Back to Journals » OncoTargets and Therapy » Volume 12
Inhibition Of Glycogen Synthase Kinase 3 Beta Suppresses The Growth And Survival Of Skull Base Chordoma Cells By Downregulating Brachyury Expression
Authors Yan X , Li Z, Li H, Liu P, Zhao Z, Cheng S, Wang Z, Zhang Q
Received 11 June 2019
Accepted for publication 31 October 2019
Published 18 November 2019 Volume 2019:12 Pages 9783—9791
DOI https://doi.org/10.2147/OTT.S218930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Xudong Yan,1 Zhiyuan Li,2 Hong Li,3 Pei Liu,4 Zehang Zhao,5 Shan Cheng,5 Zhenlin Wang,1 Qiuhang Zhang1
1Department of Otolaryngology-Head and Neck Surgery, Skull Base Surgery Center, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Key Laboratory, Department of Otolaryngology-Head and Neck Surgery, Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 3Department of Pathology, Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 4Department of Anesthesiology, Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 5Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Qiuhang Zhang
Department of Otolaryngology-Head and Neck Surgery, Skull Base Surgery Center, Xuanwu Hospital, Capital Medical University, 45 Changchun Street, Xicheng, Beijing 100053, People’s Republic of China
Email [email protected]
Purpose: Chordomas are locally aggressive tumors arising from notochordal remnants. Brachyury, a protein coded by T-gene, is crucial for chordoma cell proliferation. The aim of this study was to evaluate the effects of glycogen synthase kinase 3 beta (GSK3β) activity on brachyury expression and on the growth and survival of skull base chordoma cells.
Patients and methods: In this study, 16 paraffin-embedded specimens of primary skull base chordomas were analyzed for the expression of phosphorylated GSK3β and brachyury using immunohistochemistry. The UM-Chor1 cell line derived from a clival chordoma was treated with AR-A014418 (AR), an inhibitor of GSK3β, and brachyury expression was analyzed by qRT-PCR and Western blotting. The possible mechanism by which brachyury regulates the Wnt/β-catenin signaling pathway was investigated by immunocytochemistry. The effects of AR on cell proliferation as well as sensitivity to chemotherapeutic drugs were also examined.
Results: The results suggested that phosphorylated GSK3β and brachyury were upregulated in chordoma tissues. The GSK3β inhibitor (AR) decreased brachyury expression and suppressed the growth and survival of the chordoma cells, possibly via regulation of the Wnt/β-catenin signaling pathway. Moreover, AR increased the sensitivity of chordoma cells to chemotherapeutic drugs in vitro.
Conclusion: This study provides evidence for the clinical development of the GSK3β inhibitor (AR-A014418) as a potential chemotherapeutic adjuvant for the treatment of chordoma.
Keywords: GSK3β inhibitor, skull base chordoma, brachyury, Wnt/β-catenin signaling pathway
Introduction
Chordoma, which is a rare and locally aggressive tumor arising from notochordal remnants,1 occurs frequently along the cranial-spinal axis. A recent comprehensive analysis showed that 42% of all chordomas are cranial.2 Although surgery is the main therapy for chordoma at present, a large tumor burden at the time of diagnosis, poor margination and impingement on surrounding structures make gross total resection difficult.3 Especially in cases of skull base chordoma, wide local excision is usually not an option.4 Combined with the insensitivity to conventional radiotherapy and chemotherapy, the recurrence rates for cranial chordoma have been reported to be high as 68%.5 In secondary patients, surgery is more difficult and is associated with a high rate of incomplete resection.6 Moreover, local recurrence has become the major cause of mortality in chordoma patients.4 Improvements in chordoma treatment require a better understanding of the molecular biology and oncogenesis of chordomas to identify and develop efficient targeted chemotherapies.6
Brachyury, a core T-box transcription factor coded by the T-gene, is thought to be the vital protein in chordomas.7 Recent reports revealed that T-gene duplication is a major susceptibility factor for familial chordoma8 and suppression of brachyury expression in a chordoma cell line suppressed growth in vitro.9 On the other hand, brachyury plays a vital role in the development of early embryonic notochord formation,10 which is subsequently downregulated in late-stage embryos and eventually becomes undetectable in the majority of normal adult tissues. To date, brachyury expression in normal adult human tissues has been reported only in scattered cells in seminiferous tubules11 and isolated cells in the thyroid.12 However, almost 100% of chordomas express high levels of brachyury protein.13,14 Studies in stem cells have shown that Wnt signaling regulates brachyury expression;15 therefore, we investigated the role of this pathway in chordomas. Glycogen synthase kinase 3 beta (GSK3β) regulates β-catenin destabilization and consequently, plays a central role in Wnt/β-catenin signaling.16 Studies have confirmed that the potent and selective GSK3β inhibitor (AR-A014418; AR) reduces the expression of β-catenin.17,18 Thus, in the present study, we investigated the effects of GSK3β activity on brachyury expression and the role of Wnt signaling pathway in the skull base chordoma.
Materials And Methods
Clinical Data And Materials
Sixteen formalin-fixed paraffin-embedded samples were collected when tumors were resected from patients diagnosed with skull base chordoma at the Xuanwu Hospital between 2012 and 2018. All the patients were treated primarily with surgery and had not undergone chemotherapy or radiotherapy.
Cell Lines
The UM-Chor1 (ATCC® CRL-3270™) cell line, which was the first chordoma cell line to be generated and originates from the clivus, was purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)1640 4:1 supplemented with 10% fetal calf serum, penicillin, streptomycin (Sigma–Aldrich, Poole, Dorset, UK) at 37°C under 5% CO2 and 95% humidity.
Immunohistochemical (IHC) Staining
Chordoma samples were fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned. After dewaxing, antigen retrieval was achieved by treating the sections with 1% hot citric acid buffer. Sections were then incubated with primary detection antibodies for 1 h at room temperature, followed by incubation for 30 min with secondary detection antibodies. Phosphorylated GSK3β(Ser9) (1:50) antibody was purchased from Santa Cruz (California, USA) and brachyury (1:1000) antibody was purchased from Abcam (Cambridge, UK). Immunohistochemical staining was visualized using the DAB chromogenic substrate. After counter-staining using hematoxylin and dehydration, images were captured under a light-field microscope at 200× magnification. Immunoreactive in the cytoplasm/nucleus was defined as positive for p-GSKβ, while in the immunoreactive in the nucleus was defined as positive for brachyury. Positive immunoreactivity in more than 95% of the neoplastic cells was defined as positive. The adjacent normal tissues were used as negative controls.
RNA Isolation And Quantitative PCR (qRT-PCR)
Total RNA was isolated from the chordoma cell line using TRIzol® Reagent (Sigma–Aldrich). RNA was converted to cDNA using iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories Ltd., Hercules, CA, USA). Following reverse transcription, brachyury gene expression was determined by quantitative real-time quantitative PCR (qRT-PCR) using SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA) on the Applied Biosystems 7500 Real-Time PCR System. The qRT-PCR conditions were as follows: 95°C for 10 min, followed by 30 cycles of 95°C for 10 s, 55°C for 35 s, and 72°C for 10 s. The following primers were used in the study: GAPDH sense: 5ʹ-GGGAAGGTGAAGGTCGGAGT-3ʹ, antisense: 5ʹ-TTGAGGTCAATGAAGGGGTCA-3ʹ; brachyury sense: 5ʹ-CTATTCTGACAACTCACCTGCAT-3ʹ, antisense: 5ʹ-CTATTCTGACAACTCACCTGCAT-3ʹ. GAPDH was used as an internal control to normalize brachyury gene expression. Reactions were performed in triplicate with a set of standards and negative controls.
Western Blot Analysis
Cells were treated with AR-A014418 (2.5, 5, 10, 20, 30 μM) or DMSO, as a control, for 24 h. Then protein expression in the chordoma cell lysates was assessed by standard sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. GAPDH expression was used as an internal control. GSK3β (1:500), phosphorylated GSK3β(Ser9) (1:500) and GAPDH (1:2000) antibodies were purchased from Santa Cruz (California, USA). β-catenin (1:800) and brachyury (1:5000) antibodies were purchased from Abcam (Cambridge, UK).
Immunofluorescence Staining
UM-Chor1 cells were plated on coverslips in 6-well plates containing complete medium. Cells were treated with AR-A014418 (30 μM) or DMSO, as a control, for 24 h. Coverslips were rinsed with PBS and permeabilized with 4% paraformaldehyde (containing 0.2% Triton-100) for 30 min, then washed three times with PBS and incubated with brachyury and β-catenin antibodies overnight at 4°C. After washing three times with PBS, cells were incubated with Alexa Fluor ®488-conjugated anti-mouse IgG and Alexa Fluor ® 594-conjugated anti-rabbit IgG (Cell Signaling Technology) for 30 min. The nucleus was stained with Hoechst. Images of fixed cells were acquired using a confocal microscope with Laser Sharp software.
Cell Viability Assay
Cell viability was measured using the the CCK-8 tetrazolium salt (WST-8)-based colorimetric assay. Briefly, cells were seeded in 96-well plates at an initial density of 5000 cells per well. At the time points indicated, spent medium was replaced with fresh medium containing 100 μl CCK-8 solution, and the plate was incubated for 1 h. Cell viability was detected by scanning at 450 nm using a spectrophotometric microplate reader.
Colony Formation Assay
Cells were seeded into 6-well plates (500 cells/well) and treated with AR-A014418 (0.1, 1 or 10μM) or DMSO, as a control, for 12 days. Cells were then stained with 0.01% (w/v) crystal violet, and cell colonies were counted. The assays were performed in triplicate with at least three replicates per treatment.
FACS (Fluorescence-Activated Cell sorting) Analysis
Cells were seeded into 6-well plates (5×105 cells/well) and treated with AR-A014418, rapamycin or DMSO, as a control, for 48 h. Then cells were harvested and washed twice with ice-cold PBS. Cells were then resuspended in binding buffer and stained with annexin V-FITC and propidium iodide (PI) in the dark. The samples were analyzed using a Muse® Cell Analyzer (Merck).
Statistical Analysis
Statistical analyses were performed using the SPSS 22.0 (SPSS Inc, Chicago, IL, USA) and GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA). Data were presented as mean±SD of at least three independent experiments. Two tailed Student’s t-test was used for comparisons between two groups, and one-way analysis of variance (ANOVA) was used for comparisons among more than two groups. A value of P<0.05 was considered to indicate statistical significance.
Results
Phosphorylated GSK3β And Brachyury Were Expressed At High Levels In Chordoma
Patient characteristics are presented in Table 1. Sixteen paraffin-embedded specimens of primary skull base chordomas were collected and the expression of p-GSK3β and brachyury was analyzed using immunohistochemical methods. The results showed that the levels of p-GSK3β and brachyury proteins were elevated in samples of chordomas tissues, compared to those in the adjacent normal tissues (Figure 1A and B).
 |
Table 1 Clinical Features Of Chordoma Patients |
The GSK3β Inhibitor AR-A014418 Treatment Decreased Brachyury Expression
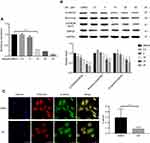
The chordoma cell lines were treated with AR (GSK3β inhibitor) for 24 h. The expression of brachyury mRNA significantly decreased in chordoma cells in a dose-dependent manner (Figure 2A). Western blotting confirmed the decreased brachyury expression after AR treatment. Downregulated expression of β-catenin was also observed in the AR-treated cells (Figure 2B). In addition, immunofluorescent staining showed that the nuclear accumulation of β-catenin was reduced after AR (30 μM) treatment for 24 h (Figure 2C). Taken together, our results suggested that brachyury expression is regulated by GSK3β via a mechanism that involves the Wnt/β-catenin signaling pathway.
AR Suppressed The Growth And Survival Of Chordoma Cells In Vitro
Cell viability assays showed that the growth of UM-Chor1 chordoma cells was markedly suppressed after treatment with AR (30 μM) (Figure 3A). In addition, AR treatment of chordoma cell lines for 14 days strongly inhibited colony formation in a dose-dependent manner (Figure 3B). Taken together, these findings demonstrated that AR effectively reduces survival and growth of chordoma cells in vitro, possibly due to the inhibition of brachyury expression.
AR Induced Apoptosis Of Chordoma Cells And Increased The Sensitivity To The Rapamycin Chemotherapy In Vitro
To determine the effects of AR on the cellular response, we performed cell apoptosis assays on chordoma cells after AR treatment. Compared to the control group (treated with DMSO), chordoma cell apoptosis increased in a dose-dependent manner after AR treatment, and the effect reached the level of statistical significance in the 40μM and 50μM groups. No significant increase in apoptosis was after treatment with high-dose rapamycin (500 nM). However, the combined treatment with 500 nM rapamycin and 40 μM AR showed a statistically significant increase in cell apoptosis compared to that in the single treated cells (Figure 4). The results showed that AR induced apoptosis of chordoma cells and increased the sensitivity to the rapamycin chemotherapy.
Discussion
At present, surgery remains the major treatment for chordoma and the endoscopic endonasal approach (EEA) is becoming the preferred surgical approach.19,20 However, achieving oncology resection is usually difficult due to the complexity of the growth site, especially in cases of skull base chordoma. Total resection is achieved has been reported to range from 45.1% to 83% in several series,3,21,22 which makes the recurrent rates high. Many patients will require repeated surgeries during the of survival period (average, approximately 6 years),23 which severely affects the quality of life of these patients and their families. Therefore, new and effective methods other than surgery are urgently required to reduce tumor growth and prolong surgery intervals.
In previous studies, brachyury was proposed as the vital molecule in chordomas. Increased brachyury gene copy numbers were found in both sporadic8 and familial chordoma.23 In vitro studies in chordoma cell lines confirmed that cell growth was significantly inhibited by brachyury gene-silencing.24 In addition, it has been shown that increased brachyury expression in lung cancer cell lines is associated with increased resistance to cisplatin, vinorelbine, docetaxel, and radiation in vitro and reduced brachyury expression in tumor cells resulted in increased tumor responsiveness to chemotherapy.25 Therefore, brachyury represents a potential target for drug development. Based on studies of the chordoma cell lines JHC7, UCH1, and UCH2 (all originating from sacral chordoma), Hu et al proposed that FGF/FGFR and brachyury promote chordoma cell growth and survival via a positive feedback loop.26 After analyzing frozen specimens of chordoma, Schwab et al reported that the PI3K/AKT/mTOR pathway is activated in the tumor and that the inhibitor PI-103 suppressed the growth of chordoma cell line UCH127 This theory was supported by studies of the chordoma cell line UCH2 reported by Otani et al.9 However, despite rational selection of agents based on potential therapeutic targets identified in preclinical studies, radiographic response rates have been very low.28
Chordoma is believed to originate from residual embryonic chordate tissue, and brachyury is specifically expressed in the embryonic stage as an important molecule guiding mesoderm differentiation. Previous studies of embryonic stem cell differentiation and proliferation indicated that brachyury expression is regulated by the Wnt signaling pathway;15,29 however, there is no current evidence for this mechanism in chordoma.
GSK3β is known as the central molecule in canonical Wnt signaling. In this study, we observed that phosphorylated-GSK3β is highly expressed in skull base chordoma. In biochemical studies of skull base chordoma cell lines, we observed that GSK3β inhibition downregulated the expression of brachyury. Furthermore, Western blot analysis showed a corresponding decreased in β-catenin expression. We also observed that nuclear accumulation of β-catenin was reduced, suggesting that brachyury expression is regulated by the Wnt/β-catenin pathway. The AR-induced decrease in brachyury expression was associated with reduced chordoma cell viability. Moreover, the cells become more sensitive to the chemotherapy drug rapamycin, which is consistent with cases of other cancer.30,31 These results suggest that brachyury expression in chordoma is regulated by the Wnt/β-catenin pathway and inhibition of this pathway suppresses the growth and survival of chordoma cells. Thus, the GSK3β inhibitor AR-A014418 is implicated as a potential adjuvant chemotherapeutic agent in chordoma. Interestingly, GSK3 activity is also dysregulated in numerous neurodegenerative disorders such as Alzheimer’s Disease and Parkinson’s Disease;32 thus, GSK3 is also a promising therapeutic target in these conditions. Independent studies have demonstrated that direct or indirect inhibition of GSK3β improves the condition of neurological disorders.33–39 It is reported that AR-A014418 can prevent dopaminergic neurons from MPTP-induced apoptosis and be candidates for PD treatment.38 However, changes in GSK3β activity can leads to several side-effects because of the pleiotropic function of the kinase in cellular physiology and a variety of functional cross-talk. Thus, GSK3β inhibition as a treatment approach remains a challenge in clinical practice.
Conclusion
This study provides evidence that, similar to embryonic stem cells, the Wnt/β-catenin signaling pathway is activated in chordoma cells, which are known to originate from residual embryonic chordate tissue. Inhibition of Wnt/β-catenin by a GSK3β inhibitor (AR-A014418) downregulated brachyury expression, leading to suppression of the growth and survival of skull base chordoma cells in vitro. Furthermore, AR significantly increased the sensitivity of chordoma cells to the rapamycin chemotherapy. Overall, this study provides evidence for the clinical development of the GSK3β inhibitor AR-A014418 as a potential adjuvant chemotherapeutic agent in the treatment of chordoma.
Ethics Approval And Informed Consent
This study was approved by the Ethics Committee of the Xuanwu Hospital (Beijing, China). All patients (or a parent/legal guardian of any patients under the age of 18 years) provided written informed consent for their tissues to be used for future research, which was conducted in accordance with the Declaration of Helsinki.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Professor Jisheng Zhang and Yaxin Bo for their generous help.
Author Contributions
XY, SC, ZW and QZ conceived and designed the study. ZZ performed cell culture. HL performed immunostaining. XY collected clinical data and performed qRT-PCR, Western blotting, and immunofluorescence staining. PL performed cell viability and colony formation assays. ZL performed FACS analysis. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Di Maio S, Rostomily R, Sekhar LN. Current surgical outcomes for cranial base chordomas: cohort study of 95 patients. Neurosurgery. 2012;70(6):1355–1360. discussion 1360. doi:10.1227/NEU.0b013e3182446783
2. Chambers KJ, Lin DT, Meier J, Remenschneider A, Herr M, Gray ST. Incidence and survival patterns of cranial chordoma in the United States. Laryngoscope. 2014;124(5):1097–1102. doi:10.1002/lary.24420
3. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–76. doi:10.1016/S1470-2045(11)70337-0
4. Stacchiotti S, Casali PG, Lo Vullo S, et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol. 2010;17(1):211–219. doi:10.1245/s10434-009-0740-x
5. Jian BJ, Bloch OG, Yang I, et al. Adjuvant radiation therapy and chondroid chordoma subtype are associated with a lower tumor recurrence rate of cranial chordoma. J Neurooncol. 2010;98(1):101–108. doi:10.1007/s11060-009-0068-1
6. George B, Bresson D, Herman P, Froelich S. Chordomas: a review. Neurosurg Clin N Am. 2015;26(3):437–452. doi:10.1016/j.nec.2015.03.012
7. Kitamura Y, Sasaki H, Kimura T, et al. Molecular and clinical risk factors for recurrence of skull base chordomas: gain on chromosome 2p, expression of brachyury, and lack of irradiation negatively correlate with patient prognosis. J Neuropathol Exp Neurol. 2013;72(9):816–823. doi:10.1097/NEN.0b013e3182a065d0
8. Yang XR, Ng D, Alcorta DA, et al. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet. 2009;41(11):1176–1178. doi:10.1038/ng.454
9. Otani R, Mukasa A, Shin M, et al. Brachyury gene copy number gain and activation of the PI3K/Akt pathway: association with upregulation of oncogenic Brachyury expression in skull base chordoma. J Neurosurg. 2018;128(5):1428–1437. doi:10.3171/2016.12.JNS161444
10. Shah SR, David JM, Tippens ND, et al. Brachyury-YAP regulatory axis drives stemness and growth in cancer. Cell Rep. 2017;21(2):495–507. doi:10.1016/j.celrep.2017.09.057
11. Miettinen M, Wang Z, Lasota J, Heery C, Schlom J, Palena C. Nuclear brachyury expression is consistent in chordoma, common in germ cell tumors and small cell carcinomas, and rare in other carcinomas and sarcomas: an immunohistochemical study of 5229 cases. Am J Surg Pathol. 2015;39(10):1305–1312. doi:10.1097/PAS.0000000000000462
12. Roselli M, Fernando RI, Guadagni F, et al. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res. 2012;18(14):3868–3879. doi:10.1158/1078-0432.CCR-11-3211
13. Vujovic S, Henderson S, Presneau N, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209(2):157–165. doi:10.1002/(ISSN)1096-9896
14. Tirabosco R, Mangham DC, Rosenberg AE, et al. Brachyury expression in extra-axial skeletal and soft tissue chordomas: a marker that distinguishes chordoma from mixed tumor/myoepithelioma/parachordoma in soft tissue. Am J Surg Pathol. 2008;32(4):572–580. doi:10.1097/PAS.0b013e31815b693a
15. Singh AM, Reynolds D, Cliff T, et al. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell. 2012;10(3):312–326. doi:10.1016/j.stem.2012.01.014
16. Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35(3):161–168. doi:10.1016/j.tibs.2009.10.002
17. Carter YM, Kunnimalaiyaan S, Chen H, Gamblin TC, Kunnimalaiyaan M. Specific glycogen synthase kinase-3 inhibition reduces neuroendocrine markers and suppresses neuroblastoma cell growth. Cancer Biol Ther. 2014;15(5):510–515. doi:10.4161/cbt.28015
18. Kunnimalaiyaan S, Gamblin TC, Kunnimalaiyaan M. Glycogen synthase kinase-3 inhibitor AR-A014418 suppresses pancreatic cancer cell growth via inhibition of GSK-3-mediated Notch1 expression. HPB (Oxford). 2015;17(9):770–776. doi:10.1111/hpb.12442
19. Stippler M, Gardner PA, Snyderman CH, Carrau RL, Prevedello DM, Kassam AB. Endoscopic endonasal approach for clival chordomas. Neurosurgery. 2009;64(2):268–277. discussion 277–268. doi:10.1227/01.NEU.0000338071.01241.E2
20. Koutourousiou M, Gardner PA, Tormenti MJ, et al. Endoscopic endonasal approach for resection of cranial base chordomas: outcomes and learning curve. Neurosurgery. 2012;71(3):614–624. discussion 624–615. doi:10.1227/NEU.0b013e31825ea3e0
21. Di Maio S, Temkin N, Ramanathan D, Sekhar LN. Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J Neurosurg. 2011;115(6):1094–1105. doi:10.3171/2011.7.JNS11355
22. Koutourousiou M, Snyderman CH, Fernandez-Miranda J, Gardner PA. Skull base chordomas. Otolaryngol Clin North Am. 2011;44(5):1155–1171. doi:10.1016/j.otc.2011.06.002
23. McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12(1):1–11. doi:10.1023/A:1008947301735
24. Hsu W, Mohyeldin A, Shah SR, et al. Generation of chordoma cell line JHC7 and the identification of Brachyury as a novel molecular target. J Neurosurg. 2011;115(4):760–769. doi:10.3171/2011.5.JNS11185
25. Hamilton DH, David JM, Dominguez C, Palena C. Development of cancer vaccines targeting brachyury, a transcription factor associated with tumor epithelial-mesenchymal transition. Cells Tissues Organs. 2017;203(2):128–138. doi:10.1159/000446495
26. Hu Y, Mintz A, Shah SR, Quinones-Hinojosa A, The HW. FGFR/MEK/ERK/brachyury pathway is critical for chordoma cell growth and survival. Carcinogenesis. 2014;35(7):1491–1499. doi:10.1093/carcin/bgu014
27. Schwab J, Antonescu C, Boland P, et al. Combination of PI3K/mTOR inhibition demonstrates efficacy in human chordoma. Anticancer Res. 2009;29(6):1867–1871.
28. Heery CR. Chordoma: the quest for better treatment options. Oncol Ther. 2016;4(1):35–51. doi:10.1007/s40487-016-0016-0
29. Arnold SJ, Stappert J, Bauer A, Kispert A, Herrmann BG, Kemler R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev. 2000;91(1–2):249–258. doi:10.1016/S0925-4773(99)00309-3
30. Larocca C, Cohen JR, Fernando RI, Huang B, Hamilton DH, Palena C. An autocrine loop between TGF-beta1 and the transcription factor brachyury controls the transition of human carcinoma cells into a mesenchymal phenotype. Mol Cancer Ther. 2013;12(9):1805–1815. doi:10.1158/1535-7163.MCT-12-1007
31. Huang B, Cohen JR, Fernando RI, et al. The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis. 2013;4:e682. doi:10.1038/cddis.2013.208
32. Duda P, Wisniewski J, Wojtowicz T, et al. Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin Ther Targets. 2018;22(10):833–848.
33. Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77(1):94–102. doi:10.1046/j.1471-4159.2001.t01-1-00251.x
34. Li YZ, Tang XH, Wang CY, et al. Glycogen synthase kinase-3beta inhibition prevents remifentanil-induced postoperative hyperalgesia via regulating the expression and function of AMPA receptors. Anesth Analg. 2014;119(4):978–987. doi:10.1213/ANE.0000000000000365
35. Prati F, De Simone A, Bisignano P, et al. Multitarget drug discovery for Alzheimer’s disease: triazinones as BACE-1 and GSK-3beta inhibitors. Angew Chem Int Ed Engl. 2015;54(5):1578–1582. doi:10.1002/anie.201410456
36. Sereno L, Coma M, Rodriguez M, et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35(3):359–367. doi:10.1016/j.nbd.2009.05.025
37. Ren ZX, Zhao YF, Cao T, Zhen XC. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol Sin. 2016;37(10):1315–1324. doi:10.1038/aps.2016.42
38. Wang W, Yang Y, Ying C, et al. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52(8):1678–1684. doi:10.1016/j.neuropharm.2007.03.017
39. Morales-Garcia JA, Susin C, Alonso-Gil S, et al. Glycogen synthase kinase-3 inhibitors as potent therapeutic agents for the treatment of Parkinson disease. ACS Chem Neurosci. 2013;4(2):350–360. doi:10.1021/cn300182g
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.