Back to Journals » OncoTargets and Therapy » Volume 11
Inhibition of miR-10a-5p suppresses cholangiocarcinoma cell growth through downregulation of Akt pathway
Authors Gao L, Yang X, Zhang H, Yu M, Long J, Yang T
Received 1 August 2018
Accepted for publication 24 September 2018
Published 15 October 2018 Volume 2018:11 Pages 6981—6994
DOI https://doi.org/10.2147/OTT.S182225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Lili Gao,1,* Xiaoping Yang,2,* Hao Zhang,2 Minghua Yu,3 Jianting Long,4 Tao Yang1
1Center for Medical Research and Innovation, 2Department of General Surgery, 3Department of Medical Oncology, Shanghai Pudong Hospital, Fudan University Pudong Medical Center, Shanghai 201399, People’s Republic of China; 4Department of Medical Oncology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510080, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Backgrounds: Cholangiocarcinoma (CCA) is epithelial cell malignancy with very poor prognosis. A lot of patients were diagnosed at advanced stage of CCA and no risk factors were identified. There are limited treatment options available for the management of CCA patients. It is urgent to develop effective targeted therapies for the treatment of CCA. miRNAs are small noncoding RNAs that negatively regulate the target genes. In this study, we investigated the role and mechanism of miR-10a-5p in CCA.
Methods: Human CCA cell lines (CCLP1 and SG-231) were transfected with miR-10a-5p mimic or miR-10a-5p inhibitor. qRT-PCR was performed to detect the miR-10a-5p level. Proliferation, colony formation, and apoptosis were analyzed. Luciferase reporter assay was used to explore the targeting of miR-10a-5p on PTEN. For in vivo tumorigenesis assay, CCLP1 cells with stable knockdown of miR-10a-5p or control CCLP1 cells were injected subcutaneously into the flank of the SCID mice and animals were monitored for tumor growth.
Results: miR-10a-5p expression was significantly upregulated in human CCA cell lines (CCLP1 and SG-231). Inhibition of miR-10a-5p significantly suppressed the proliferation and induced apoptosis in CCLP1 and SG-231. PTEN is a direct target of miR-10a-5p in CCA cells.
Conclusion: Inhibition of miR-10a-5p can decrease CCA cells growth by downregulation of Akt pathway. These results indicate that miR-10a-5p may serve as a potential target for the treatment of CCA and help to develop effective therapeutic strategies.
Keywords: miR-10a-5p, cholangiocarcinoma, PTEN, Akt, liver, proliferation
Introduction
Cholangiocarcinoma (CCA) is the second most common primary liver malignancy.1 CCA represents a diverse group of epithelial cell malignancy that develops along the biliary tract.2,3 CCA are classified into intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA) depending on their site of origin.4 Different types of CCA have different features and require specific treatments. Primary sclerosing cholangitis is considered to be the principal risk factor for CCA.5 Other risk factors include hepatitis C virus, human immunodeficiency virus, liver cirrhosis, and diabetes.6 However, in most CCAs, no risk factors are identified. The incidence of ICC in the US continues to rise. Between 1973 and 2012, the reported US incidence of ICC increased from 0.44 to 1.18 cases per 100,000.7 Patients with CCA often present symptoms with biliary obstruction or non-specific abdominal pain, a high proportion of patients were diagnosed at advanced stage of CCA.8 At early stage, curative options are available in the form of surgical resection and/or liver transplantation.9 The most frequently used treatment modality is chemotherapy. Due to high rate of recurrence after liver transplantation, distant metastasis and invasion, as well as the chemoresistance, CCA patients represent a very poor prognosis. The average 5-year survival rate for CCA patients is 5%–10%.10 It is urgent to develop new specific effective targeted therapies for the treatment of CCA.
miRNAs are small noncoding RNAs which are short single-stranded molecules about 21–23 nucleotides in length.11 miRNAs regulate gene expression at posttranscriptional level. miRNAs inhibit the target genes expression by binding to 3′ untranslated regions (3′UTRs) of target mRNAs which cause mRNA degradation and destabilization.12 miRNAs play important roles in a broad range of biological processes, such as embryonic development,13 apoptosis,14 stem cell differentiation,15 hematopoiesis,16 and immune response.17 Dysregulation of miRNA expression has been reported in cancer, including CCA. For example, miR-29a has emerged as a tumor suppressor, miR-29a level was found significantly decreased in both CCA tissues and tumor cell lines.18 miR-34a was rhythmically expressed in CCA cells. Inhibition of miR-34a decreased proliferation, migration, and invasion in CCA cells.19 miR-21 and miR-221 levels significantly upregulated in CCA serum. Circulating plasma levels of miR-21 and miR-221 can serve as a diagnostic and prognostic biomarkers for CCA.20,21
miR-10 family including miR-10a and miR-10b has attracted attention because of its conservation and the position of the miR-10 genes within the Homeobox (HOX) clusters.22 Hox genes are a group of evolutionarily conserved genes that encode a class of important transcription factors that regulate early developmental morphogenetic processes and continue to be expressed into adulthood.23 Hox genes organized into four distinct clusters. These clusters, labeled HOXA, HOXB, HOXC, and HOXD, are located on chromosomes 7p14, 17q21, 12q13, and 2q31.23 miR-10a was located within the HOX B cluster on 17q21 and miR-10b was located at HOX D cluster 2q31.24 miR-10 family members are deregulated in numerous types of cancers including uterine sarcomas,25 breast cancer,26 and hepatocellular carcinoma (HCC).27 miR-10a has been reported to be associated with liver regeneration,28 regulates human mesangial cells proliferation and chemokine expression by targeting IL-8.29 Plasma miR-10a levels were decreased in patients with coronary artery disease (CAD) and negatively associated with the presence and severity of CAD.30 miR-10a serves as a switch to control miR-10a-NF-kB regulatory circuit that promotes the excessive secretion of NF-κB-mediated inflammatory cytokines and the proliferation and migration of fibroblast-like synoviocytes of rheumatoid arthritis (RA).31 miR-10a-5p is overexpressed in human pancreatic ductal adenocarcinoma (PDAC) and acts as an oncogene to promote the metastatic behavior of PDAC cells.32 Abnormal high expression of miR-10a was also found in patients with acute myeloid leukemia (AML). miR-10a promotes proliferation of immature blood progenitors and repression of mature blood cell differentiation and maturation in AML.33
The expression of miR-10 was upregulated in CCA.34 However, the function of miR-10a-5p in CCA is largely unknown. In the present study, we explored the role of miR-10a-5p in CCA. We found that PTEN is a direct target of miR-10a-5p in CCA cell lines. Inhibition of miR-10a-5p suppressed proliferation and promoted apoptosis in CCA cells through downregulation Akt pathway.
Methods
Cell culture
Human intrahepatic bile duct epithelial cell line HIBEC was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Human CCA cell lines CCLP1 and SG-231 were obtained from Cell Bank of Chinese Academy of Sciences (Shanghai, People’s Republic of China). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% FBS, L-glutamine, and antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin). All cells were maintained in a 37°C humidified incubator with 5% CO2.
Transfections
CCLP1 and SG-231 cells were seeded in six-well plate and transfected with scramble control or miR-10a-5p mimic or miR-10a-5p inhibitor GenePharma (Shanghai, People’s Republic of China) using Oligofectamine reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Final concentration of scramble or miR-10a-5p mimic or miR-10a-5p inhibitor is 100 nM. At the indicated time point, cells samples were collected.
qRT-PCR
Total RNA was extracted from cells using Trizol (Thermo Fisher Scientific). Reverse transcription was performed using the miScript RT Kit (TaKaRa, Dalian, People’s Republic of China). qRT-PCR was performed using miScript SYBR Green PCR Kit (Qiagen NV, Venlo, the Netherlands) on a C1000 thermal cycler (Bio-Rad Laboratories Inc., Hercules, CA, USA). The primers of miR-10a-5p and U6 were obtained from Qiagen NV. U6 was used as an internal control.
Cell proliferation assay
Proliferation assays were conducted using WST-1 assay (Beyotime, Shanghai, People’s Republic of China). After CCLP1 and SG-231 cells were transfected with miR-10a-5p mimic or miR-10a-5p inhibitor or scramble control for 6 hours, cells were seeded in 96-well plates (2,000 cells/well). At 0, 24, 48, and 72 hours, culture medium was removed and 100 μL fresh medium containing 10 μL of WST-1 reagents was added into the wells. After 2–3 hours, the absorbance was measured at 450 nm by using ELISA Microplate Reader (Biocompare, San Francisco, CA, USA).
Western blot analysis
Total protein was extracted from cells using a protein extraction kit (Beyotime). Protein concentrations were measured using the BCA Protein Assay Kit (Beyotime). Protein fractions were separated on SDS-PAGE gel electrophoresis (Bio-Rad Laboratories Inc.) and transferred to a nitrocellulose membrane (Bio-Rad Laboratories Inc.). After blocking in 5% skim milk in PBS for 1 hour at room temperature, the membranes were incubated overnight at 4°C with primary antibodies. Primary antibodies against PARP, cleaved caspase-3, PTEN, p-Akt (ser473), and Akt were obtained from Cell Signaling Technology (Danvers, MA, USA). Primary antibody against β-actin was obtained from Abcam (Cambridge, MA, USA). Secondary antibodies IRDye800CW Goat anti-Mouse IgG and IRDye800CW Goat anti-Rabbit IgG were obtained from LI-COR (LI-COR Biosciences, Lincoln, NE, USA). Western bolt images were detecting by using Li-COR Odyssey 9120 Imaging System (LI-COR Biosciences).
Luciferase reporter assays
PTEN 3′-UTR was obtained from GeneCopoeia (Rockville, MD, USA). We mutated two nucleotides of the PTEN 3′-UTR by using Site-Directed Mutagenesis kit (Stratagene, Shanghai, People’s Republic of China). These vectors also express the Renilla luciferase serving as internal controls for the dual-luciferase assay. CCLP1 and SG231 were co-transfected with miR-10a-5p mimic (100 nM) or scramble (100 nM) with PTEN 3′-UTR or its mutant (Mut) using lipofectamine 2000 transfection reagent (Thermo Fisher Scientific). After 48 hours of transfection, the luciferase activity was measured using the dual luciferase reporter assay kit (Promega Corporation, Madison, WI, USA).
Colony formation assay
Lentiviral plasmid vector expresses miR-10a-5p inhibitor (LV-miR-10-5p-inhibitor) and scramble control lentivirus vector (LV-con) were obtained from ABM Industries Inc. (New York, NY, USA). We established CCLP1 cell line with stable knockdown of miR-10a-5p by transfecting cells with LV-miR-10-5p-inhibitor. The control CCLP1 cells were transfected with LV-con. Cells were seeded in 10 cm dishes at 2,000 cells/dish and cultured for 14 days. After fixation with methanol for 20 minutes, the colonies were stained with 0.1% crystal violet.
Mouse xenograft model
For tumorigenesis assays, 6 weeks old, male SCID mice were purchased from Wei Tong Li Hua Experimental Animal Technology Co., Ltd (Beijing, People’s Republic of China) (n=3). In total, 1 × 106 miR-10a-5p stable knockdown CCLP1 cells (LV-miR-10a-5p-inhibitor) or control CCLP1 cells (LV-con) were injected subcutaneously into the flank of the mice. Mice were observed for 30 days for tumor formation. Tumor diameters are measured with digital calipers, and the tumor volume in mm3 is calculated by the formula: Volume = (width)2 × length ÷ 2. All animal studies were approved by the Ethics Committee of Fudan University Pudong Medical Center. The handling of the mice and all experimental procedures were carried out in strict accordance with Fudan University Guidelines for the Care and Use of Laboratory Animals.
Statistical analysis
Data represent the mean ± SD. Experiments were repeated at least three times. Statistical analysis was performed using GraphPad Prism (version 5.0, GraphPad Software, Inc., La Jolla, CA, USA). One-way ANOVA along with Bonferroni adjustment and Student’s t-test were used to evaluate the differences between groups. A P-value < 0.05 was considered statistically significant.
Results
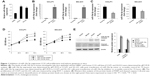
Inhibition of miR-10a-5p suppresses proliferation and promotes apoptosis in CCA cells
We evaluated the expression of miR-10a-5p in human intrahepatic bile duct epithelial cell line HIBEC and human CCA cell lines (CCLP1 and SG-231) by qRT-PCR analysis. Results showed that miR-10a-5p was upregulated significantly in CCA cells compared with HIBEC (Figure 1A). To evaluate the role of miR-10a-5p on CCA cells growth, human CCA cell lines CCLP1 and SG-231 were transfected with miR-10a-5p mimic or miR-10a-5p inhibitor or scramble control. The expression of miR-10a-5p was determined by qRT-PCR. As shown in Figure 1B, compared with scramble control, transfection of miR-10a-5p mimic for 72 hours led to a dramatic increase expression of miR-10a-5p in both CCLP1 and SG231 cells, whereas transfection of miR-10a-5p inhibitor for 72 hours led to a significant inhibition of the miR-10a-5p level in these cells (Figure 1C). Cell viability was measured using WST-1 assay. As shown in Figure 1D, upregulated miR-10a-5p level by miR-10a-5p mimic significantly increased the proliferation in both of CCLP1 and SG-231 cells, whereas a significant decrease in cell viability was detected when cells transfected with miR-10a-5p inhibitor compared with scramble control. Western blot analysis revealed that the cleaved PARP and cleaved caspase-3 were significantly increased in CCLP1 and SG-231 cells transfected with miR-10a-5p inhibitor (Figure 1E). These results indicated that miR-10a-5p promoted CCA cells proliferation, while inhibition of miR-10a-5p suppressed cell growth and induced apoptosis in CCA cells.
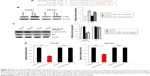
PTEN is a direct target of miR-10a-5p in CCA cells
To explore the tumor suppressive mechanism of miR-10a-5p inhibition, the potential target genes of miR-10a-5p were analyzed using miRNA target prediction programs TargetScan (http://www.targetscan.org). There are 287 transcripts with conserved sites, containing a total of 302 conserved sites and 99 poorly conserved sites. The predicted targets of human miR-10a-5p are shown in Table S1. We found that there was a predicted miR-10a-5p binding site in the 3′-UTR of PTEN (PTEN, phosphatase, and tensin homologue deleted on chromosome ten) (Figure 2A). To determine whether PTEN was regulated by miR-10a-5p, CCLP1 and SG-231 cells were transfected with miR-10a-5p inhibitor, Western blot analysis showed that inhibition of miR-10a-5p significantly upregulated the protein levels of PTEN (Figure 2B) and decreased the expression of p-Akt (ser473) (Figure 2C). To further verify whether PTEN is a direct target of miR-10a-5p, we generated PTEN reporter construct containing 3′-UTR with mutations of miR-10a-5p binding site (indicated in Figure 2A). CCLP1 and SG-231 cells were transfected with wild type or Mut PTEN 3′-UTR and miR-10a-5p mimic, luciferase reporter assay showed that miR-10a-5p mimic remarkably decreased the 3′-UTR luciferase reporter activity of PTEN, this effect was abolished when miR-10a-5p binding site was mutated (Figure 2D). These findings suggested that PTEN was a direct target of miR-10a-5p in CCA cells.
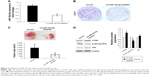
Inhibition of miR-10a-5p suppresses CCA growth in SCID mice
To further evaluate the effects of miR-10a-5p on CCA growth in vivo, we generated CCLP1 cells with stable knockdown of miR-10a-5p. CCLP1 cells were transfected with LV-mir-10a-5p-inhibitor or LV-con. As shown in Figure 3A, the downregulation of miR-10a-5p was confirmed by qRT-PCR. Knockdown of miR-10a-5p led to a significantly decreased colony formation in CCLP1 cells compared with control cells (Figure 3B). CCLP1 cells with stable knockdown of miR-10a-5p and control cells were injected subcutaneously into the flank of SCID mice to establish a xenograft model. Compared with the control group, knockdown of miR-10a-5p resulted in a significant reduction of tumor size and tumor volume (Figure 3C). Western blot analysis of the tumor tissues confirmed upregulated PTEN and decreased p-Akt (ser473) in miR-10a-5p knockdown tumors (Figure 3D). Taken together, these results suggested that inhibition of miR-10a-5p played an important role suppressed CCA cell proliferation.
Discussion
CCA is an aggressive tumor with very poor prognosis. The majority of patients present with unresectable disease and have a survival of less than 12 months following diagnosis.35 It is crucial to understand the pathogenesis of CCA, find out the effective, targeted, individualized therapies, and improve the quality of patient’s life. In our study, we investigated the effect of miR-10a-5p on CCA cells proliferation in vitro and in vivo. We found that overexpression of miR-10a-5p promoted CCA cells proliferation, whereas inhibition of miR-10a-5p suppressed proliferation and induced apoptosis in CCA cells. In a mouse xenograft model, inhibition of miR-10a-5p significantly suppressed tumorigenicity. PTEN is a direct target of miR-10a-5p in CCA cells. Inhibition of miR-10a-5p led to the downregulation of Akt pathway. miRNA expression has been reported to be involved in tumor progression and prognosis, including CCA.36 It has been reported that overexpression of miR-10a-5p promoted the migration and invasion of human HCC cell lines (QGY-7703 and HepG2) in vitro but suppressed metastasis in vivo.37 EphA4 (Eph tyrosine kinase receptor) was identified as the direct target of miR-10a. miR-10a promotes HCC cell migration and invasion through targeting EphA4, thereby regulating epithelial–mesenchymal transition and cell adhesion.37 Downregulation of miR-10a-5p has been shown to promote proliferation and restricts apoptosis via targeting T-box transcription factor 5 (TBX5) in inflamed synoviocytes.38 In our study, we found that inhibition of miR-10a-5p suppressed CCA cells proliferation through regulating PTEN-Akt pathway.
Akt pathway has been well established as an important signaling intermediate controlling cell survival, growth, proliferation, and other cellular processes.39 Activation of Akt pathway is an important survival pathway activated in cancer. Increased activation of AKT signaling was reproducibly observed in both CCA cell lines and CCA tissues.40 PTEN is a tumor suppressor and is a major negative regulator of the Akt signaling pathway. PTEN can be regulated by posttranslational modifications that include oxidation, acetylation, phosphorylation, ubiquitination, and proteolytic cleavage and by protein–protein interactions.41 PTEN can also be regulated by miRNAs. miRNAs may function as either oncogenes or tumor suppressors depending on their downstream targets.42 For example, miR-21 contributes both HCC and CCA growth by targeting PTEN.43,44 miR-22145 and miR-17-92 cluster46 promote CCA growth by targeting PTEN. In our study, we found that PTEN is a direct target of miR-10a-5p in CCA cells. Inhibition of miR-10a-5p promotes apoptosis in CCA cells through regulating PTEN.
Increasing evidences have shown that miRNAs are potential targets for human cancer treatment.47 Our study provided insight into the mechanism of inhibition of miR-10a-5p suppressed CCA cells proliferation. miR-10a-5p may be serve as a potential target for CCA. These findings may help to better understand the tumorigenesis of CCA and develop effective therapeutic strategies.
Acknowledgment
The work was financially supported by National Natural Science Foundation of China (grant nos 81572518 and 81372750) to TY.
Disclosure
The authors report no conflicts of interest in this work.
References
Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48(1):308–321. | ||
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. | ||
Ko KS, Peng J, Yang H. Animal models of cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29(3):312–318. | ||
Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13.e1–21.e1. | ||
Vogel A, Wege H, Caca K, Nashan B, Neumann U. The diagnosis and treatment of cholangiocarcinoma. Dtsch Arztebl Int. 2014;111(44):748–754. | ||
Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128(3):620–626. | ||
Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594–599. | ||
Najran P, Lamarca A, Mullan D, et al. Update on treatment options for advanced bile duct tumours: radioembolisation for advanced cholangiocarcinoma. Curr Oncol Rep. 2017;19(7):50. | ||
Qureshi K, Jesudoss R, Al-Osaimi AM. The treatment of cholangiocarcinoma: a hepatologist’s perspective. Curr Gastroenterol Rep. 2014;16(10):412. | ||
Strand DS, Cosgrove ND, Patrie JT, et al. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80(5):794–804. | ||
Felekkis K, Touvana E, Stefanou C, Deltas C. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14(4):236–240. | ||
Fang Z, Rajewsky N. The impact of miRNA target sites in coding sequences and in 3′UTRs. PLoS One. 2011;6(3):e18067. | ||
Liu Q, He H, Zeng T, Huang Z, Fan T, Wu Q. Neural-specific expression of miR-344-3p during mouse embryonic development. J Mol Histol. 2014;45(4):363–372. | ||
Kim JS, Choi DW, Kim CS, et al. MicroRNA-203 induces apoptosis by targeting Bmi-1 in YD-38 oral cancer cells. Anticancer Res. 2018;38(6):3477–3485. | ||
Hu T, Chong Y, Lu S, et al. miR-339 promotes development of stem cell leukemia/lymphoma syndrome via downregulation of the BCL2L11 and BAX pro-apoptotic genes. Cancer Res. 2018;78(13):3522–3531. | ||
Liu Y, Huang X, Timani KA, Broxmeyer HE, He JJ. MicroRNA-124 Targets Tip110 Expression and Regulates Hematopoiesis. Stem Cells Dev. 2015;24(17):2009–2017. | ||
Lind EF, Elford AR, Ohashi PS. Micro-RNA 155 is required for optimal CD8+ T cell responses to acute viral and intracellular bacterial challenges. J Immunol. 2013;190(3):1210–1216. | ||
Wang H, Li C, Jian Z, Ou Y, Ou J. TGF-β1 reduces miR-29a expression to promote tumorigenicity and metastasis of cholangiocarcinoma by targeting HDAC4. PLoS One. 2015;10(10):e0136703. | ||
Han Y, Meng F, Venter J, et al. miR-34a-dependent overexpression of Per1 decreases cholangiocarcinoma growth. J Hepatol. 2016;64(6):1295–1304. | ||
Liu CH, Huang Q, Jin ZY, et al. Circulating microRNA-21 as a prognostic, biological marker in cholangiocarcinoma. J Cancer Res Ther. 2018;14(1):220–225. | ||
Correa-Gallego C, Maddalo D, Doussot A, et al. Circulating plasma levels of MicroRNA-21 and MicroRNA-221 are potential diagnostic markers for primary intrahepatic cholangiocarcinoma. PLoS One. 2016;11(9):e0163699. | ||
Lund AH. miR-10 in development and cancer. Cell Death Differ. 2010;17(2):209–214. | ||
Quinonez SC, Innis JW. Human HOX gene disorders. Mol Genet Metab. 2014;111(1):4–15. | ||
Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101(9):2999–3004. | ||
Gonzalez Dos Anjos L, de Almeida BC, Gomes de Almeida T, et al. Could miRNA signatures be useful for predicting uterine sarcoma and carcinosarcoma prognosis and treatment? Cancers (Basel). 2018;10(9):E315. | ||
Kim C, Go EJ, Kim A. Recurrence prediction using microRNA expression in hormone receptor positive breast cancer during tamoxifen treatment. Biomarkers. Epub 2018 Aug 23. | ||
Zhu Q, Gong L, Wang J, et al. miR-10b exerts oncogenic activity in human hepatocellular carcinoma cells by targeting expression of CUB and sushi multiple domains 1 (CSMD1). BMC Cancer. 2016;16(1):806. | ||
Luo L, Yu ZP, Qin H, et al. Exosomal MicroRNA-10a Is Associated with Liver Regeneration in Rats through Downregulation of EphA4. Chin Med J (Engl). 2018;131(4):454–460. | ||
Tangtanatakul P, Thammasate B, Jacquet A, et al. Transcriptomic profiling in human mesangial cells using patient-derived lupus autoantibodies identified miR-10a as a potential regulator of IL8. Sci Rep. 2017;7(1):14517. | ||
Luo L, Chen B, Li S, et al. Plasma miR-10a: a potential biomarker for coronary artery disease. Dis Markers. 2016;2016:3841927. | ||
Mu N, Gu J, Huang T, et al. A novel NF-κB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci Rep. 2016;6:20059. | ||
Xiong G, Huang H, Feng M, et al. MiR-10a-5p targets TFAP2C to promote gemcitabine resistance in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2018;37(1):76. | ||
Bi L, Sun L, Jin Z, Zhang S, Shen Z. MicroRNA-10a/b are regulators of myeloid differentiation and acute myeloid leukemia. Oncol Lett. 2018;15(4):5611–5619. | ||
Haga H, Yan I, Takahashi K, Wood J, Patel T. Emerging insights into the role of microRNAs in the pathogenesis of cholangiocarcinoma. Gene Expr. 2014;16(2):93–99. | ||
Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol. 2016;34(3):219–226. | ||
Chen X, Chen J, Liu X, Guo Z, Sun X, Zhang J. The real-time dynamic monitoring of microRNA function in cholangiocarcinoma. PLoS One. 2014;9(6):e99431. | ||
Yan Y, Luo YC, Wan HY, et al. MicroRNA-10a is involved in the metastatic process by regulating Eph tyrosine kinase receptor A4-mediated epithelial-mesenchymal transition and adhesion in hepatoma cells. Hepatology. 2013;57(2):667–677. | ||
Hussain N, Zhu W, Jiang C, et al. Down-regulation of miR-10a-5p promotes proliferation and restricts apoptosis via targeting T-box transcription factor 5 in inflamed synoviocytes. Biosci Rep. 2018;38(2):BSR20180003. | ||
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405. | ||
Yothaisong S, Dokduang H, Techasen A, et al. Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol. 2013;34(6):3637–3648. | ||
Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1(12):1170–1177. | ||
Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. | ||
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–658. | ||
Wang LJ, He CC, Sui X, et al. MiR-21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTEN. Oncotarget. 2015;6(8):5932–5946. | ||
Li J, Yao L, Li G, et al. miR-221 promotes epithelial-mesenchymal transition through targeting PTEN and forms a positive feedback loop with β-catenin/c-Jun signaling pathway in extra-hepatic cholangiocarcinoma. PLoS One. 2015;10(10):e0141168. | ||
Zhu H, Han C, Lu D, Wu T. miR-17-92 cluster promotes cholangiocarcinoma growth: evidence for PTEN as downstream target and IL-6/Stat3 as upstream activator. Am J Pathol. 2014;184(10):2828–2839. | ||
Ji W, Sun B, Su C. Targeting microRNAs in cancer gene therapy. Genes (Basel). 2017;8(1):E21. |
Supplementary material
    | Table S1 Predicted targets of human miR-10a-5p |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.