Back to Journals » Journal of Inflammation Research » Volume 15
Inhibition of miRNA-1-Mediated Inflammation and Autophagy by Astragaloside IV Improves Lipopolysaccharide-Induced Cardiac Dysfunction in Rats
Authors Wang Q, Chen W, Yang X, Song Y, Sun X, Tao G, Wang H, Zhao N, Huang Y, Chai E, Tang F
Received 14 February 2022
Accepted for publication 5 April 2022
Published 23 April 2022 Volume 2022:15 Pages 2617—2629
DOI https://doi.org/10.2147/JIR.S362368
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Qiuning Wang,1,* Weiying Chen,2,* Xuefeng Yang,3 Ying Song,4 Xiaowei Sun,5 Guizhou Tao,6 Hong Wang,7 Nan Zhao,7 Yue Huang,1 Erqing Chai,8 Futian Tang9
1Department of Pharmacology, Jinzhou Medical University, Jinzhou, Liaoning Province, People’s Republic of China; 2Department of Drug Quality Analysis, Jiuquan Drug Inspection and Testing Center, Jiuquan, Gansu Province, People’s Republic of China; 3Department of Physiology, Jinzhou Medical University, Jinzhou, Liaoning Province, People’s Republic of China; 4Cardiovascular Laboratory, the First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning Province, People’s Republic of China; 5Department of Neurosurgery, China Resources Liaojian Group, General Hospital of Fuxin Mining Group (10th Clinical College of China Medical University), Fuxin, Liaoning Province, People’s Republic of China; 6Internal Medicine-Cardiovascular Department, the First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning Province, People’s Republic of China; 7Allergy and Clinical Immunology Center, the First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning Province, People’s Republic of China; 8Neurointerventional Department, Emergency General Hospital, Beijing, People’s Republic of China; 9Department of Cardiovascular Disease and Key Laboratory of Digestive System Tumor of Gansu Province, Lanzhou University Second Hospital, Lanzhou, Gansu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Futian Tang, Department of Cardiovascular Disease and Key Laboratory of Digestive System Tumor of Gansu Province, Lanzhou University Second Hospital, Lanzhou, Gansu Province, People’s Republic of China, Email [email protected] Erqing Chai, Neurointerventional Department, Emergency General Hospital, Beijing, People’s Republic of China, Email [email protected]
Introduction: Astragaloside IV (AS-IV) is one of the main active components isolated from the traditional Chinese medicinal herb, Astragalus membranaceus. The present study was designed to investigate whether the regulation of microRNA-1 (miR-1)-mediated inflammation and autophagy contributes to the protective effect of AS-IV against cardiac dysfunction in rats treated with lipopolysaccharides (LPS).
Methods: Animal model of cardiac dysfunction in rats or cellular model of injured H9c2 heart cell line was established by using LPS. Echocardiography, electron microscopy, enzyme-linked immunosorbent assay, immunofluorescence, quantitative RT-PCR, and Western blotting were used to determine the cardiac function and expression of inflammation- and autophagy-related proteins at both the mRNA and protein levels.
Results: LPS caused cardiac dysfunction in rats or injury in H9c2 cells and induced inflammation and autophagy. Compared with LPS treatment, AS-IV treatment attenuated cardiac dysfunction or cell injury, accompanied by inhibition of inflammation and autophagy. However, the miR-1 mimics partly abolished the effects of AS-IV. In addition, the effect of the miR-1 inhibitor was similar to that of AS-IV in the LPS model. Further analyses showed that AS-IV treatment decreased the mRNA expression of miR-1 in the heart tissue of rats and H9c2 cells treated with LPS.
Conclusion: These results suggest that AS-IV attenuated cardiac dysfunction caused by LPS by inhibiting miR-1-mediated inflammation and autophagy, thereby providing a novel mechanism for the protection against cardiac diseases.
Keywords: lipopolysaccharides, astragaloside IV, cardiac dysfunction, inflammation, autophagy, miRNA-1
Introduction
Astragali Radix is prepared from the roots of Astragalus membranaceus (Fisch.) Bunge.1 As a kind of traditional Chinese medicine, Astragali Radix has a wide range of pharmacological effects, especially on cardiovascular diseases, such as coronary heart disease,2 heart failure,3 viral myocarditis,4 and hypertension.5 Astragaloside IV (AS-IV), one of the main active ingredients in A. membranaceus, exerts various protective effects against cardiovascular diseases, such as preventing the endothelial dysfunction of the aortas in streptozotocin-induced diabetic mice,6 ameliorating lead-related cognitive impairments in mice,7 attenuating hypoxia-induced pulmonary vascular remodeling,8 and restoring myocardial infarction-induced cardiac dysfunction.9 Many studies have shown that several mechanisms contribute to the protective effects of AS-IV against the aforementioned diseases. For example, AS-IV can improve oxidative stress,6 induce Nrf2 nuclear translocation,7 regulate the Notch signaling pathway,8 and inhibit apoptosis by inactivating the JNK and p38 signaling pathways.9 In summary, these results suggest that AS-IV exerts multiple effects on preventing cardiovascular diseases through different mechanisms. Nevertheless, the exact mechanisms are largely unknown and require further investigation.
Lipopolysaccharides (LPS) can lead to sepsis-like conditions, causing systemic tissue damage, such as myocardial dysfunction.10 Inflammation has been linked to LPS-induced myocardial dysfunction.11 Many studies have shown that AS-IV attenuates myocardial dysfunction caused by LPS by inhibiting inflammation. For example, AS-IV inhibits LPS-induced myocardial damage via the toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) signaling pathway.12 AS-IV has protective effects against injury in H9c2 cardiomyocyte cell lines treated with LPS by reducing the levels of interleukin (IL)-6 and tumor necrosis factor (TNF)-α and decreasing the expression of NF-κB.13 In addition, the protective effect of AS-IV on cardiac dysfunction in mice induced by LPS is contributed to the inhibition of inflammatory cytokines, such as IL-6 and TNF-α.14 However, the mechanisms by which AS-IV prevents cardiac dysfunction caused by LPS through the inhibition of inflammation remain to be investigated.
Autophagy plays a critical role in cardiac dysfunction and inflammation.15,16 The significance of regulation of autophagy and inflammation in the preventive effect of AS-IV against cardiac dysfunction is inconsistent. A line of evidence has shown that AS-IV exerts protection by inhibiting both inflammation and autophagy.17–19 For example, studies have revealed that AS-IV reduces a driamycin-induced myocardial injury by inhibiting autophagy.18 In addition, AS-IV inhibits high glucose-induced oxidative stress and autophagy and protects cardiomyocytes from injury.19 In contrast, researchers found that AS-IV prevents mechanical stress-induced myocardial hypertrophy in the aortic banding model by activating autophagy and inhibiting inflammation.16 Taken together, these findings reveal that the regulation of autophagy and inflammation by AS-IV depends on the heart injury model. Therefore, the significance of both autophagy and inflammation in the protective effect of AS-IV against cardiac dysfunction caused by LPS requires further investigation.
MicroRNAs (miRNAs, miRs) regulate gene expression post-transcriptionally.20,21 miR-1 has been identified as a muscle-specific miRNA and has been extensively investigated and confirmed to be a key regulator of cardiac development and disease.20,22–24 However, the expression pattern of miR-1 varies in different cardiac diseases. Increased expression of miR-1 was found in a rat model of myocardial infarction,25 in individuals with coronary artery disease,23 and the remote myocardium of patients with myocardial infarction.24 On the contrary, Sayed et al reported that miR-1 was downregulated after aortic constriction-induced hypertrophy in a mouse model.20 These results suggest that the patterns of miR-1 expression are model- and/or disease-dependent and require further investigation. However, the significance of miR-1 in LPS-induced cardiac dysfunction and in the effect of AS-IV on miR-1 remains largely unknown.
AS-IV has been shown to inhibit inflammatory diseases by regulating miRNAs. For instance, AS-IV attenuates renal fibrosis by inhibiting miR-192 expression.26 In diabetic peripheral neuropathy, AS-IV attenuates Schwann cell injury through the regulation of miR-155-mediated autophagy. In a recent study, the effect of miR-1 on cardiac hypertrophy and cardiomyocyte inflammation was suggested.27 Given that both inflammation and autophagy play essential roles in cardiac dysfunction, this study aimed to explore whether the inhibition of miR-1-mediated inflammation and autophagy also contributes to the protective effect of AS-IV against cardiac dysfunction in rats treated with LPS.
Materials and Methods
Materials
Fetal bovine serum (FBS) and Dulbecco’s Modified Eagle’sMedium (DMEM) were provided by Gibco (Grand Island, NY, USA). AS-IV (purity >98%; chemical formula: C41H68O14; molecular weight: 785, shown in Figure 1 as structure) was purchased from Nanjing Jingzhu Bio-technology Co., Ltd (CAS No: 84687-43-4; Nanjing, China). LPS was purchased from Sigma (St. Louis, MO, USA). miR-1 mimics and inhibitor lentiviruses were obtained from Shanghai Genechem Gene Technology (Shanghai, China). Antibodies against HPS70, TLR4, IKBα, IKKβ, p65, Bax, Bcl-2, LC3-I, LC3-II, beclin 1, P62, and GAPDH were obtained from ABclonal (Boston, USA).
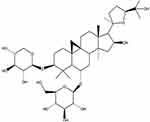 |
Figure 1 AS-IV chemical structure. |
Animals and Groups
Sprague-Dawley (SD) rats (male, approximately 220 g) were provided by the Animal Research Center of Jinzhou Medical University (Jinzhou, China). The Ethics Committee of Animal Experiments of the Jinzhou Medical University approved the study protocol (approval number: JZMU‑2020‑168; Liaoning, China), which was conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Rats were assigned into five groups containing six rats each: 1) Control, 2) LPS, 3) LPS + AS-IV, 4) LPS + AS-IV+ miR-1 mimics, and 5) LPS + miR-1 inhibitor. The dosages of AS-IV and LPS, which were selected according to previous studies, were 80 mg/kg and 10 mg/kg, respectively.28,29 We used only one dose of AS-IV in the present study because we confirmed the protective effect of AS-IV against cardiac dysfunction using different doses in previous studies.30–33 miR-1 mimics and inhibitors were injected into the myocardium. AS-IV was intragastrically administered once per day for 15 days, followed by either an LPS or saline injection. Twenty-four hours after LPS administration, echocardiographic measurements were obtained under anesthesia. This was followed by blood collection and heart removal for histological or RNA and protein analyses.
Echocardiographic Assessments
Rats were anesthetized with isoflurane, and echocardiographic images were collected using the Mindray Resona 7 imaging system (Shenzhen, China). Ejection fraction (EF), left ventricle internal diameter in systole (LVIDs), and diastole (LVIDd) were determined. Fractional shortening (FS) was defined as (LVIDd − LVIDs)/LVIDd.
Histological Analysis
The left ventricle of the heart was fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin-eosin (HE).
Enzyme-Linked Immunosorbent Assay (ELISA)
The serum was collected after blood centrifugation. The concentrations of IL-6, TNF-α, and IL-β were measured using ELISA according to the manufacturer’s instructions.
Immunofluorescence
Immunofluorescence was performed to measure the expression of related proteins.
H9c2 Cell Culture and Treatment
H9c2 cells purchased commercially (Wuhan Punuosai Life Technology Co., Ltd, Wuhan, China) were assigned into five groups: 1) Control, 2) LPS, 3) LPS + AS-IV, 4) LPS + AS-IV+ miR-1 mimics, and 5) LPS + miR-1 inhibitor. The concentrations of both LPS and AS-IV were 10 μg/mL, respectively. The miR-1 inhibitor and mimics were transfected into H9c2 cells. AS-IV was incubated with the cells for 48 h, followed by treatment with LPS.
Autophagosomes Detected by Transmission Electron Microscopy
Autophagosomes were detected using transmission electron microscopy. Briefly, after the LPS treatment, the vesicle structures of the autophagosomes in the bilayer membrane of cells were observed.
mRNA Expression Determined by Quantitative RT-PCR
Total RNA from heart tissue and cells was extracted and quantified, followed by complementary DNA synthesis and qRT-PCR. The relative mRNA expression of each gene was normalized to that of the control group. The gene primers used are listed in Table 1.
 |
Table 1 List of Primers Used for Real-Time RT-PCR |
Protein Expression Measured by Western Blot
Proteins from heart tissue or cells were extracted using RIPA buffer followed by electrophoresis, transferred to PVP membranes, blocked with skim milk, and incubated with antibodies against Bax, Bcl-2, Beclin 1, LC3II/LC3I, P62, HPS70, TLR4, NF-κB inhibitor (IKB)α, inhibitor of NF-κB kinase (IKKβ), NF-κB subunit p65, and GAPDH. The membrane was incubated with secondary antibodies, followed by detection with ECL reagents.
Statistical Analysis
Values are presented as mean ± SD and analyzed by one-way ANOVA. Statistical significance was set at p <0.05.
Results
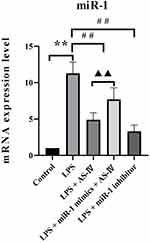
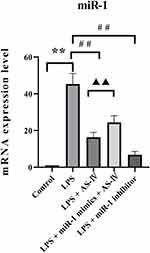
AS-IV Decreased the miR-1 mRNA Expression in the Heart Tissue of LPS-Treated Rats
We first used qRT-PCR to determine the miR-1 mRNA expression in the heart tissue. The results showed that the LPS treatment alone increased miR-1 expression compared to the control (Figure 2). Compared with the LPS treatment, supplementation with AS-IV significantly decreased miR-1 expression, and this decrease was partly abolished by the combination with miR-1 mimics. In addition, miR-1 inhibitor had an effect similar to that of AS-IV on miR-1 expression. These results suggest that miR-1 might play a critical role in heart diseases caused by LPS, and AS-IV could protect against heart diseases by targeting miR-1.
AS-IV Improved Heart Dysfunction and Attenuated the Pathological Changes in LPS-Treated Rats
To explore the effect of AS-IV on cardiac dysfunction, we established a rat model of cardiac dysfunction by injecting LPS and confirmed the success of the model by echocardiographic assessment. The results revealed that the LPS treatment alone increased the LVIDd and LVIDs values while decreasing the EF and FS values compared to the control (Figure 3A and B). Compared with the LPS treatment, the AS-IV treatment decreased of the LVIDd and LVIDs values, increasing the EF and FS values. However, all the effects of AS-IV against cardiac dysfunction were partly offset by the combination with miR-1 mimics. In addition, the miR-1 inhibitor had effects similar to those of AS-IV on cardiac function. The results also showed that rats treated with LPS exhibited infiltration of inflammatory cells and structural damage in the heart tissue (Figure 3C). Compared with LPS treatment, AS-IV treatment reduced the degree of inflammatory cell infiltration and structural damage. However, the combination with miR-1 mimics partly offset all AS-IV effects. In addition, the miR-1 inhibitor had similar effects as AS-IV. These results revealed that AS-IV improved cardiac dysfunction and attenuated the infiltration of inflammatory cells and structural damage induced by LPS, which might be related to the downregulation of miR-1.
AS-IV Reduced the Levels of Inflammatory Cytokines in the Serum of LPS-Treated Rats
Inflammation has been implicated in pathological changes associated with heart diseases. We measured the levels of cytokines IL-6, TNF-α, and IL-β in the serum of rats treated with LPS to explore whether AS-IV improved cardiac dysfunction by reducing inflammation. LPS increased the levels of IL-6, TNF-α, and IL-β compared to that in the control (Figure 4). Compared to the LPS treatment, AS-IV treatment reduced the levels of IL-6, TNF-α, and IL-β; however, the reduction of the levels of these cytokines was partly abolished by the combination withmiR-1 mimics. In addition, the miR-1 inhibitor had similar effects as AS-IV on the production of cytokines. These findings suggest that AS-IV improves cardiac dysfunction induced by LPS, at least partly through the reduction of miR-1-mediated inflammation.
AS-IV Regulated the Protein Expression of Inflammatory Signaling Molecules in the Heart Tissue of LPS-Treated Rats
Signaling molecules involved in inflammation include TLR4, IKKβ, and NF-κB. Next, we investigated the mechanisms underlying the inhibition of cytokine production by AS-IV through investigating the protein expression of TLR4, IKKβ, p65, HPS70, and IKBα in the heart tissue of LPS-treated rats. We found that LPS alone significantly upregulated the protein expression of HPS70, TLR4, IKKβ, and p65, and downregulated that of IKBα in comparison with the control (Figure 5A and B). We also found that compared with the LPS treatment, AS-IV treatment significantly downregulated the protein expression of HPS70, TLR4, IKKβ, and p65, and upregulated that of IKBα. However, the upregulation and downregulation of these signaling molecules by AS-IV were partly abolished by the combination with miR-1 mimics. In addition, the miR-1 inhibitor had similar effects as AS-IV on the protein expression of these molecules. The protein expression of HPS70 and p65 was confirmed by immunofluorescence (Figure 5C). These results revealed that AS-IV reduced the production of cytokines induced by LPS, at least partly through regulating these inflammatory signaling molecules.
AS-IV Regulated Autophagy in the Heart Tissue of LPS-Treated Rats
The crosstalk between inflammation and autophagy plays an essential role in cardiac dysfunction. Next, we determined the protein expression of autophagy-related proteins Bax, Bcl-2, Beclin 1, LC3-II/LC3-I, and P62 to determine whether AS-IV regulates autophagy and inflammation. We found that the LPS treatment alone significantly upregulated the protein expression of Bax, Beclin 1, and LC3-II/LC3-I, but downregulated the expression of Bcl-2 and P62 compared to those in the control (Figure 6A and B). Compared to the LPS treatment, AS-IV treatment significantly downregulated the protein expression of Bax, Beclin 1, and LC3-II/LC3-I, and upregulated the expression of Bcl-2 and P62. However, the upregulation and downregulation of autophagy-related proteins by AS-IV were partly abolished by the combination with miR-1 mimics. In addition, the miR-1 inhibitor had similar effects as AS-IV. LC3-II and LC3-I protein expression was confirmed by immunofluorescence (Figure 6C). These results demonstrate that the autophagy regulation also contributes to the improvement of cardiac dysfunction by AS-IV and that inflammation is accompanied by autophagy.
AS-IV Decreased miR-1 mRNA Expression in LPS-Treated Heart Cells
To clarify the results of the animal study, we performed an experiment at the cellular level using H9c2 cells. We found that the LPS treatment alone significantly increased miR-1 expression in H9c2 cells compared to that in the control (Figure 7). Compared to the LPS treatment, the addition of AS-IV reduced miR-1 expression, which was partly abolished by the combination of miR-1 mimics. Similarly, the miR-1 inhibitor reduced miR-1 expression in H9c2 cells treated with LPS. These results are consistent with the findings of the animal studies.
AS-IV Regulated the Protein Expression of Inflammation Signaling Molecules in LPS-Treated Heart Cells
We investigated the mRNA and protein expression levels of TLR4, IKKβ, p65, HPS70, and IKBα. We found that compared with the LPS treatment, the addition of AS-IV downregulated the expression of HPS70, TLR4, IKKβ, and p65, and upregulated the expression of IKBα in H9c2 cells (Figure 8A–C). However, the upregulation or downregulation of these signaling molecules by AS-IV was partly abolished by the combination with miR-1 mimics. In addition, the miR-1 inhibitor had similar effects as AS-IV on the mRNA and protein expression of these molecules.
AS-IV Regulated Autophagy in LPS-Treated Heart Cells
We investigated the mRNA and protein expression of Bax, Bcl-2, Beclin 1, LC3-II/LC3-I, and P62 in cells. Compared with the LPS treatment, the addition of AS-IV significantly decreased the mRNA and protein expression of Bax, Beclin 1, and LC3-II/LC3-I, and increased that of Bcl-2 and P62 in H9c2 cells (Figure 9A–C). However, the upregulation and downregulation of autophagy-related proteins by AS-IV was partly abolished by the combination with miR-1 mimics. In addition, the miR-1 inhibitor had similar effects as AS-IV. Autophagosomes observed under an electron microscope yielded similar results (Figure 9D), where the Beclin 1 expression is indicated by a red arrow.
Discussion
In the present study, we found that AS-IV and miR-1 inhibitors attenuated cardiac dysfunction in LPS-treated rats, inhibited inflammation and autophagy, and decreased miR-1 mRNA expression. However, the protective effect of AS-IV against cardiac dysfunction was partly offset by the addition ofmiR-1 mimics. The findings of the animal study were confirmed at the cellular level. These results revealed that AS-IV prevented cardiac dysfunction caused by LPS through the attenuation of miR-1-mediated inflammation and autophagy. The results of the present study stressed the roles of miR-1-mediated inflammation and autophagy in LPS-induced cardiac dysfunction and provided the novel mechanisms by which AS-IV attenuates cardiac dysfunction.
To study the protective effect of AS-IV on cardiac dysfunction, we established a rat model of cardiac dysfunction by injecting rats with LPS and confirmed the success of the model by echocardiographic assessment. These results are in agreement with those of previous reports, as they reveal that compromised cardiac function is associated with inflammation, apoptosis, and excess autophagy.34,35 Additionally, the ablation of CD74, the MIF receptor, protects against LPS-induced cardiac anomalies, inflammation, and apoptosis by suppressing autophagy in a Skp2-SUV39H1-mediated mechanism.34 Similarly, ALDH2 protects against LPS-induced cardiac anomalies by suppressing ER stress and autophagy in a CAMKKβ/AMPK/mTOR-dependent manner.35 In the present study, we found that AS-IV improved cardiac dysfunction by decreasing the values of LVIDd and LVIDs and increasing the values of EF and FS. Consistent with the findings from our previous study,36 these results suggest that AS-IV improved cardiac dysfunction induced by LPS. The results of our present and previous studies showing that AS-IV improved cardiac dysfunction are in agreement with those of previous studies.28,37–41 For example, Zhang et al found that AS-IV increases the cardiac function index LVEF and decreases hypertrophy indices LVPWd, LVPWs, IVSd, and IVSs in an aortic banding model.16 Mei et al reported that AS-IV inhibits the isoproterenol-induced apoptosis of hypertrophic cardiomyocytes by inhibiting oxidation and calpain-1.28 Nevertheless, the mechanisms by which AS-IV attenuates cardiac dysfunction require further investigation.
Inflammation has been implicated in the pathological changes of cardiac dysfunction, as reflected by the increased levels of inflammatory factors, including IL-6, IL-β, and TNF-α. In the present study, we found that compared with the LPS treatment, AS-IV reduced the levels of IL-6, IL-β, and TNF-α in the serum of LPS-treated rats. These results suggest that AS-IV improves cardiac dysfunction induced by LPS, at least partly through the inhibition of inflammation. However, the mechanism by which AS-IV decreases the levels of these inflammatory factors remains unknown. Signaling molecules involved in inflammation include TLR4, IKKβ, and NF-κB. In the present study, we found that AS-IV downregulated the protein expression of HPS70, TLR4, IKKβ, and NF-κB, and upregulated that of IKBα in the heart tissue of LPS-treated rats. These findings were confirmed in terms of both mRNA and protein expression at the cellular level. Taken together, these results suggest that AS-IV reduced the production of inflammatory cytokines induced by LPS, at least partly through the regulation of these inflammatory signaling molecules. In agreement with our studies, many studies have revealed that AS-IV has strong anti-inflammatory properties.14,30,42,43 One study reported that AS-IV inhibits inflammation by inhibiting theTLR4/NF-κB pathway in acute myocardial infarction-induced heart failure in rats.43 Another study found that in rat cardiac hypertrophy caused by isoproterenol, AS-IV decreased the ratio of nuclear/cytosolic NF-κB p65 protein and increased IκB-α protein expression, resulting in the downregulation of IL-1β, IL-6, and TNF-α.30 In LPS-induced heart failure in mice, AS-IV inactivates the NF-кB signaling pathway, decreases the levels of TNF-α, IL-6, andIL-1β, and improves cardiac function.14
The crosstalk between inflammation and autophagy plays an important role in cardiac dysfunction. However, the significance of the regulation of autophagy and inflammation in the protective effect of AS-IV against cardiac dysfunction remains unclear. In the present study, we found that AS-IV downregulated the protein expression of Bax, Beclin 1, and LC3-II/LC3-I, and upregulated that of Bcl-2 and P62. These findings were verified in terms of both mRNA and protein expression at the cellular level. Together with that the AS-IV-induced inflammation inhibition, these results demonstrate that the inhibition of autophagy also contributes to the improvement of cardiac dysfunction by AS-IV and that inflammation is accompanied by autophagy. The roles of autophagy in AS-IV protection against cardiac dysfunction were incompatible. A line of evidence has shown that AS-IV exerts protective effects by inhibiting autophagy accompanied by reduction of inflammation. For example, studies have revealed that AS-IV alleviates adriamycin-induced myocardial injury by inhibiting autophagy.18 In addition, AS-IV inhibits high glucose-induced autophagy and protects cardiomyocytes from injury.19 In contrast, researchers found that AS-IV prevents mechanical stress-induced myocardial hypertrophy in the aortic banding model by activating autophagy and reducing inflammation.16 Taken together, these results suggest that the significance of autophagy in the protective effect of AS-IV against cardiac dysfunction is largely model-dependent. Similarly, the relationship between inflammation and autophagy in cardiac dysfunction is also model-dependent. Luo et al demonstrated the pronounced inflammatory responses in LPS-challenged hearts that were possibly downstream responses to autophagy.34 Consistently, their findings showed that the ablation of CD74 protected against LPS-induced cardiac anomalies and inflammation by suppressing autophagy. The mechanism underlying the inhibition of inflammation and autophagy by AS-IV in cardiac dysfunction caused by LPS remains unclear.
miRs regulate gene expression post-transcriptionally.20,21 Cardiac enriched miR-1 regulates cardiac diseases.20,23,25,44 However, the expression pattern of miR-1 varies in different cardiac diseases. Shan et al found upregulated miR-1 expression in a rat model of myocardial infarction.25 Similarly, Yang et al reported that miR-1 is overexpressed in individuals with coronary artery disease, while the overexpression of miR-1 in normal or infarcted rat hearts exacerbates arrhythmogenesis.23 In addition, miR-1 is upregulated in the remote myocardium of patients with myocardial infarction.24 On the contrary, Sayed et al reported that miR-1 was downregulated after aortic constriction-induced hypertrophy in a mouse model.20 These results suggest that the patterns of miR-1 expression are model- and/or disease-dependent and require further investigation. The effect of miR-1 on cardiomyocytic inflammation and cardiac hypertrophy was suggested in a recent study.27 In the present study, we found that the protective effect of AS-IV against cardiac dysfunction and the inhibition of inflammation and autophagy by AS-IV were partly offset by miR-1 mimics. In addition, the miR-1 inhibitor attenuated cardiac dysfunction in LPS-treated rats, inhibited inflammation and autophagy, and decreased miR-1 mRNA expression. The animal study findings were consistent with those in the cells. These results suggested that miR-1-mediated inflammation and autophagy contributed, at least partly, to the protection of cardiac dysfunction by AS-IV.
Conclusion
The results of this study demonstrated that AS-IV improved the cardiac dysfunction of rats treated with LPS by inhibiting miR-1-mediated inflammation and autophagy. These results suggest that AS-IV prevents cardiac dysfunction from LPS injury through inhibiting inflammation and autophagy by targeting miR-1.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by National Natural Science Foundation of China (81960673, 81870329); the Key Projects of Natural Science Foundation of Liaoning Province (No. 20170540358); Project for Young Scientists Fund of Education Department of Liaoning Province (No. JYTQN201706); Natural Science Foundation of Gansu Province (21JR1RA135); Cuiying Technological Innovation Foundation of Lanzhou University Second Hospital (CY2019-MS03); Industrial Support Program for Colleges and Universities in Gansu Province (2020C-04); Special Research Project of Lanzhou University Serving the Economic and Social Development of Gansu Province (054000282).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Liu J, Chen HB, Guo BL, et al. Study of the relationship between genetics and geography in determining the quality of Astragali Radix. Biol Pharm Bull. 2011;34:1404. doi:10.1248/bpb.34.1404
2. Jin C, Dai RH. [Effect of Astragalus membranaceus on erythrocyte sodium content and sodium transport in the coronary heart disease]. Zhong Xi Yi Jie He Za Zhi. 1991;11:651. Chinese.
3. Yang QY, Lu S, Sun HR. [Effects of Astragalus on cardiac function and serum tumor necrosis factor-alpha level in patients with chronic heart failure]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:699. Chinese.
4. Huang ZQ, Qin NP, Ye W. [Effect of Astragalus membranaceus on T-lymphocyte subsets in patients with viral myocarditis]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15:328. Chinese.
5. Xu GH, Yuan L, Li Y, et al. [Clinical observation of Astragalus injection in treatment of renal injury in patients with primary hypertension]. Zhong Xi Yi Jie He Xue Bao. 2008;6:530. Chinese. doi:10.3736/jcim20080519
6. Zhang Y, Mao XD, Cao AL, et al. Astragaloside IV prevents endothelial dysfunction by improving oxidative stress in streptozotocin-induced diabetic mouse aortas. Exp Ther Med. 2021;22:1197. doi:10.3892/etm.2021.10631
7. Yu C, Zhang J, Li X, et al. Astragaloside IV-induced Nrf2 nuclear translocation ameliorates lead-related cognitive impairments in mice. Biochim Biophys Acta Mol Cell Res. 2021;1868:118853. doi:10.1016/j.bbamcr.2020.118853
8. Yao J, Fang X, Zhang C, et al. Astragaloside IV attenuates hypoxia-induced pulmonary vascular remodeling via the Notch signaling pathway. Mol Med Rep. 2021;23:1.
9. Sun C, Zeng G, Wang T, et al. Astragaloside IV ameliorates myocardial infarction induced apoptosis and restores cardiac function. Front Cell Dev Biol. 2021;9:671255. doi:10.3389/fcell.2021.671255
10. Ebner N, Foldes G, Schomburg L, et al. Lipopolysaccharide responsiveness is an independent predictor of death in patients with chronic heart failure. J Mol Cell Cardiol. 2015;87:48. doi:10.1016/j.yjmcc.2015.07.029
11. Van Tassell BW, Toldo S, Mezzaroma E, et al. Targeting interleukin-1 in heart disease. Circulation. 2013;128(17):1910. doi:10.1161/CIRCULATIONAHA.113.003199
12. Zhang X, Li M, Wang H. Astragaloside IV alleviates the myocardial damage induced by lipopolysaccharide via the toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-kappaB)/proliferator-activated receptor alpha (PPARalpha) signaling pathway. Med Sci Monit. 2019;25:7158. doi:10.12659/MSM.916030
13. Wang SG, Xu Y, Xie H, et al. Astragaloside IV prevents lipopolysaccharide-induced injury in H9C2 cardiomyocytes. Chin J Nat Med. 2015;13:127. doi:10.1016/S1875-5364(15)60016-4
14. Zhao P, Wang Y, Zeng S, et al. Protective effect of astragaloside IV on lipopolysaccharide-induced cardiac dysfunction via downregulation of inflammatory signaling in mice. Immunopharmacol Immunotoxicol. 2015;37(5):428. doi:10.3109/08923973.2015.1080266
15. Gao Y, Zhang Y, Fan Y. Eupafolin ameliorates lipopolysaccharide-induced cardiomyocyte autophagy via PI3K/AKT/mTOR signaling pathway. Iran J Basic Med Sci. 2019;22:1340. doi:10.22038/ijbms.2019.37748.8977
16. Zhang T, Wang H, Lu M, et al. Astragaloside IV prevents myocardial hypertrophy induced by mechanical stress by activating autophagy and reducing inflammation. Am J Transl Res. 2020;12:5332.
17. Pei C, Wang F, Huang D, et al. Astragaloside IV protects from PM2.5-induced lung injury by regulating autophagy via inhibition of PI3K/Akt/mTOR signaling in vivo and in vitro. J Inflamm Res. 2021;14:4707. doi:10.2147/JIR.S312167
18. Luo LF, Qin LY, Wang JX, et al. Astragaloside IV attenuates the myocardial injury caused by adriamycin by inhibiting autophagy. Front Pharmacol. 2021;12:669782. doi:10.3389/fphar.2021.669782
19. Zhu Y, Qian X, Li J, et al. Astragaloside-IV protects H9C2 (2-1)cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artif Cells Nanomed Biotechnol. 2019;47:4172. doi:10.1080/21691401.2019.1687492
20. Sayed D, Hong C, Chen IY, et al. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416. doi:10.1161/01.RES.0000257913.42552.23
21. Perez-Cremades D, Mompeon A, Vidal-Gomez X, et al. Role of miRNA in the regulatory mechanisms of estrogens in cardiovascular ageing. Oxid Med Cell Longev. 2018;2018:6082387. doi:10.1155/2018/6082387
22. Lagos-Quintana M, Rauhut R, Yalcin A, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735. doi:10.1016/S0960-9822(02)00809-6
23. Yang B, Lin H, Xiao J, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486. doi:10.1038/nm1569
24. Bostjancic E, Zidar N, Stajner D, et al. MicroRNA miR-1 is up-regulated in remote myocardium in patients with myocardial infarction. Folia Biol. 2010;56:27.
25. Shan ZX, Lin QX, Fu YH, et al. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem Biophys Res Commun. 2009;381:597. doi:10.1016/j.bbrc.2009.02.097
26. Cao Y, Zhang L, Wang Y, et al. Astragaloside IV attenuates renal fibrosis through repressing epithelial-to-mesenchymal transition by inhibiting microRNA-192 expression: in vivo and in vitro studies. Am J Transl Res. 2019;11:5029.
27. Tomaniak M, Sygitowicz G, Blaszczyk O, et al. miR-1, miR-21, and galectin-3 in hypertensive patients with symptomatic heart failure and left ventricular hypertrophy. Kardiol Pol. 2018;76:1009. doi:10.5603/KP.2018.0117
28. Mei M, Tang F, Lu M, et al. Astragaloside IV attenuates apoptosis of hypertrophic cardiomyocyte through inhibiting oxidative stress and calpain-1 activation. Environ Toxicol Pharmacol. 2015;40:764. doi:10.1016/j.etap.2015.09.007
29. Zhang N, Feng H, Liao HH, et al. Myricetin attenuated LPS induced cardiac injury in vivo and in vitro. Phytother Res. 2018;32:459. doi:10.1002/ptr.5989
30. Yang J, Wang HX, Zhang YJ, et al. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-small ka, CyrillicB signaling pathway in isoproterenol-induced myocardial hypertrophy. J Ethnopharmacol. 2013;150:1062. doi:10.1016/j.jep.2013.10.017
31. Lu M, Leng B, He X, et al. Calcium sensing receptor-related pathway contributes to cardiac injury and the mechanism of astragaloside IV on cardioprotection. Front Pharmacol. 2018;9:1163. doi:10.3389/fphar.2018.01163
32. Lu M, Tang F, Zhang J, et al. Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/nuclear factor-kappaB signaling pathway. Phytother Res. 2015;29:599. doi:10.1002/ptr.5297
33. Lu M, Wang H, Wang J, et al. Astragaloside IV protects against cardiac hypertrophy via inhibiting the Ca2+/CaN signaling pathway. Planta Med. 2014;80:63. doi:10.1055/s-0033-1360129
34. Luo Y, Fan C, Yang M, et al. CD74 knockout protects against LPS-induced myocardial contractile dysfunction through AMPK-Skp2-SUV39H1-mediated demethylation of BCLB. Br J Pharmacol. 2020;177:1881. doi:10.1111/bph.14959
35. Pang J, Peng H, Wang S, et al. Mitochondrial ALDH2 protects against lipopolysaccharide-induced myocardial contractile dysfunction by suppression of ER stress and autophagy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1627. doi:10.1016/j.bbadis.2019.03.015
36. Wang Q, Yang X, Song Y, et al. Astragaloside IV-targeting miRNA-1 attenuates lipopolysaccharide-induced cardiac dysfunction in rats through inhibition of apoptosis and autophagy. Life Sci. 2021;275:119414. doi:10.1016/j.lfs.2021.119414
37. Sui YB, Wang Y, Liu L, et al. Astragaloside IV alleviates heart failure by promoting angiogenesis through the JAK-STAT3 pathway. Pharm Biol. 2019;57:48. doi:10.1080/13880209.2019.1569697
38. Dong Z, Zhao P, Xu M, et al. Astragaloside IV alleviates heart failure via activating PPARalpha to switch glycolysis to fatty acid beta-oxidation. Sci Rep. 2017;7:2691. doi:10.1038/s41598-017-02360-5
39. Xu XL, Chen XJ, Ji H, et al. Astragaloside IV improved intracellular calcium handling in hypoxia-reoxygenated cardiomyocytes via the sarcoplasmic reticulum Ca-ATPase. Pharmacology. 2008;81:325. doi:10.1159/000121335
40. Tang B, Zhang JG, Tan HY, et al. Astragaloside IV inhibits ventricular remodeling and improves fatty acid utilization in rats with chronic heart failure. Biosci Rep. 2018;38. doi:10.1042/BSR20171036
41. Liu T, Yang F, Liu J, et al. Astragaloside IV reduces cardiomyocyte apoptosis in a murine model of coxsackievirus B3-induced viral myocarditis. Exp Anim. 2019;68:549. doi:10.1538/expanim.19-0037
42. Zhao Z, Wang W, Wang F, et al. Effects of Astragaloside IV on heart failure in rats. Chin Med. 2009;4:6. doi:10.1186/1749-8546-4-6
43. Cheng S, Yu P, Yang L, et al. Astragaloside IV enhances cardioprotection of remote ischemic conditioning after acute myocardial infarction in rats. Am J Transl Res. 2016;8:4657.
44. Tang Y, Zheng J, Sun Y, et al. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50:377. doi:10.1536/ihj.50.377
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.