Back to Journals » OncoTargets and Therapy » Volume 13
Inhibition of ERKs/Akt-Mediated c-Fos Expression Is Required for Piperlongumine-Induced Cyclin D1 Downregulation and Tumor Suppression in Colorectal Cancer Cells
Authors Gao F, Zhou L, Li M, Liu W, Yang S, Li W
Received 25 February 2020
Accepted for publication 20 May 2020
Published 15 June 2020 Volume 2020:13 Pages 5591—5603
DOI https://doi.org/10.2147/OTT.S251295
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Feng Gao,1,2,* Li Zhou,3,* Ming Li,1,4,5,* Wenbin Liu,6 Shuting Yang,2 Wei Li1,7
1Cell Transplantation and Gene Therapy Institute, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China; 2Department of Ultrasonography, The Third Xiangya Hospital of Central South University, Changsha, Hunan 410013, People’s Republic of China; 3Department of Pathology, Xiangya Hospital of Central South University, Changsha, Hunan 410013, People’s Republic of China; 4Changsha Stomatological Hospital, Changsha, Hunan 410004, People’s Republic of China; 5School of Stomatology, Hunan University of Chinese Medicine, Changsha, Hunan 410208, People’s Republic of China; 6Department of Pathology, Hunan Cancer Hospital, Changsha, Hunan 410013, People’s Republic of China; 7Department of Radiology, The Third Xiangya Hospital of Central South University, Changsha, Hunan 410013, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Li Email [email protected]
Background: Deregulation of Cyclin D1 and cell cycle progression plays a critical role in tumorigenesis. The natural compound piperlongumine (PL) exhibits potential anticancer effects in various cancer models, but the underlying mechanism needs further elucidation.
Methods: The inhibitory effect of PL on colorectal cancer (CRC) cells was determined by anchorage-dependent and -independent assays. The protein level of Cyclin D1 was examined by immunoblot (IB) and immunohistochemical staining (IHC). The mRNA level was determined by qRT-PCR. Phosphorylation of histone H3 was analyzed by immunofluorescence (IF). The cell cycle was examined by flow cytometry. The in vivo antitumor effect was validated by the xenograft mouse model.
Results: Cyclin D1 was overexpressed in CRC tissues and cells, and was required for maintaining cell growth, colony formation, and in vivo tumorigenesis. PL decreased the protein level of c-Fos, which eventually reduced the transcriptional activity of AP-1 and the mRNA level of Cyclin D1. Mechanism study showed that PL impaired EGF-induced activation of ERK1/2 and Akt signalings, which resulted in a reduction of c-Fos transcription. Furthermore, PL reduced the half-life of c-Fos and caused the ubiquitination-dependent degradation of c-Fos. Finally, the in vivo antitumor effect of PL on CRC cells was examined using a xenograft mouse model.
Conclusion: Our data indicate that PL is a promising antitumor agent that deserves further study for CRC treatment.
Keywords: colorectal cancer, piperlongumine, c-Fos, Cyclin D1
Introduction
Colorectal cancer (CRC) is one of the most common types of human malignancies. Each year, nearly 9% of cancer-related deaths were caused by CRC.1,2 Currently, the surgery treatment remains the mainstay of treatment for early cases. However, most CRC patients are frequently diagnosed at an advanced stage, and metastasis is the major reason to cause therapy failure.3,4 Although the fluorouracil (5-FU) based systemic chemotherapy and the combination with radiotherapy or targeting therapy increased the overall survival rate of CRC patients, the outcome has not improved at a satisfactory rate over the past decades. The majority of the patients receiving chemotherapy will eventually experience tumor recurrence due to drug resistance, and this has become a key barrier for the clinical treatment of colorectal cancer.5,6 Thus, revealing the underlying mechanism of colorectal tumorigenesis and identify novel therapeutic targets are necessary for the development of effective therapies for CRC patients.
Cell cycle progression is regulated by two families of proteins called cyclins and cyclin-dependent kinases (CKDs). Cyclins bind with CDKs and form complexes to activate the kinase activity of CDKs and phosphorylate the downstream target proteins that are required for cell-cycle progression and transition.7 Previous reports have shown that the induction of Cyclin D1 and the subsequent interaction with CDK4/CDK6 is a rate-limiting step for cell cycle progression in the early G1 phase. Given the crucial role of Cyclin D1 for cell cycle regulation, it’s not surprising that Cyclin D1 is overexpressed in human cancers.8 Previous studies revealed that highly expressed Cyclin D1 promoted tumor cell growth and correlated with poor prognosis in human lung cancer,9 colorectal cancer,10 gastric cancer,11 and liver cancer.12 The expression of Cyclin D1 is tightly regulated at multiple levels, including transcriptional, translational, and post-translational. A panel of transcription factors, such as AP-1, NF-κB, epidermal growth factor receptor (EGFR), and Egr1, have been identified to be required for Cyclin D1 transcription in various tumor models.8,13 Targeting the transcription or translation of Cyclin D1 is considered as a promising anti-tumor strategy for clinical treatment.
In this study, we showed that Cyclin D1 is highly expressed in human CRC tumor tissues and cell lines. Knockout of Cyclin D1 attenuated the malignant phenotype of CRC cells both in vitro and in vivo. Importantly, we found a natural compound, piperlongumine (PL), suppressed CRC cells by inhibition of AP-1-mediated Cyclin D1 expression. We investigated the anti-tumor effect of PL in CRC cells and revealed the underlying mechanism.
Materials and Methods
Reagents and Antibodies
The natural product piperlongumine (>99%) was purchased from Selleck Chemicals (Houston, TX). The primary antibodies against Cyclin D1, c-Jun, Jun B, Jun D, Fos B, Fra1, c-Fos, p-EGFR Tyr1068, p-ERK1/2, β-actin, and p-Akt were obtained from Cell Signaling Technology, Inc. (Beverly, MA). The anti-ki67 antibody for Immunohistochemical was a product of Abcam (Cambridge, United Kingdom). The jetPEI (Qbiogene, Inc., Montreal, Canada) was used for plasmid transfection according to the manufacturer’s instructions.
Cell Culture
Human colorectal cancer cells, including LOVO, SW480, HCT116, HT29, HCT8, SW620, and the immortalized colorectal epithelial cells FHC and CCD 841, were purchased from American Type Culture Collection (ATCC, Manassas, VA). All cells were maintained in a humidified incubator with 5% CO2 at 37°C following the ATCC protocols. Cell culture medium, fetal bovine serum (FBS), and antibiotics were purchased from Invitrogen (Grand Island, NY). All cells were subjected to mycoplasma analysis every two months.
Immunoblotting
The immunoblotting (IB) was performed as described previously.14 Briefly, the whole-cell extract (WCE) was prepared with the RIPA buffer (Thermo Fisher, Waltham, MA) supplied with protease inhibitors and concentrated by BCA protein assay. A total of 30 μg WCE was mixed with loading buffer and boiled at 95°C for 5 min, followed by SDS-PAGE electrophoresis and electrotransfer. The non-fat milk (5%) was used for membrane blocking at room temperature for 30 min, and the membrane was incubated with the primary antibody at 4°C overnight. After incubation with anti-rabbit/mouse IgG HRP second antibody, the target protein was visualized by chemiluminescence.
MTS Assay
MTS assay was performed as described previously.15 Human colorectal cancer cells were suspended and seeded (2×103/well/100 μL) into 96-well plates. After 24 h, the cells were treated with various concentrations of piperlongumine and maintained for 72 h. Cell viability was examined by MTS assay (Promega, Madison, WI) following the standard protocol provided.
Soft Agar Assay
The soft agar assay was performed as described previously.16 Briefly, the agar base in a 6-well plate was prepared with 3 mL of Eagle’s basal medium containing 0.6% agar, 10% FBS, and various dose of piperlongumine. Human colorectal cancer cells were suspended and counted at the concentration of 8000 cells/mL in 1 mL of Eagle’s basal medium containing 0.3% agar, 10% FBS, and piperlongumine overlaid into a 6-well plate with 0.6% agar base. The cells were maintained in a 5% CO2 incubator at 37 °C for 2 weeks. The colony number was counted using the microscope.
Construct the Knockout Stable Cell Line
The CRISPR-Cas9-mediated gene knockout was performed as described previously.17 Briefly, the sgRNAs (#1, GTTCGTGGCCTCTAAGATGA, #2, GAAGCGTGTGAGGCGGTAGT) targeting Cyclin D1 were used for stable cell construction. Briefly, the CRC cells were transfected with Cyclin D1 sgRNA and selected by 1μg/mL puromycin for three weeks. Single colony was chosen for further study.
Flow Cytometry
The flow cytometry was performed as described previously.18 Briefly, the cells were treated with piperlongumine or DMSO control as indicated. Cells were centrifuged and then suspended at a concentration of 1×106 cells/mL with PBS. The Propidium Iodide staining buffer containing RNAse was added to the cell suspension and incubated for 15 min in the dark at room temperature. The cells were suspended with PBS and analyzed by FACSort Flow Cytometer (BD, San Jose, CA, USA).
Immunofluorescence (IF)
The IF analysis was performed as described previously.19 Briefly, HT29 cells were treated with the compound for 24 h and fixed in 4% paraformaldehyde and permeabilized in 0.5% Triton X-100 for 30 minutes. The cells were blocked with 50% goat serum albumin in PBS and incubated with p-histone H3 Ser10 antibody in a humidified chamber overnight at 4°C, followed by incubation with the second antibody at room temperature for 40 min. DAPI was used for counterstaining. The stained cells were viewed and captured with the fluorescence microscope.
qRT–PCR
RNA was isolated using the RNAstormTM RNA isolation kit and converted into cDNA. qRT–PCR assay was performed by mixing equal amounts of cDNAs, iQ SYBR Green Supermix and Cyclin D1 (Forward, TCTACACCGACAACTCCATCCG; Reverse, TCTGGCATTTTGGAGAGGAAGTG) or c-Fos (Forward, GCCTCTCTTACTACCACTCACC; Reverse, AGATGGCAGTGACCGTGGGAAT) primers. All real-time data were normalized to β-actin.
Dual Reporter Assay
The dual-luciferase reporter assay was performed as described previously.20 Briefly, the renilla luciferase reporter construct pRL-SV40 plasmid was co-transfected with the pGL3-Basic control or the pGL3-AP-1 (#40,342, Addgene) construct into human colorectal cancer cells. The compound, piperlongumine, was added to the cell culture medium and maintained for another 24 h. The cell lysates were collected following the standard protocol and subjected to dual reporter assay using the Dual-Luciferase reporter assay kit (#E1910; Promega, Madison, WI, USA).
Clinical Tissue Sample Collection
Colorectal cancer patients were diagnosed by the Department of Pathology at The Third Xiangya Hospital following the WHO guidelines. All subjects provided written informed consent for entry into this study, a total of 40 cases of primary adenocarcinomas and adjacent non-tumor tissues were collected.
In vivo Tumor Growth
The in vivo animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of Central South University (Changsha, China). HT29 (1 × 106/100 μL) cells in RPMI-1640 were injected into the right flank of 6-week-old female athymic nude mice. Mouse body weight was recorded, and the tumor volume was determined by vernier caliper every two days. Piperlongumine treatment was initiated when tumor volume reached 100 mm3. The piperlongumine administrated group was given an i.p. injection of piperlongumine at a dose of 10 mg/kg every two days, whereas the control group was administered the vehicle control. Tumor volume was calculated following the formula of A × B2 × 0.5, wherein A is the longest diameter of the tumor, B is the shortest diameter, and B2 is B squared. The mouse was euthanized by CO2 and tumor mass was dissected.
Immunohistochemical Staining (IHC)
The tissues from xenograft tumors were fixed in 10% neutral-buffered formalin and subjected to immunohistochemical staining as described previously.21 Briefly, the tissue slides were deparaffinized, followed by rehydration using various concentrations of ethanol. Antigen retrieval was performed by boiling the slides with the sodium citrate buffer (10 mM, pH 6.0) for 10 min. The slides were incubated with 3% H2O2 in methanol for 10 min to quench the endogenous peroxidase. After washing with PBS, slides were blocked with 50% goat serum albumin in PBS for 1 h at room temperature and incubated with primary antibody at 4°C overnight. Tissue slides were then incubated with the second antibody at room temperature for 45 min and visualized with DAB substrate. Hematoxylin was used for counterstaining.
Statistical Analysis
The statistical analysis was conducted with GraphPad Prism 5 (San Diego, CA). The Student’s t-test or one-way ANOVA was used to evaluate the difference between tested groups, and a probability value of p< 0.05 was used as the criterion for statistical significance. The experiment was performed triplicate, and all quantitative data are expressed as mean ± sd.
Results
Cyclin D1 Is Overexpressed in CRC Tumor Tissues and Required for Maintaining of Tumorigenic Properties of CRC Cells
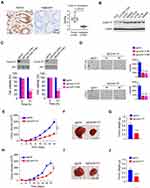
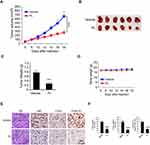
To investigate the function of Cyclin D1 in CRC cells, we first examined the protein level of Cyclin D1 in 40 cases of paired CRC tissue and adjacent non-tumor tissues. The result showed that Cyclin D1 is highly expressed in CRC tumor tissues when compared to that of the matched adjacent non-tumor tissues (Figure 1A). Moreover, the IB data indicated that Cyclin D1 is upregulated in all tested CRC cell lines, including LOVO, SW480, HCT116, HT29, HCT8, and SW620 (Figure 1B). The two sgRNAs targeting Cyclin D1 decreased the protein level of Cyclin D1 in HCT116 and HT29 cells. Furthermore, the MTS data revealed that depletion of Cyclin D1 in HCT116 and HT29 cells significantly reduced cell viability (Figure 1C). We next determined the colony formation efficacy of Cyclin D1 deficient and proficient CRC cells. As shown in Figure 1D, knockout of Cyclin D1 reduced the colony number and colony size of HCT116 and HT29 cells. To validate the crucial oncogenic role of Cyclin D1 in CRC cells, we performed the in vivo xenograft mouse model. The results indicated that knockout of Cyclin D1 significantly delayed the in vivo growth of the HCT116- sgCyclin D1 (Figure 1E–G) and HT29-sgCyclin D1 (Figure 1H–J) cells, as the tumor volume and tumor size of the sgCyclin D1 xenograft tumors were significantly reduced when compared to that of the sgCtrl expressing stable cells. These results demonstrate that Cyclin D1 is highly expressed in CRC tissues and cell lines, and knockout of Cyclin D1 suppresses the malignant phenotype of CRC cells, indicating that targeting Cyclin D1 is a promising strategy for CRC prevention and therapy.
Piperlongumine (PL) Inhibits the Growth of CRC Cells
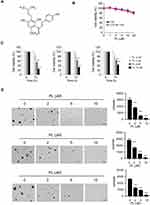
Previous reports showed that the natural product, piperlongumine (Figure 2A), suppresses a panel of human cancer cells.22 However, the anti-tumor effect of PL in CRC cells, and the underlying mechanism remains elusive. Our data indicated that PL exhibited a negligible impact on the cell viability of immortalized colorectal epithelial cells (Figure 2B), indicating that PL is well-tolerated in non-tumor colorectal epithelial cells. To determine the anti-tumor activity of PL on CRC cells, we examined the cell viability of PL-treated HCT116, HT29, and SW620 cells. Our result showed that PL significantly decreased cell viability of all these tested CRC cells in a dose-dependent manner (Figure 2C), and the inhibitory ratio of PL on CRC cells was promoted from 20% to 60% (Figure 2C). We then tested the colony formation of CRC cells using the soft agar assay. The data revealed that PL significantly reduced the colony number of CRC cells in soft agar. Moreover, 2 μM of PL reduced the colony number over 40% in HCT116, HT29, and SW620 cells. The inhibitory efficacy of PL was enhanced in a dose-dependent manner, and 10 μM of PL reduced the colony number over 95% of all these examined CRC cells (Figure 2D). These data suggest that PL inhibits the growth of CRC cells dose-dependently.
Piperlongumine Decreases the Cyclin D1 Protein Level Through Suppression of c-Fos in CRC Cells
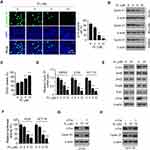
Phosphorylation of histone H3 on Ser10 is a protein marker for cell proliferation. The immunofluorescence result indicated that treatment with PL significantly reduced the population of histone H3 Ser10 positive cells (Figure 3A), further confirmed that PL inhibited the growth of tumor cells. The IB result revealed that the PL decreased the protein level of Cyclin D1 in HT29, HCT116, and SW620 cells dose-dependently (Figure 3B). Likewise, the flow cytometry data showed that PL induced cell cycle arrest at the G0/G1 phase (Figure 3C). To determine the mechanisms of how PL reduced Cyclin D1 expression, we examined the mRNA level of CRC cells with PL treatment. As shown in Figure 3D, the qRT-PCR results revealed that the mRNA of Cyclin D1 was reduced significantly in response to PL treatment, indicating that the transcription of Cyclin D1 was suppressed. Previous reports showed that the transcription factor, AP-1, is required for Cyclin D1 expression in multiple human cancer cells.13 Indeed, our data showed that PL slightly decreased the protein level of c-Jun, but robustly reduced the expression of Fos family member, c-Fos, in HCT116 and HT29 cells (Figure 3E). PL inhibited AP-1 luciferase activity significantly, 10 μM PL reduced the activity over 70% in CRC cells (Figure 3F). As shown in Figure 3G and H, transfection with c-Fos compromised PL-induced Cyclin D1 downregulation. These results indicate that PL decreases the Cyclin D1 protein level through suppression of c-Fos in CRC cells.
Inhibition of Akt and ERK1/2 Signaling is Required for PL-Induced c-Fos Downregulation in CRC Cells
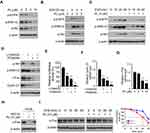
To determine the underlying mechanism of how PL reduced c-Fos protein level, we examined the signaling transduction of EGFR kinase in PL-treated CRC cells. The IB data revealed that PL dose-dependently reduced the phosphorylation of EGFR on Tyr1068. Consistently, the activity of EGFR downstream kinases, Akt, and ERK1/2, were inhibited (Figure 4A). We further examined the inhibitory effect of PL on EGF-induced EGFR activation. As shown in Figure 4B, EGF promoted the activation of EGFR signaling. However, the phosphorylation of EGFR, Akt, and ERK1/2 was suppressed with PL pre-treatment. Furthermore, EGF increased the activity of EGFR signaling time-dependently, whereas PL compromised this efficacy (Figure 4C). Furthermore, treatment with the kinase inhibitors LY294002 and PD98059, which can specifically reduce the activity of Akt and ERK1/2 kinases, respectively, caused a robustly decrease of c-Fos and Cyclin D1 in HT29 cells (Figure 4D). Importantly, the combination of LY294002 and PD98059 nearly blocked the protein expression of c-Fos and Cyclin D1 (Figure 4D). Consistently, the Dual-reporter assay showed that treated with LY294002 and PD98059 alone, or in combination, significantly reduced the AP-1 luciferase activity (Figure 4E). Likewise, the qRT-PCR result showed that the mRNA of Cyclin D1 was reduced with these inhibitors treatment (Figure 4F). These results suggest that the expression of c-Fos and Cyclin D1 in CRC cells is dependent on ERK1/2 and Akt signaling. To better understand how PL regulates c-Fos expression, we examined the mRNA level of c-Fos in PL-treated HT29 cells. The result revealed that PL inhibited c-Fos transcription dose-dependently (Figure 4G). Interestingly, pretreated with proteasome inhibitor, MG132, partially restored c-Fos protein level in PL-treated HT29 cells (Figure 4H). In addition, PL shortened the half-life of c-Fos from 40 min to 20 min (Figure 4I), indicating that PL reduced c-Fos protein stability. Overall, these results suggest that PL inhibited Akt and ERK1/2 signalings, and decreased c-Fos expression by suppression of mRNA transcription and reduction of protein stability.
Piperlongumine Suppresses in vivo Tumor Growth
To further confirm the in vivo antitumor effect of PL on CRC cells, we performed a xenograft mouse model using HT29 cells. Our data showed that PL significantly decreased the growth of xenograft tumor, as the tumor volume of the vehicle- and PL-treated group was 663 ± 121 mm3 and 267 ± 48 mm3, respectively (Figure 5A). Moreover, the tumor weight was reduced by over 60% in the PL-treated group (Figure 5B and C). Our result revealed that treatment with PL did not cause a significant bodyweight loss of tumor-bearing mice (Figure 5D), indicating that PL is well-tolerated in tumor-bearing mice. The IHC staining results indicated that PL decreased the population of Ki-67 positive cells. Consistently, the population of c-Fos and Cyclin D1 positive cells was significantly decreased in PL-treated xenograft tumors (Figure 5E and F), further confirmed that PL inhibited in vivo tumor growth.
Discussion
Piperlongumine (PL) exhibits significant antitumor effect in various human cancer models, including colorectal,23 lung,24 liver,25 and prostate cancer.26 Previous reports revealed that PL inhibits the activity of multiple protein kinases, attenuates angiogenesis and tumor invasion/metastasis,27,28 and suppresses of glycolysis.24 Furthermore, treatment with PL induced autophagy-dependent cell death of human cancer cells.29 PL enhanced the tumor-killing effect of traditional chemotherapy and radiotherapy and overcomes drug resistance in human cancer cells.30,31 Although treatment with PL resulted in cell cycle arrest, the mechanism underlying such a role of PL in CRC cells remains elusive. In the present study, we found that PL dose-dependently suppressed the expression of Cyclin D1 and caused cell cycle G0/G1 arrest in CRC cells. Further study showed that PL-induced Cyclin D1 reduction was dependent on the suppression of AP-1 (c-Fos)-mediated transcription. Overexpression of c-Fos restored the protein level of Cyclin D1 in PL-treated CRC cells. Our data extend our understanding of the antitumor mechanism of PL and suggest that a decrease of the mRNA level of Cyclin D1 might offer a potential antitumor strategy for CRC prevention and treatment.
Targeting cell cycle progression and mitosis is a highly successful strategy for clinical antitumor treatment.7,32 A panel of chemotherapy drugs, such as taxol and vincristine, which eliminates the tumors via directly induced cell cycle arrest or enhances the tumor-killing effect of other therapeutic agents, exhibit a significant antitumor efficacy in clinical treatment.7 Currently, multiple small inhibitors that specifically targeting the kinase activity of CDK4/CDK6 are in clinical trials for both solid tumors and hematological malignancies.33–35 Furthermore, various cell cycle-related protein kinases, including Aurora A/B, CDK7, and CDK1, are considered as potential antitumor targets.7,36 Because the activation of CDK is dependent on the expression of Cyclins, reduction of protein level of Cyclin D1 transcriptionally is an alternative strategy for CDK4/6 activity suppression. In addition, targeting both Cyclins and CDKs to block cell cycle progression through combination treatment is a promising antitumor strategy that deserves further study.
The transcriptional factor AP-1 regulates gene expression in response to various stimuli and stress.37 AP-1 is a dimeric transcriptional regulator composed of proteins mainly belonging to the Jun and Fos families. Previous reports have shown that AP-1 is involved in the regulation of multiple cellular events, such as proliferation, survivin, differentiation, and apoptosis.37,38 Deregulation of AP-1 activity is implicated in the tumorigenesis of both solid tumors and lymphomas.39,40 Importantly, the proto-oncogene c-Fos is the human homolog of the retroviral oncogene v-fos, which is linked to cancer due to the in vitro neoplastic transformation of normal cells. Overexpression of c-Fos promotes proliferation, angiogenesis, and survival of cancer cells.41,42 Furthermore, the high protein level of c-Fos is related to drug resistance in different human cancer models.43,44 As an “immediate-early” gene, the transcription of c-Fos is induced in response to a panel of extracellular stimuli, such as growth factors. Previous studies have revealed that the mitogen-activated protein kinases (MAPKs), such as the Ras-Raf-MEK-ERK signaling, play a crucial role in growth factors-induced c-Fos expression.45,46 Our data showed that PL dose-dependently decreased AP-1 luciferase activity and c-Fos expression. Further study revealed that PL-induced c-Fos reduction is dependent on the suppression of Akt and ERK1/2 signaling. The combination of Akt and ERK1/2 inhibitor blocked the expression of c-Fos in CRC cells. Strikingly, treatment with PL decreased both mRNA and protein levels of c-Fos, as the proteasome inhibitor MG132 partially restored c-Fos expression. Also, the CHX assay showed that PL shortened the half-life of c-Fos from nearly 40 min to 20 min.
In summary, the present study identified PL as a novel antitumor agent to regulate Cyclin D1 expression in CRC cells. PL inhibited the activation of EGF-induced ERK1/2 and Akt kinases and decreased the protein level of c-Fos, which eventually attenuated AP-1-mediated Cyclin D1 expression. This evidence might provide an alternative option for the clinical combination treatment of PL for CRC.
Abbreviations
CRC, colorectal cancer; IB, immunoblot; 5-FU, fluorouracil; CKDs, cyclin-dependent kinases; EGFR, epidermal growth factor receptor; PL, piperlongumine; MAPKs, mitogen-activated protein kinases.
Ethics and Consent Statement
The use and care of experimental animals were approved by the Institutional Animal Care and Use Committee of Central South University (2018-S116) according to the Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC). All surgical specimens were collected in accordance with an Institutional Review Board-approved protocol (2018-S116-1). All subjects provided written informed consent for entry into this study, and 40 cases of primary adenocarcinomas and adjacent non-tumor tissues were collected.
Author Contributions
All authors, Feng Gao, Li Zhou, Ming Li, Wenbin Liu, Shuting Yang, and Wei Li, contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med. 2019;7(21):609. doi:10.21037/atm.2019.07.91
3. Dorr NM, Bartels M, Morgul MH. Current treatment of colorectal liver metastasis as a chronic disease. Anticancer Res. 2020;40(1):1–7. doi:10.21873/anticanres.13921
4. Li Y, Qin Y. Peri-operative chemotherapy for resectable colorectal lung metastasis: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2020;146(3):545–553. doi:10.1007/s00432-020-03142-9
5. Chan GHJ, Chee CE. Making sense of adjuvant chemotherapy in colorectal cancer. J Gastrointest Oncol. 2019;10(6):1183–1192. doi:10.21037/jgo.2019.06.03
6. Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 2020;206:107447. doi:10.1016/j.pharmthera.2019.107447
7. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. doi:10.1038/nrc.2016.138
8. Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11(8):558–572. doi:10.1038/nrc3090
9. Luangdilok S, Wanchaijiraboon P, Chantranuwatana P, Teerapakpinyo C, Shuangshoti S, Sriuranpong V. Cyclin D1 expression as a potential prognostic factor in advanced KRAS-mutant non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):959–966. doi:10.21037/tlcr.2019.12.01
10. Li Y, Wei J, Xu C, Zhao Z, You T, Katoh M. Prognostic significance of cyclin D1 expression in colorectal cancer: a meta-analysis of observational studies. PLoS One. 2014;9(4):e94508. doi:10.1371/journal.pone.0094508
11. Fan HN, Zhu MY, Peng SQ, Zhu JS, Zhang J, Qu GQ. Dihydroartemisinin inhibits the growth and invasion of gastric cancer cells by regulating cyclin D1-CDK4-Rb signaling. Pathol Res Pract. 2020;216(2):152795. doi:10.1016/j.prp.2019.152795
12. Zhang H. CCND1 silencing suppresses liver cancer stem cell differentiation through inhibiting autophagy. Hum Cell. 2020;33(1):140–147. doi:10.1007/s13577-019-00295-9
13. Klein EA, Assoian RK. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci. 2008;121(Pt 23):3853–3857. doi:10.1242/jcs.039131
14. Li W, Yu X, Ma X, et al. Deguelin attenuates non-small cell lung cancer cell metastasis through inhibiting the CtsZ/FAK signaling pathway. Cell Signal. 2018;50:131–141. doi:10.1016/j.cellsig.2018.07.001
15. Li W, Yu X, Tan S, Liu W, Zhou L, Liu H. Oxymatrine inhibits non-small cell lung cancer via suppression of EGFR signaling pathway. Cancer Medicine. 2018;7(1):208–218. doi:10.1002/cam4.1269
16. Yu X, Li W, Xia Z, et al. Targeting MCL-1 sensitizes human esophageal squamous cell carcinoma cells to cisplatin-induced apoptosis. BMC Cancer. 2017;17(1):449. doi:10.1186/s12885-017-3442-y
17. Zhou L, Yu X, Li M, et al. Cdh1-mediated Skp2 degradation by dioscin reprogrammes aerobic glycolysis and inhibits colorectal cancer cells growth. EBioMedicine. 2020;51:102570. doi:10.1016/j.ebiom.2019.11.031
18. Yu X, Liang Q, Liu W, Zhou L, Li W, Liu H. Deguelin, an aurora B kinase inhibitor, exhibits potent anti-tumor effect in human esophageal squamous cell carcinoma. EBioMedicine. 2017;26:100–111. doi:10.1016/j.ebiom.2017.10.030
19. Liu W, Li W, Liu H, Yu X. Xanthohumol inhibits colorectal cancer cells via downregulation of hexokinases II-mediated glycolysis. Int J Biol Sci. 2019;15(11):2497–2508. doi:10.7150/ijbs.37481
20. Yu X, Li W, Deng Q, et al. MYD88 L265P mutation in lymphoid malignancies. Cancer Res. 2018;78(10):2457–2462. doi:10.1158/0008-5472.CAN-18-0215
21. Yu X, Wang R, Zhang Y, et al. Skp2-mediated ubiquitination and mitochondrial localization of Akt drive tumor growth and chemoresistance to cisplatin. Oncogene. 2019;38(50):7457–7472. doi:10.1038/s41388-019-0955-7
22. Piska K, Gunia-Krzyzak A, Koczurkiewicz P, Wojcik-Pszczola K, Pekala E. Piperlongumine (piplartine) as a lead compound for anticancer agents - synthesis and properties of analogues: a mini-review. Eur J Med Chem. 2018;156:13–20. doi:10.1016/j.ejmech.2018.06.057
23. Chen W, Lian W, Yuan Y, Li M. The synergistic effects of oxaliplatin and piperlongumine on colorectal cancer are mediated by oxidative stress. Cell Death Dis. 2019;10(8):600. doi:10.1038/s41419-019-1824-6
24. Zhou L, Li M, Yu X, Gao F, Li W. Repression of hexokinases II-mediated glycolysis contributes to piperlongumine-induced tumor suppression in non-small cell lung cancer cells. Int J Biol Sci. 2019;15(4):826–837. doi:10.7150/ijbs.31749
25. Chen Y, Liu JM, Xiong XX, et al. Piperlongumine selectively kills hepatocellular carcinoma cells and preferentially inhibits their invasion via ROS-ER-MAPKs-CHOP. Oncotarget. 2015;6(8):6406–6421. doi:10.18632/oncotarget.3444
26. Piska K, Koczurkiewicz P, Wnuk D, et al. Synergistic anticancer activity of doxorubicin and piperlongumine on DU-145 prostate cancer cells - the involvement of carbonyl reductase 1 inhibition. Chem Biol Interact. 2019;300:40–48. doi:10.1016/j.cbi.2019.01.003
27. Lee HL, Hwang SC, Nah JW, et al. Redox- and pH-responsive nanoparticles release piperlongumine in a stimuli-sensitive manner to inhibit Pulmonary metastasis of colorectal carcinoma cells. J Pharm Sci. 2018;107(10):2702–2712. doi:10.1016/j.xphs.2018.06.011
28. Chen D, Ma Y, Guo Z, et al. Two natural alkaloids synergistically induce apoptosis in breast cancer cells by inhibiting STAT3 activation. Molecules. 2020;25(1):216.
29. Liu J, Liu W, Lu Y, et al. Piperlongumine restores the balance of autophagy and apoptosis by increasing BCL2 phosphorylation in rotenone-induced parkinson disease models. Autophagy. 2018;14(5):845–861. doi:10.1080/15548627.2017.1390636
30. Zhang C, He LJ, Zhu YB, et al. Piperlongumine inhibits Akt phosphorylation to reverse resistance to cisplatin in human non-small cell lung cancer cells via ROS regulation. Front Pharmacol. 2019;10:1178. doi:10.3389/fphar.2019.01178
31. Wang H, Jiang H, Corbet C, et al. Piperlongumine increases sensitivity of colorectal cancer cells to radiation: involvement of ROS production via dual inhibition of glutathione and thioredoxin systems. Cancer Lett. 2019;450:42–52. doi:10.1016/j.canlet.2019.02.034
32. Dominguez-Brauer C, Thu KL, Mason JM, Blaser H, Bray MR, Mak TW. Targeting mitosis in cancer: emerging strategies. Mol Cell. 2015;60(4):524–536. doi:10.1016/j.molcel.2015.11.006
33. Chong QY, Kok ZH, Bui NL, et al. A unique Cdk4/6 inhibitor: current and future therapeutic strategies of abemaciclib. Pharmacol Res;2020:104686. doi:10.1016/j.phrs.2020.104686
34. Scheinberg T, Kench J, Stockler M, et al. Pharmacodynamics effects of CDK4/6 inhibitor LEE011 (ribociclib) in high-risk, localised prostate cancer: a study protocol for a randomised controlled Phase II trial (LEEP study: LEE011 in high-risk, localised prostate cancer). BMJ Open. 2020;10(1):e033667. doi:10.1136/bmjopen-2019-033667
35. Hartkopf AD, Muller V, Wockel A, et al. Translational highlights in breast and ovarian cancer 2019 - immunotherapy, DNA repair, PI3K inhibition and CDK4/6 therapy. Geburtshilfe Frauenheilkd. 2019;79(12):1309–1319. doi:10.1055/a-1039-4458
36. Goldenson B, Crispino JD. The aurora kinases in cell cycle and leukemia. Oncogene. 2015;34(5):537–545. doi:10.1038/onc.2014.14
37. Mirzaei H, Khodadad N, Karami C, Pirmoradi R, Khanizadeh S. The AP-1 pathway; a key regulator of cellular transformation modulated by oncogenic viruses. Rev Med Virol. 2020;30(1):e2088. doi:10.1002/rmv.2088
38. Atsaves V, Leventaki V, Rassidakis GZ, Claret FX. AP-1 transcription factors as regulators of immune responses in cancer. Cancers (Basel). 2019;11:7. doi:10.3390/cancers11071037
39. Zhang J, Wu Z, Savin A, et al. The c-Jun and JunB transcription factors facilitate the transit of classical Hodgkin lymphoma tumour cells through G1. Sci Rep. 2018;8(1):16019. doi:10.1038/s41598-018-34199-9
40. Bejjani F, Evanno E, Zibara K, Piechaczyk M, Jariel-Encontre I. The AP-1 transcriptional complex: local switch or remote command? Biochim Biophys Acta Rev Cancer. 2019;1872(1):11–23. doi:10.1016/j.bbcan.2019.04.003
41. Mahner S, Baasch C, Schwarz J, et al. C-Fos expression is a molecular predictor of progression and survival in epithelial ovarian carcinoma. Br J Cancer. 2008;99(8):1269–1275. doi:10.1038/sj.bjc.6604650
42. Papoudou-Bai A, Hatzimichael E, Barbouti A, Kanavaros P. Expression patterns of the activator protein-1 (AP-1) family members in lymphoid neoplasms. Clin Exp Med. 2017;17(3):291–304. doi:10.1007/s10238-016-0436-z
43. Muhammad N, Bhattacharya S, Steele R, Phillips N, Ray RB. Involvement of c-Fos in the promotion of cancer stem-like cell properties in head and neck squamous cell carcinoma. Clin Cancer Res. 2017;23(12):3120–3128. doi:10.1158/1078-0432.CCR-16-2811
44. Misawa A, Katayama R, Koike S, Tomida A, Watanabe T, Fujita N. AP-1-Dependent miR-21 expression contributes to chemoresistance in cancer stem cell-like SP cells. Oncol Res. 2010;19(1):23–33. doi:10.3727/096504010X12828372551759
45. Gazon H, Barbeau B, Mesnard JM, Peloponese JM
46. Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117(Pt 25):5965–5973. doi:10.1242/jcs.01589
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.