lypersia and Hippobosca.1,3 Transmission by tabanid flies is efficient and achieved when there is a short time lapse between two interrupted blood meals that is less than 30 mins.3,4 Transmission can be in several ways; via biting insects, sucking insects, and the vampire bats (Desmodus rotundus). Generally, mechanical transmission by biting insects is the most important mode of transmission in livestock and other large animals.1,5 In Africa, the production losses in cattle due to trypanosome infections have been estimated to be up to 20% across a range of parameters, including mortality, calving rate, meat and milk production.6 So far, there is no vaccine for the disease. The clinical effects of the disease can be reduced by the application of trypanocide and the introduction of trypanotolerant cattle breeds.7
As a consequence of the mechanical transmission of Trypanosoma evansi by blood-feeding insects, especially the tabanids, the disease has spread beyond its original sub-Saharan Africa to South and Asian countries.8 Trypanosoma evansi evades the host immune system by modifying their surface coat; a homogeneous protein coat comprising a single variant surface glycoprotein (VSG). The VSG is a glycophosphatidylinositol-anchored glycosylated protein that protects the invariant antigens on the parasite surface from the immune system. As a result, the parasite undergoes rapid multiplication in the blood of the host, producing waves of parasitaemia that characterize the disease.9,10 The impact of this surface membrane modification has resulted in the appearance of strains of the parasite that are resistant to drugs.11 In the absence of effective vaccination, due to the surface membrane modification, control of trypanosomiasis could be achieved principally by chemoprophylactic or chemotherapeutic agents,7 therefore, highlighting the need to explore novel plant metabolites for new and effective drug to tackle the disease.
Protein-tyrosine phosphatase is implicated in the disease of trypanosomiasis.12,13 Trypanosome differentiation into procyclic forms which lack the immune evasion mechanisms for survival is prevented in the bloodstream by tyrosine dephosphorylation which is catalyzed by protein-tyrosine phosphatase, thereby promoting survival of the parasites in the hosts.12,14–17 Basically, three developmental stages accompany the life cycle of trypanosomes; the stumpy form which exists in the mammalian hosts, the procyclic and the metacyclic forms which exist in the tsetse fly vector. When an infected tsetse fly bites a mammalian host, metacyclic forms are inoculated into the blood.18 These metacyclic forms develop into slender forms that undergo rapid asexual replication, maintaining infection in the hosts.18 As the parasite density increases, a parasite-derived factor known as stumpy induction factor (SIF) accumulate and causes the parasites to undergo cell cycle arrest and differentiate into stumpy forms.19 Stumpy forms have a limited life expectancy in the blood because they no longer replicate or productively switch their variant surface glycoprotein (VSG) coat, but they are infective to tsetse flies and therefore provide the potential for transmission in another mammalian host,20 then, the transmission cycle continues. Hence, stumpy forms are the forms of the parasite that tsetse fly ingests during a blood meal from an infected mammalian host. In the bloodstream of the hosts, differentiation from stumpy forms to procyclic forms is prevented by well-conserved cell signaling events of tyrosine dephosphorylation catalyzed by protein-tyrosine phosphatase. Differentiation to procyclic forms would cause the parasite to loss its glycoprotein coat (immune evasion mechanisms that allow survival in the host), thereby exposing the parasite to the eminent attack by the host immune system. This validates that protein-tyrosine phosphatase is a key regulator that propagates the survival of trypanosomes in the host, hence, a potential target for therapeutic interference against trypanosomiasis.12 If the activity of protein-tyrosine phosphatase is inhibited, differentiation to procyclic forms would occur spontaneously,12 this would result to the killing of the parasites in the bloodstream by the host's immune system.
Although the scientific bases for the use of Khaya senegalensis and Tamarindus indica in the treatment of trypanosomiasis have been reported,21–23 to gain a better insight into the trypanocidal properties of the plants, the inhibitory effects of their stem bark extracts on the enzymatic activity of protein-tyrosine phosphatase were explored by testing the phytochemicals (inhibitors) in the stem bark extracts on the enzyme as well as the determination of the type of inhibition. In a survey that evaluated the use of plants in folklore medicine for the treatment of trypanosomiasis in domestic animals in Nigeria, it was found that Khaya senegalensis and Tamarindus indica were among the most commonly used plants.21 In a similar study, the in vitro anti-trypanosoma activity of aqueous stem bark extract of Khaya senegalensis showed that the extract was trypanocidal against Trypanosoma brucei brucei; at a minimum concentration of 8.3 mg/mL, the parasites were immotile within 30 mins of incubation.22 More so, it was revealed that ethanolic stem bark extract of Khaya senegalensis possessed both in vitro and in vivo anti Trypanosoma evansi activity.23 Incubating 40 µL of Trypanosoma evansi infected blood (109 T. evansi per mL of blood) with 20 µL of ethanolic stem bark extract of Khaya senegalensis at 37°C eliminated the parasites within 5 mins of post-incubation,23 while treatment of Trypanosoma evansi-infected rats with 80 mg/kg body weight of the same extract significantly lowered the parasitemia level within 13 days of post-infection.23 Protein-tyrosine phosphatase was previously isolated from Trypanosoma evansi and characterized,24 in continuation, we studied the inhibitory effect of stem bark extracts of Khaya senegalensis and Tamarindus indica on the enzyme.
Materials and Methods
Plant Material
The stem bark of Khaya senegalensis and Tamarindus indica were collected during the dry season of October from Kudigi village in Zaria, 11°5ʹ7.9476ʺ N and 7°43ʹ11.8020ʺ E, Sabon-gari Local Government Area, Kaduna state, Nigeria. They were authenticated by a taxonomist at the Herbarium, Department of Botany, Ahmadu Bello University, Zaria. Their respective voucher number 900181 and 026 were obtained.
Preparation and Extraction of Samples
The stem bark of Khaya senegalensis and Tamarindus indica were thoroughly washed with clean water and air-dried at room temperature for 3 weeks. The dried stem barks were grounded into fine powder using mortar and pestle. Exactly 100g of each of the power obtained was weighed using a weighing balance (Contech® Instruments Ltd India, Model CAC-224) and initially extracted by maceration with 500 mL of methanol for 48 hrs. Few drops of chloroform were added to ensure there was no fungi growth during the 48 hrs of extraction, they were then concentrated by freeze-drying. The methanolic extract obtained was exhaustively extracted using ethyl acetate, n-butanol, and chloroform for partitioning into individual fractions. Exactly 0.5g of the methanolic extract was reconstituted in 100 mL of water and vigorously extracted in triplicates by ethyl acetate (50 mL) in a 250 mL separating funnel. After extraction, the solution was left for phase separation. The organic fraction of ethyl acetate was filtered using Whatman filter paper No.1 and concentrated by freeze-drying using bench top freeze dryer (Labconco™) at 4°C for 2 and half hours. A similar partitioning process was also carried out to obtain n-butanol and chloroform fractions. They were stored in air-tight bottles and kept in the refrigerator until used. These served as the crude extracts.
Bioassay-Guided Fractionation and Determination of the Active Plant Extract
The activity of each of the fractions (ethyl acetate, n-butanol, and chloroform) obtained was determined by bioassay-guided fractionation. An aliquot (50 mg) of each of the extracts was measured and dissolved in 10 mL of distilled water. Each extract was incubated with the enzyme under the standard assay conditions for the enzyme. The n-butanolic extracts showed activity against the enzyme and were further used. The technique of preparative thin-layer chromatography (TLC) was used to narrow down the search for the particular bioactive phytochemical in the n-butanolic extract.
Fractionation of n-Butanolic Stem Bark Extracts of Khaya senegalensis and Tamarindus indica by Preparative Thin Layer Chromatographic (TLC)
The n-butanolic stem bark extracts of Khaya senegalensis and Tamarindus indica were partially purified by fractionation on preparative thin-layer chromatography (TLC) using silica gel (60 F254) coated aluminum-backed TLC plate (EMD MilliporeTH). Using sterile capillary tube, a spot of about 1mm of the n-butanolic extract was made horizontally on the TLC plate (1ʺ × 3ʺ). Then, ascending thin layer chromatography was carried out by placing the plate vertically in a chromatographic tank-containing n-butanol, acetic acid, and water (6:2:1) as the solvent system for stem bark extract of Khaya senegalensis. The same procedure was followed for the stem bark extract of Tamarindus indica using n-hexane and methanol (3:2) as solvent system. At the end of the chromatography, the TLC plates were viewed under UV light for the detection of the separated compounds which appeared as bands. These procedures were carried out in a fume-hood to prevent inhalation of chemicals. The individual bands were scraped and suspended in 2 mL of the respective solvent systems and centrifuged at 3000 ×g for 10 mins. The supernatants were concentrated to dryness by subjecting them to rotary evaporator (RE-20ID, Xingyan Kori, China) at 40°C and reconstituted with 2 mL of distilled water. Thereafter, they were freeze-dried using a bench top freeze dryer (Labconco™) at 4°C for 2 hrs. Reconstitution with 2 mL of distilled water was carried out in order to ensure that there was no trace of the extracting solvents in any of the scrapped bands. Each of the bands was verified for inhibitory effects against Protein-tyrosine phosphatase. The bands with inhibitory activity were submitted to Gas chromatography-mass spectrometry analysis (GCMS-QP2010 PLUS Shimadzu, Japan) to identify the bioactive compounds in the test sample.
Isolation of Protein-Tyrosine Phosphatase
Protein-tyrosine phosphatase was previously isolated from trypanosoma evansi and characterized.24 Here, we focus on the inhibition of the enzyme.
Enzyme Assay
The assay of protein-tyrosine phosphatase (PTPase) was carried out following the standard method as described.25 The assay was done with 10 mmol/L para-nitrophenylphosphate (pNPP) as substrate in assay buffer (10 mM sodium phosphate, pH 8.0, 1 mmol/L dithiothreitol, 1 mmol/L EDTA, and 10% glycerol), to a final reaction volume of 1 mL (700 µL assay buffer, 250 µL para-nitrophenylphosphate, 50 µL PTPase) at 37°C for 10 mins. The reaction was terminated after 10 mins by the addition of 1 mL of stop reagent (0.2 M NaOH). The para-nitrophenol produced was determined by measuring the absorbance at 410 nm. The assay specificity for PTPase was verified by the addition of sodium orthovanadate (PTPase inhibitor) to the reaction mixture at a final volume of 5 µL, 15 µL, and 25 µL. The Extinction coefficient of para-nitrophenol was 19.03 at 410 nm.25 One unit of activity is equivalent to 1 nmol/L of para-nitrophenylphosphate hydrolyzed per minute.
Inhibition Study
The inhibition study was carried out by incubating varying concentrations; 0 mg/mL, 0.5 mg/mL, 1 mg/mL, and 2 mg/mL (Table 1) of the bioactive band from n-butanolic extract. Para-nitrophenylphosphate was used as substrate for the measurement of enzymatic activity. The buffer solution was a mixture of 25 mM Tris–HCl (pH 7.5), 2 mM b-mercaptoethanol, 1 mM ethylenediaminetetraacetic acid (EDTA), and 1 mM dithiothreitol (DTT). The inhibition assay was performed by adding 25 µL of each of the varying concentration to 675 µL assay buffer, 50 µL PTPase, 250 µL of 10 mM para-nitrophenylphosphate to a final reaction volume of 1 mL. Each of the reaction mixtures was incubated at 37°C for 10 mins. The reaction was terminated by adding 200 µL of stop reagent (0.2 M NaOH). Para-nitrophenol produced following the dephosphorylation of para-nitrophenylphosphate by protein-tyrosine phosphatase was monitored using table top Shimadzu spectrophotometer at 410 nm. Sodium orthovanadate (Na3VO4) was used as positive control of the inhibition. The readings obtained from spectrophotometer were used for Lineweaver–Burk plots26 using sigma plot software (SPCC Inc., Chicago, IL) in order to determine the type of inhibition and to calculate the kinetic parameters.
 |
Table 1 Incubation Pattern Using the Bioactive Stem Bark Fraction of the Extracts |
Assay Specificity of PTPase by Inhibition with Sodium Orthovanadate (Na3VO4)
A working stock solution (0.1 M) of sodium orthovanadate was prepared by dissolving 0.92 g Sodium orthovanadate (Sigma Aldrich, USA) in 30 mL of distilled water and placed on a bench top shaker (Thermo Scientific MaxQ™ 4000 orbital shaker) at room temperature until the yellowish solution turned colorless. Protein-tyrosine phosphatase was incubated on ice in the presences of varying concentrations (0.2 mg/mL, 0.5 mg/mL, and 1 mg/mL) of sodium orthovanadate. The assay was performed with 10 mmol/L para-nitrophenylphosphate as substrate in a buffer (25 mM, 4-2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), pH 7.3, and 10% glycerol), to a final reaction volume of 1 mL (695 µL assay buffer, 50 µL PTPase, 5 µL of 0.2 mg/mL sodium orthovanadate, 250 µL para-nitrophenylphosphate) at 37°C for 10 mins. This was followed for the 15 µL of 0.5 mg/mL and 25 µL of 1 mg/mL of sodium orthovanadate. The reaction was terminated after 10 mins by the addition of 10 mM dithiothreitol (10 µL). The activity was measured using table top Shimadzu spectrophotometer at 410 nm. Data obtained was used for Lineweaver–Burk plot as described above.
Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Aliquots of each bioactive fraction of the extracts were subjected to analysis using GCMS-QP 2010 plus Shimadzu, Japan. Standard analytical conditions for the GC-MS were followed. The chromatographic conditions were as follows: flow rate was 1.58 mL/min pressure with split ratio of 1:0. The machine was programmed with the injection syringe positioned at 250ºC temperature and 100.2 pressures and at 60% column oven. The carrier gas was helium at a flow rate of 1.58 mL/min. The transfer line and source temperature were set at 280ºC. The oven temperature was initially at 80ºC for 2 mins, then increased to 200ºC at a rate of 9ºC per min until 280ºC and held at 280ºC at a flow rate of 10ºC for 5 mins. The chromatograms were acquired in full scan mode (m/z 40–800). The NIST/EPA/NIH Mass Spectral Library (NIST/EPA/NIH 11, version 2.0) was used for identification of the compounds.
Results
Analogues of myristic acid isolated from the stem bark extracts of Khaya senegalensis and Tamarindus indica proved to be potent inhibitors of Trypanosoma evansi protein-tyrosine phosphatase. Interestingly, the Lineweaver–Burk plot of the inhibition indicated that the analogues of myristic acid present in the stem bark extracts of Khaya senegalensis and Tamarindus indica inhibited the enzyme non-competitively as shown in Figures 1 and 2, respectively. This implies that analogues of myristic acid are non-competitive inhibitors of protein-tyrosine phosphatase. The inhibitor binding constant (Ki) was 0.67 mg/mL for Khaya senegalensis and 2.17 mg/mL for Tamarindus indica. The enzyme kinetic parameters for the cleavage of para-nitrophenylphosphate substrate (Figure 3) showed a KM of 3.44 mM and Vmax of 0.19 µmol/min. Sodium orthovanadate (specific inhibitor of protein-tyrosine phosphatase) inhibited the enzyme competitively with a Ki of 0.20 mg/mL.
 |
Figure 1 Lineweaver–Burk plot showing non-competitive inhibition of Trypanosoma evansi protein-tyrosine phosphatase by the stem bark bioactive fraction of Khaya senegalensis. |
 |
Figure 2 Lineweaver–Burk plot showing non-competitive inhibition of Trypanosoma evansi protein-tyrosine phosphatase by the stem bark bioactive fraction of Tamarindus indica. |
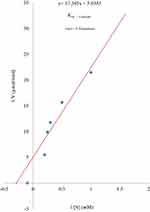 |
Figure 3 Lineweaver–Burk plot for the cleavage of para-nitrophenylphosphate by Trypanosoma evansi protein-tyrosine phosphatase. |
Gas Chromatography–Mass Spectroscopy (GC-MS) Analysis
Results of the gas chromatography-mass spectrometry analysis of the bioactive stem bark fractions of Khaya senegalensis and Tamarindus indica showed that seven compounds were present in the bioactive fraction of Khaya senegalensis, namely, 4-hydroxy-methylolacetone, methyltrimethylene glycol, 2-methylpropyl ester, n-tridecanoic acid, n-tetratriconate, n-hexadecanioc acid, and 14-pentadecanoic acid, with their corresponding molecular weight and structures as shown in Figure 4. Compounds present in the bioactive fraction of Tamarindus indica were five, namely, n-decanoic acid, n-tridecanoic acid, 1-(+)-ascorbic acid 2,6-dihexadecanoate, methyl trans-9-octadecanoate, and octa-9-enoic acid, with their corresponding molecular weight and structures as presented in Figure 5. The GC-MS revealed that these compounds are analogues of myristic acid.
 |
Figure 4 Gas chromatography–mass spectrometry spectra of the stem bark bioactive fraction of Khaya senegalensis. |
 |
Figure 4 Continued. |
 |
Figure 4 Continued. |
Discussion
Protein-tyrosine phosphatases are enzymes that dephosphorylate proteins on tyrosine residues and together with protein-tyrosine kinases that phosphorylate proteins on tyrosine residues are responsible for the regulation of signaling events that control fundamental biological processes in living cells. As a key mechanism for trypanosome survival in which differentiation to procyclic forms is prevented in the bloodstream by tyrosine dephosphorylation,12 protein-tyrosine phosphatase plays major role in the disease of trypanosomiasis, hence, an important therapeutic target. This study investigated the inhibitory effect of stem bark extracts of Khaya senegalensis and Tamarindus indica on the enzymatic activity of protein-tyrosine phosphatase, with the view to establishing a novel and potent trypanocide that targets the activity of protein-tyrosine phosphatase in trypanosomes. This was inspired by the use of the plants in folklore medicine for the treatment of African Animal Trypanosomiasis.21–23 During this study; it was found that the bioactive fractions of the extracts inhibited trypanosoma evansi protein-tyrosine phosphatase dose-dependently. This may probably be the scientific rationale for the use of stem bark extracts of the two plants for the treatment of animal trypanosomiasis in folklore medicine. Considering its dephosphorylation mechanism which aids the survival of the stumpy forms of the parasite in the host’s bloodstream and for uptake into tsetse flies,19,27 the inhibition of Trypanosoma evansi protein-tyrosine phosphatase by the bioactive fractions suggests that the compounds in the fractions could serve as potential candidates for effective trypanocide.
 |
Figure 5 Gas chromatography–mass spectrometry spectra of the stem bark bioactive fraction of Tamarindus indica. |
 |
Figure 5 Continued. |
The non-competitive inhibition of Trypanosoma evansi protein-tyrosine phosphatase indicated that the inhibitors were allosterically bound to a site on the enzyme-substrate complex other than the enzyme active site. The observed reductions in the enzyme activity could be attributed to the effectiveness of the phytochemicals in the bioactive fractions, which were identified as analogues of myristic acid. This shows that the phytochemicals (analogues of myristic acid) are capable of interacting efficiently with the enzyme; suggesting that the agents are potential trypanocides.
A hallmark of the classical protein-tyrosine phosphatase is the strictly conserved primary structure, including cysteine and arginine residues in the catalytic domain which constitutes a phosphate-binding pocket.28–31 The inhibition of the enzymatic activity by the analogues of myristic acid could probably be through the oxidation mechanism; particularly through oxidation of the cysteine residue, leading to the inactivation of the enzyme. The cysteine residue localized in the catalytic center exists in a thiolate anion form and is susceptible to oxidation.28 The myristic acid analogues were bound irreversibly to the enzyme as evident by the non-competitive inhibition, suggesting that they may be highly oxidizing. Highly oxidizing conditions can induce oxidation to the sulfinic and sulfonic acid residues of protein-tyrosine phosphatase, which is considered irreversible under physiological conditions.32
In the band regions of the GC-MS spectra of bioactive stem bark fraction of Khaya senegalensis, the percentage abundance of 4-hydroxy-methylolacetone was 3.63%, methyltrimethylene glycol was 3.31%, 2-methylpropyl ester was 1.61%, n-tridecanoic acid was 4.01%, n-tetratriconate was 25.11%, n-hexadecanioc acid was 25.06%, and 14-pentadecanoic acid was 37.26% as shown in Figure 4. Also, for the compounds present in the bioactive fraction of Tamarindus indica, the percentage abundance of n-decanoic acid was 2.47%, n-tridecanoic acid was 6.17%, 1-(+)-ascorbic acid 2.6-dihexadecanoate was 35.78%, methyl trans-9-octadecanoate was 4.30%, and octadec-9-enoic acid was 51.27 as shown in Figure 5. From the percentage abundance of the various compounds, for Khaya senegalensis, 14-pentadecanoic acid was the highest in abundance while 2-methylpropyl ester was the least in abundance. For Tamarindus indica, octa-9-enoic acid had highest percentage of 51.27% while n-decanoic acid had the lowest abundance of 2.47%. Probably, the proportions of the percentage abundance of the compounds may not be a significant factor that determines the compound that exhibited the highest degree of inhibition; the compounds may have acted synergistically towards inhibition of the enzyme.
Incubation of the analogues of myristic acids with trypanosoma evansi protein-tyrosine phosphatase showed that the enzymatic activity was irreversibly inhibited. It has been established that myristoylation is an irreversible and co-translational protein modification essential for membrane targeting and signal transduction.33 In a previous study that investigated the effects of peroxytetradecanoic acid, an analogue of myristic acid on the enzymatic activity of Protein-tyrosine phosphatase CD45, it was found that peroxytetradecanoic acid is a potent inhibitor of protein-tyrosine phosphatase CD45. Peroxytetradecanoic acid was found to be effective in nanomolar concentrations of 50 nM which caused 50% decrease in the enzymatic activity.33 The observation supports the findings of this study; incubation of the enzyme with a low concentration of 0.2 mg/mL of the bioactive fraction of the extracts caused potent inhibition with an inhibitor binding constant (Ki) of 0.67 mg/mL for Khaya senegalensis and 2.17 mg/mL for Tamarindus indica. The low Ki which indicates that the inhibitors in the extracts bound the enzyme with high affinity, suggests that they would be effective inhibitors. In a related study, it was found that the growth of bloodstream forms of Trypanosoma evansi in axenic culture was inhibited by incubation with 11-oxatetradecanoic acid, an analogue of myristic acid.34,35 It was shown that the concentration of 11-oxatetradecanoic acid that inhibited trypanosome growth by 50% (LD50) was 3.7±0.2 µM as measured by direct counting of survivors using a haemocytometer, 5.1±2.0 µM in a colorimetric test based on the formation of a formazan product, and 8.8±3.7 µM by estimation of pyruvate.34 This also supports the observed inhibition of Trypanosoma evansi protein-tyrosine phosphatase by low concentrations of the analogues of myristic acids isolated from stem bark of the plants. In a study of the utilization of heteroatom-containing analogues of myristate both in a cell-free system and in vivo,35 the result indicated that 10-(propoxy)decanoic acid, myristic acid analogue was highly toxic to Trypanosoma brucei in culture, although it was nontoxic to mammalian cells.35 The study reported that at 10 µM, 11-oxatetradecanoic acid killed >99% of cultured trypanosomes within 24 hrs, 13-oxatetradecanoic acid inhibited the growth rate by 40%, and 6-oxatetradecanoic acid inhibited the growth rate by 10%.35 In another study that assayed 244 different fatty acid analogues, with chain lengths comparable to that of myristate for trypanocidal effects, in addition to their trypanocidal effects upon incorporation in the parasite’s variant surface glycoprotein (VSG), it was found that myristate analogues are useful for studying the mechanism of glycosyl phosphatidylinositol (GPI) myristoylation, and they are candidates for antitrypanosomal chemotherapy.36 The usefulness of these agents reported in the previously mentioned studies as candidates for antitrypanosomal chemotherapy correlates with the findings of this study which indicated that the myristic acid analogues isolated from the bioactive fractions could be the antidote to trypanosomiasis and could as well constitute a novel and effective trypanocide without side effects. Although the aforementioned studies have demonstrated that myristic acid analogues are effective inhibitor of protein-tyrosine phosphatase, but being synthetic products would most likely pose the challenge of toxicity and adverse effects known to be associated with synthetic agents,37,38 unlike inhibitor from natural sources that have little or no side effects and adverse reactions.39–41
In a side-by-side comparison of inhibition of protein-tyrosine phosphatase by the bioactive fraction of the stem bark extracts and sodium orthovanadate (specific inhibitor of protein-tyrosine phosphatase), results showed that while the inhibitors in the extracts inhibited the enzyme non-competitively, sodium orthovanadate inhibited the enzyme competitively as shown in Figures 1, 2 and 6, respectively. Compared to the competitive inhibition by sodium orthovanadate, it implies that the non-competitive inhibitors in the bioactive fraction of the stem bark extracts of Khaya senegalensis and Tamarindus indica are better inhibitors of the enzyme. This is because increase in substrate concentration would not reverse the inhibition unlike in competitive inhibition where increase in substrate concentration reverses inhibition. 4-2-Hydroxyethyl-1-piperazineethanesulfonic acid (HEPES) was used in the inhibition assay of protein-tyrosine phosphatase because it does not complex with sodium orthovanadate, unlike other buffers, hence, an ideal buffer. Although ethylenediaminetetraacetic acid (EDTA) is commonly included in protein-tyrosine phosphatase assays, it was excluded here because it is known to chelate and form a complex with sodium orthovanadate.42 Protein-tyrosine phosphatase is specific for dephosphorylating phosphotyrosyl residues of proteins and peptides,43 making it a key regulator of a variety of fundamental cellular processes. This work has introduced naturally occurring myristic analogues that are potent inhibitors of protein-tyrosine phosphatase which can be possibly used to attenuate the disease of trypanosomiasis.
 |
Figure 6 Lineweaver–Burk plot for the inhibition of protein-tyrosine phosphatase by sodium orthovanadate. Orthovanadate inhibited the enzyme competitively, while the bioactive fraction of the plant extracts inhibited the enzyme non-competitively in a dose-dependent fashion as shown in Figures 1 and 2. |
Conclusion
The experiment described in this paper has shown that analogues of myristic acid isolated from stem bark of Khaya senegalensis and Tamarindus indica are non-competitive inhibitors of Trypanosoma evansi protein-tyrosine phosphatase. If harnessed, the myristic acid analogues could be developed as effective and potent trypanocides that would inhibit the enzymatic activity of protein-tyrosine phosphatase in trypanosomes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Desquesnes M, Holzmuller P, Lai DH, Dargantes A, Lun ZR, Jittaplapong S. Trypanosoma evansi and Surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed Res Int. 2013;2013:1–22. doi:10.1155/2013/194176
2. Fong IW. New and emerging parasitic zoonoses, in emerging zoonoses. Fong IW, Editor. Cham, Switzerland. Springer Int Publishing. 2017;211–239.
3. Singla LD, Juya PD, Sharma NS. Immune responses to haemorrhagic septicaemia (HS) vaccination in Trypanosoma evansi infected buffalo-calves. Trop Anim Hlth Prod. 2010;42:589–595. doi:10.1007/s11250-009-9461-1
4. Sumba AL, Mihok S, Oyieke FA. Mechanical transmission of Trypanosoma evansi and T. congolense by Stomoxys niger and S. taeniatus in a laboratory mouse model. Med Vet Entomol. 1998;12:417–422. doi:10.1046/j.1365-2915.1998.00131.x
5. Muhanguzi D, Picozzi K, Hattendorf J, et al. The burden and spatial distribution of bovine African trypanosomes in small holder crop-livestock production systems in Tororo District, south-eastern Uganda. Parasites Vectors. 2014. doi:10.1186/s13071-014-0603-6
6. Swallow BM. Impacts of Trypanosomiasis on African Agriculture. Programme Against African Trypanosomiasis (PAAT) Technical and Scientific Series 2. FAO; 2000.
7. Holt HR, Selby R, Mumba C, Napier GB, Guitian J. Assessment of animal African trypanosomiasis (AAT) vulnerability in cattle-owning communities of sub-saharan Africa. Parasites Vectors. 2016;9:53–64. doi:10.1186/s13071-016-1336-5
8. Vanhollebeke B, Truc P, Poelvoorde P, et al. Human Trypanosoma evansi infection linked to a lack of apolipoprotein 1. New Engl J Med. 2006;355:2752–2756.
9. Nok AJ, Nzelibe HC, Yako SK. Trypanosoma evansi sialidase: surface localization, properties and hydrolysis of ghost red blood cells and brain cells-implications in trypanosomiasis. Z Naturforsh. 2003;58:594–601. doi:10.1515/znc-2003-7-825
10. Habila N, Inuwa HM, Aimola IA, Udeh MU, Haruna E. Pathogenic mechanisms of trypanosoma evansi infections. Res Vet Sci. 2012;93:13–17. doi:10.1016/j.rvsc.2011.08.011
11. Delespaux V, De Koning HP. Drug and drug resistance in African trypanosomiasis. Drug Resist Update. 2007;67:30–50. doi:10.1016/j.drup.2007.02.004
12. Szoor B, Wilson J, Mcelhinney H, Tabernero H, Keith R, Matthews R. Protein-tyrosine phosphatase TbPTP1: a molecular switch controlling life cycle differentiation in trypanosomes. J Cell Biol. 2006;175:293–303. doi:10.1083/jcb.200605090
13. Seemay C, Bryan CJ, Marilyn P, Tom A, Christoph G. The trypanosome brucei life cycle switch Tbtpt1 is structurally conserved and dephosphorylates the nucleolar protein NOPP44/46. J Bio Chem. 2010;285:22075–22081. doi:10.1074/jbc.M110.108860
14. Bakalara N, Seyfang A, Davis C, Baltz T. Characterization of a life-cycle-stage-regulated membrane protein tyrosine phosphatase in Trypanosoma brucei Eur J Biochem. 1995;234:871–877. doi:10.1111/ejb.1995.234.issue-3
15. Persons M, Ledbetter JA, Schieven GL, Nel AE, Kanner SB. Developmental regulation of pp44/46, tyrosine-phosphorylated proteins associated with tyrosine/serine kinase activity in Trypanosoma brucei. Mol Biochem Parasitol. 1993;63:69–78. doi:10.1016/0166-6851(94)90009-4
16. Galán-Caridad JM, Calabokis M, Uzcanga G, Aponte F, Bubis J. Identification of casein kinase 1, casein kinase 2, and cAMP dependent protein kinase-like activities in Trypanosoma evansi. Mem Inst Oswaldo Cruz. 2004;99:845–854. doi:10.1590/S0074-02762004000800011
17. Bakalara N, Seyfang A, Baltz T, Davis C. Trypanosoma brucei and Trypanosoma cruzi: life cycle-regulated protein tyrosine phosphatase activity. Exp Parasitol. 1995;81:302–312. doi:10.1006/expr.1995.1121
18. Vassella E. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110:2661–2671.
19. Pollitt LC, MacGregor P, Matthews K, Reece SE. Malaria and trypanosome transmission: different parasites, same rules? Trends Parasitol. 2011;27:197–203. doi:10.1016/j.pt.2011.01.004
20. Roditi I, Lehane MJ. Interactions between trypanosomes and tsetse flies. Cur Op Microbiol. 2008;11:345–351. doi:10.1016/j.mib.2008.06.006
21. Atawodi SE, Ameh DA, Ibrahim S, et al. Indigenous knowledge system for treatment of trypanosomiasis in Kaduna State of Nigeria. J Ethnopharmacol. 2002;79:279–282. doi:10.1016/S0378-8741(01)00351-8
22. Wurochekke AU, Nok AJ. In vitro anti trypanosomal activity of some medicinal plants used in the treatment of trypanosomosis in northern Nigeria. Afri J Biotech. 2004;3:481–483. doi:10.5897/AJB2004.000-2094
23. Umar IA, Ibrahim MA, Fari NA, Isa S, Balogun DA. In-vitro and in-vivo anti-trypanosoma evansi activities of extracts from different parts of khaya senegalensis. J Cell Animal Bio. 2010;4:91–95.
24. Dingwoke EJ, Nzelibe HC, Sallau AB, Suleiman A. Purification and characterization of protein-tyrosine phosphatase from Trypanosoma evansi. Int J Sc Res. 2015;4:2281–2288.
25. Zhang X, Xing S, Wu X, Lin F, Fu X, Li W. Purification and characterization of protein-tyrosine phosphatase MEG1 and preparation of anti-PTP MEG1 antibody. Chem Res Chin Univ. 2010;26:591–595.
26. Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Ameri Chem Soci. 1934;56:658–666. doi:10.1021/ja01318a036
27. Mattews K, Eleanor MB, Fredrerick JD, et al. Developmental regulation and extracellular release of a VSG expression-site-associated gene product from tyrpanosoma brucei bloodstream forms. J Cell Sci. 2010;123:4301–4311. doi:10.1242/jcs.075077
28. Tabernero L, Aricescu AR, Jones EY, Szedlacsek SE. Protein tyrosine phosphatases: structure-function relationships. FEBS J. 2008;275:867–882. doi:10.1111/ejb.2008.275.issue-5
29. Zhang ZY, Wang Y, Dixon JE. Dissecting the catalytic mechanism of protein-tyrosine phosphatases. Proc Natl Acad Sci. 1994;91:1624–1627. doi:10.1073/pnas.91.5.1624
30. Andersen JN, Mortensen OH, Peters GH, et al. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol. 2001;21:7117–7136. doi:10.1128/MCB.21.21.7117-7136.2001
31. Mustelin T, Tautz L, Page R. Structure of the hematopoietic tyrosine phosphatase (HePTP) catalytic domain: structure of a KIM phosphatase with phosphate bound at the active site. J Mol Biol. 2005;354:150–163. doi:10.1016/j.jmb.2005.09.049
32. Ostman A, Frijhoff J, Sandin A, Bohmer F. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. 2011;150:345–356. doi:10.1093/jb/mvr104
33. Kuban-Jankowska A, Tuszynski JA, Winter P, Gorska M, Knap N, Wozniak M. Activation of hydrogen peroxide to peroxytetradecanoic acid is responsible for potent inhibition of protein-tyrosine phosphatase CD45. PLoS One. 2012;7:543–554. doi:10.1371/journal.pone.0052495
34. Ross CA, Taylor AM. Trypanocidal activity of a myristic acid analog in axenic cultures of trypanosoma evansi. Parasitol Res. 1994;80:147–153. doi:10.1007/BF00933783
35. Doering TL, Raper J, Buxbaum LU, et al. An analog of myristic acid with selective toxicity for African trypanosomes. Science. 1991;252(5014):1851–1854. doi:10.1126/science.1829548
36. Doering TL, Lu T, Werbovetz KA, et al. Toxicity of myristic acid analogs towards African trypanosomes. Proc Natl Acad Sci. 1994;91:919735–919739.
37. Arnold C. The new danger of synthetic drugs. Lancet. 2013;382:15–16. doi:10.1016/S0140-6736(13)61512-3
38. Gurney SM, Scott KS, Kacinko SL, Presley BC, Logan BK. Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs. Forensic Sci Rev. 2014;26:53–78.
39. Haq I. Safety of medicinal plants. Pak J Med Res. 2004;43(4):203–210.
40. Rafieian-Kopaie M. Medicinal plants for renal injury prevention. J Renal Inj Prev. 2013;2(2):63–65. doi:10.12861/jrip.2013.21
41. Karimi A, Majlesi M, Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol. 2015;4:27–30.
42. Crans DC, Simone CM. Nonreductive interaction of vanadate with an enzyme containing a thiol group in the active site: glycerol-3-phosphate dehydrogenase. Biochem. 1991;30:6734–6741. doi:10.1021/bi00241a015
43. Stone RL, Dixon JE. Protein-tyrosine phosphatases. J Biol Chem. 1994;269:31323–31326.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
