Back to Journals » International Journal of Nanomedicine » Volume 16
Inhibition of Circulating Exosomal miRNA-20b-5p Accelerates Diabetic Wound Repair
Received 22 October 2020
Accepted for publication 30 December 2020
Published 14 January 2021 Volume 2021:16 Pages 371—381
DOI https://doi.org/10.2147/IJN.S287875
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Kai Chen, Tao Yu, Xin Wang
Department of Orthopedic Surgery, Tongji Hospital, Tongji University School of Medicine, Shanghai 200065, People’s Republic of China
Correspondence: Xin Wang; Tao Yu
Department of Orthopedic Surgery, Tongji Hospital, Tongji University School of Medicine, Shanghai 200065, People’s Republic of China
Tel/Fax +86 21 8572 6525
Email [email protected]; [email protected]
Purpose: Efficient approaches to reliably improving wound healing in diabetic patients remain to be developed. Exosomes are nanomaterials from which therapeutically active microRNAs (miRNAs) can be isolated. In the present report, we therefore isolated circulating exosome-derived miRNAs from patients with diabetes and assessed the impact of these molecules on wound healing.
Patients and Methods: Exosomes were isolated from the serum of control or diabetic patients (Con-Exos and Dia-Exos, respectively), after which the effects of these exosomes on cellular activity and wound healing were assessed.
Results: We determined that miR-20b-5p was overexpressed in Dia-Exos and that it functioned by impairing wound repair by suppressing vascular endothelial growth factor A (VEGFA) expression. Consistent with such a model, the administration of Dia-Exos or this miRNA both in vivo and in vitro was sufficient to slow wound repair.
Conclusion: Dia-Exos exhibit significant increases in miR-20b-5p relative to Con-Exos, and this miRNA can be transferred into HSFs wherein it can suppress VEGFA expression and thereby slow the process of wound healing.
Keywords: nanomedicine, exosome, miRNA, diabetes, wound, fibroblast
Introduction
Diabetes is an increasingly prevalent and debilitating condition that is common in older populations.1 Diabetic foot ulcers (DFUs) are a serious complication of this metabolic disease that arises as a consequence of impaired wound healing, resulting in secondary infections and the potential need for limb amputation, thus negatively impacting patient quality of life.2 Many efforts have been made to explore the mechanistic basis for DFU onset in order to improve patient healing activity.3 In one study, the lncRNA H19/miR-152-3p/PTEN axis was shown to function as a critical regulator of wound repair in individuals with diabetes, indicating that it may be a viable therapeutic target in the context of DFU development.4 Despite these findings, however, the mechanisms that constrain diabetic wound healing remain incompletely understood, and effective treatments for affected patients are lacking.5 Novel approaches to the study of diabetic wound healing are thus required to facilitate novel therapeutic strategies.
Functional fibroblasts are integral to rapid wound healing,6 and the proper functionality of these cells is governed by diverse regulatory factors.7 For sample, WNT-inducible signaling pathway protein-1 (WISP-1) can promote the migratory activity and proliferation of dermal fibroblasts, thereby bolstering wound healing and reducing the adverse inflammatory effects of TNF-α.8 Exosomes (Exos) are small membrane-enclosed vesicles that are 30–150 nm in size and that can regulate a wide range of biological processes by delivering specific proteins, nucleic acids, and microRNAs (miRNAs) to different cells and organs in vivo.9,10 For example, human mesenchymal stem cell (MSC)-derived Exos can deliver miR-146a-5p to other cells, thereby suppressing innate lymphoid cell-dominant allergic airway inflammation.11 Such regulatory mechanisms are increasingly well understood and are linked to the ability of miRNAs, which are non-coding RNAs, to suppress target gene expression in recipient cells following Exo-mediated delivery.12 In one recent report, miR-20b-5p levels were shown to be increased in the circulating Exos of diabetes patients, and such upregulation was associated with insulin regulation in the skeletal muscle of humans.13
Herein, we explored the ability of Exo-derived miR-20b-5p to alter fibroblast functionality in the context of diabetic wound repair. Vascular endothelial growth factor A (VEGFA) is known to serve as a potent regulatory cytokine capable of promoting blood vessel permeability, enhancing cellular proliferation, and suppressing apoptotic cell death.14 VEGFA signaling is also closely linked to fibroblast functionality.15 Herein, we isolated Exos from control and diabetes patients (Con-Exos and Dia-Exos, respectively) and evaluated the impact of these molecules on human skin fibroblasts (HSFs) and animal models of wound repair. We also explored the relationship between VEGFA and the mechanisms whereby Exo-derived miR-20b-5p impacts wound healing.
Materials and Methods
Ethical Oversight
The institutional review board of Tongji Hospital, Tongji University School of Medicine approved all human research aspects of this study. The Laboratory Animal Management Committee of Tongji Hospital, Tongji University School of Medicine approved all animal studies.
Exosome Isolation and Assessment
Samples of blood were obtained from 10 control patients and 10 diabetes patients (all male) in citrate phosphate dextrose-containing tubes. After collection, blood was centrifuged for 15 min at 3000×g at 4°C. Serum was then isolated and spun again with the same settings. Supernatants were then collected and spun for 30 min at 10,000×g, after which they were spun for 70 min at 100,000×g. Isolated Exos were washed thrice with PBS and spun under the same ultracentrifugation settings prior to resuspension in 15 mL of PBS and ultrafiltration with a 15-mL Amicon Ultra-15 Centrifugal Filter (Millipore, MA, USA) by spinning the solution at 4000×g to a final 200 µL volume.
The morphological properties of isolated Exos were assessed by mixing them for 30 min with 4% osmium tetroxide and then applying them to a copper grid. Particles were then stained using 1% phosphotungstic acid and assessed via transmission electron microscopy (TEM). The expression of miR-20b-5p in Exo preparations was evaluated via qPCR. When performing experimental treatments using these Exos, Exo samples from each group were pooled to ensure consistency. All experiments were repeated in triplicate.
Animals and Treatments
C57BL/6 mice (male, 6-weeks-old, 18–20 g; 60 total) were anesthetized with pentobarbital sodium (50 mg/kg, i.p.; Sigma-Aldrich, MO, USA). Mice were then shaved, and a full-thickness cutaneous wound (10 mm in diameter) was generated on the dorsal surface for each mouse. After wounding, animals were separated at random into two experimental cohorts, with three groups per cohort. Animals in the first cohort were treated with either PBS (100 μL of PBS, applied to wounds), Con-Exos (100 μL at 50 µg/mL, applied to wounds), or Dia-Exos (100 μL at 50 µg/mL, applied to wounds). Mice in the second cohort were treated with either PBS (as above), agomiR-20b-5p (100 μL at 20 µmol/l, applied to wounds), or antagomiR-20b-5p (100 μL at 20 µmol/l, applied to wounds). For all treatments, wounds were subcutaneously injected at four different points (25 μL each) on days 0, 3, 5, 7, 9, and 11 after wound generation. In addition, the ImageJ software was used to assess wound closure on days 0, 3, 5, 7, 10, and 14 based upon the following formula:
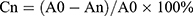
where Cn corresponds to the reduction in wound area (as a percentage) on the indicated days, A0 corresponds to the baseline wound area, and An corresponds to the wound area on the indicated day of analysis.
All mice were euthanized on day 14 after wound generation, and samples of skin were collected. For experimental models of diabetes, mice were fed a high-fat diet for 4 weeks and were then administered streptozotocin (STZ; 40 mg/kg/day i.p.) once per day for seven days. When mice had a fasting blood glucose >11.1 mmol/L in two consecutive assay measurements, they were deemed diabetic.
Cell Culture
HSFs (FuHeng Biology, Shanghai, China) were grown in high-glucose DMEM (Gibco, NY, USA) containing 10% fetal bovine serum (FBS) at 37°C in a humidified 5% CO2 incubator. Cells were transduced with agomiR/antagomiR constructs (200 mM; GenePharma, Shanghai, China) or siRNA-VEGFA (50 nM; RiboBio, Guangzhou, China) using Lipofectamine 3000 (Thermo Fisher Scientific, MA, USA) based on provided directions.
CCK-8 Assay
To evaluate proliferation and viability, HSFs were added to 96-well plates for 24, 48, or 72 h, after which the CCK-8 reagent was added to each well for 2 h in serum-free media. Absorbance was then evaluated at 450 nm.
Luciferase Reporter Assay
WT or mutant (MUT) versions of the VEGFA 3ʹ-UTR region containing the predicted binding site for miR-20b-5p were cloned into the pMIR luciferase reporter plasmid. Lipofectamine 3000 was then used to co-transfect cells with these plasmids and agomiR-20b-5p. Following a 48 h incubation, the luciferase activity in these cells was assessed with a Dual-Luciferase Reporter Assay kit (Promega, Madison, WI, USA), and data were analyzed using the SpectraMax M5 program (Molecular Devices, CA, USA).
ELISA
Basic fibroblast growth factor (bFGF) levels in HSFs were measured following a 48 h growth period in serum-free media with a sandwich ELISA kit (MN, USA). Cells in collected samples were counted, and ELISA assay results were normalized to cell counts, with bFGF levels being determined using an appropriate standard curve. This same approach was also used to measure VEGFA levels in individual samples.
Western Blotting
Lysates were prepared from cells or supernatants, were diluted 1:5 with loading buffer, and were boiled for 5 min at 95°C. Samples of protein were then separated via SDS-PAGE, transferred to PVDF membranes, and blots were blocked using 5% non-fat milk in TBST for 1 h at room temperature. Blots were then probed overnight with anti-CD81 (1:500; ab59477), anti-VEGFA (1:1000; ab52917), TSG101 (1:1000, ab83) or anti-GAPDH (1:10,000, ab37168) (all from Abcam, Cambridge, UK). Blots were then probed using HRP-linked secondary antibodies at 37°C for 1 h, and protein bands were detected via LiDE110 scanner (Canon, Tokyo, Japan), with the AlphaEaseFC software (Alpha Innotech, Genetic Technologies, Inc, FL, USA) being used to measure band density.
qPCR
TRIzol (Invitrogen) was used to extract total from samples, after which cDNA was prepared from 1 μg total of RNA per sample with the Verso cDNA Synthesis Kit (Thermo Fisher Scientific). A SeraMir Exosome RNA Purification Kit (System Biosciences, CA, USA) was utilized to isolate miRNAs from Exo preparations, after which cDNA was prepared with the TaqMan microRNA assay kit (Applied Biosystems, CA, USA). All qPCR reactions were conducted using a StepOne Real-Time PCR Instrument (Life Technologies, CA, USA). The 2–ΔΔCT approach was used to evaluate relative gene expression, while GAPDH served as a normalization control. All primers are shown in Table 1.
 |
Table 1 microRNAs and mRNA Primer Sequences |
Statistical Analysis
All assays were repeated in triplicate, and data are means ± SD. Student’s t-tests and one-way ANOVAs were employed to compare data with GraphPad Prism 8.0 (GraphPad Software, CA, USA). P<0.05 was the significance threshold.
Results
Exosome Characterization
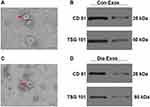
Con-Exos and Dia-Exos were assessed via TEM and Western blotting. In line with the results of prior studies, we found these particles to be cup- or sphere-like in shape (Figure 1A and C).16 Western blotting revealed these Exos to be enriched for exosomal surface marker proteins including CD81 and TSG101 (Figure 1B and D). As such, we concluded that these preparations were Exos, and they were utilized for subsequent experiments.
Dia-Exos Suppress the in vitro Functionality of HSFs
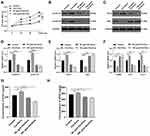
HSF morphology was assessed via fluorescent staining, revealing these cells to have ovoid nuclei and a flattened spindle-like or stellate shape. These cells were then treated with Con-Exos of Dia-Exos, and their proliferation was assessed via CCK-8 assay. This analysis revealed that HSF treatment with 50–100 μg/mL of Dia-Exos markedly suppressed the proliferation of these cells in a dose-dependent fashion relative to treatment with equivalent doses of Con-Exos (Figure 2A). Levels of Cyclin D1 and Cyclin D3, which are associated with proliferation, were also decreased following Dia-Exo treatment of HSFs (Figure 2B and D). Dia-Exos also suppressed Bcl-2 levels and enhanced Bax expression in these cells (Figure 2C and E). Dia-Exos also significantly enhanced α-SMA production and suppressed collagen I (Col 1) and collagen III (Col 3) expression (Figure 2F). ELISAs were further conducted, revealing that Dia-Exo-treated HSFs secreted lower levels of VEGFA and bFGF relative to Con-Exo-treated cells (Figure 2G and H). Together, these findings suggested that Dia-Exos can impair the functionality of HSFs in vitro.
Dia-Exos Suppress Wound Healing in vivo
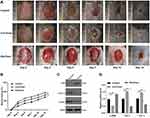
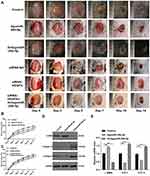
Next, we local administered Dia-Exos to mice bearing full-thickness cutaneous dorsal wounds, revealing that such treatment markedly compromised the rate of wound closure relative to control treatment (Figure 3A and B). On day 14 post-wounding, skin samples were collected and levels of collagen-related markers were assessed via qPCR and Western blotting. This analysis revealed significant increases in α-SMA and significant decreases in Collagen 1 and 3 expression in Dia-Exo-treated mice relative to other treatment groups (Figure 3C and D). As such, we concluded that Dia-Exos can suppress the wound healing process in vivo.
Suppression of Exosome-Derived miR-20b-5p Enhances the in vitro Functionality of HSFs
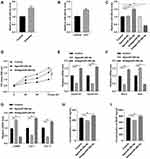
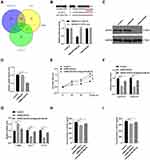
In line with prior study results, we determined that Dia-Exos exhibited significant increases in miR-20b-5p expression relative to Con-Exos (Figure 4A).14 When samples of serum were compared between patients with DFUs and patients with foot injuries that were unrelated to diabetes, we similarly observed significantly higher miR-20b-5p levels in samples from individuals with DFUs (Figure 4B). We also compared the expression of this miRNA in mice with and without diabetes (Figure S1). Next, we examined miR-20b-5p as a mediator of the impact of Dia-Exos on HSF functionality by directly transfecting these cells with mimics or inhibitors of this miRNA (agomiR-20b-5p and antagomir-20b-5p, respectively). As expected, antagomiR-20b-5p transfection suppressed the expression of this miRNA (Figure S2 and Figure 4C). Subsequent CCK-8 assays showed that cells treated with antagomiR-20b-5p were more proliferative than control cells (Figure 4D), and these cells expressed higher levels of Cyclin D1 and Cyclin D3 (Figure 4E), as well as lower levels of Bax and increased levels of Bcl-2 (Figure 4F). When we examined the impact of antagomiR-20b-5p on the secretion of soluble collagen by HSFs, we found that antagomiR-20b-5p significantly suppressed α-SMA levels and enhanced collagen 1 and 3 expression (Figure 4G). ELISAs further confirmed that inhibiting this miRNA markedly enhanced HSF secretion of VEGFA and bFGF (Figure 4H and I). As such, these data revealed that inhibiting miR-20b-5p was sufficient to enhance the functionality of HSFs in vitro.
miR-20b-5 Suppresses VEGFA Expression and Thereby Inhibits the in vitro Functionality of HSFs
We next examined the mechanistic basis for miR-20b-5p-mediated inhibition of HSF functionality. To that end, online predictive tools such as TargetScan (http://www.targetscan.org/vert_70/), miRDB (http://mirdb.org/), and miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) were used to identify predicted targets of this miRNA. In total, 24 such predicted targets were identified (Figure 5A). As VEGFA was identified as one of these target genes and has previously been linked to wound healing,17 we next explored its functional role in the context of miR-20b-5p-mediated wound repair. We began by conducting a luciferase reporter assay, which confirmed that this miRNA bound to the VEGFA 3ʹ-UTR in a WT but not mutated form (Figure 5B). We then utilized an siRNA construct to knock down VEGFA in HSFs in order to demonstrate the importance of this growth factor in the wound healing process (Figure 5C and D). Such VEGFA knockdown suppressed HSF proliferation and promoted apoptotic death. Inhibiting miR-20b-5p was able to partially reverse siRNA-VEGFA-mediated impairment of HSFs (Figure 5E and F). Knocking down VEGFA also promoted α-SMA expression while suppressing that of collagen 1 and 3 (Figure 5G). ELISAs also revealed that VEGF knockdown compromised HSF-derived VEGFA and bFGF secretion (Figure 5H and I). Overall, this indicated that miR-20b-5p disrupts HSF functionality by inhibiting VEGFA expression.
Knockdown of miR-20b-5p Improves Wound Healing
Lastly, we explored miR-20b-5p-mediated inhibition of wound healing in vivo by injecting mice bearing full-thickness dorsal wounds with PBS, agomiR-20b-5p, antagomiR-20b-5p, siRNA-NC, siRNA-VEGFA, or siRNA-VEGFA+AntagomiR-20b-5p on days 0, 3, 5, 7, 9, and 11 post-wounding. A significantly quicker wound closure rate was seen in the antagomiR-20b-5p group and a better wound closure in siRNA-VEGFA+AntagomiR-20b-5p group compared with the siRNA-VEGFA group, indicating that antagomiR-20b-5p can partially reverse the impaired effect of siRNA-VEGFA on wound repair (Figure 6A–C). When samples of wounded skin were collected on day 14 after wound generation, we found that antagomiR-20b-5p-treated mice exhibited significantly lower α-SMA levels and significantly higher collagen 1 and 3 levels as compared to other experimental groups (Figure 6D and E). This suggested that miR-20b-5p inhibition is sufficient to expedite wound healing, making it an attractive therapeutic strategy in those with diabetes.
Discussion
Plasma has been increasingly studied as a tool for tissue engineering applications in recent years.18 Plasma-derived exosomes are released by a wide range of different cells and that can be internalized by nearby or distant cells, thereby altering their functionality,19 delivering contents such as cholesterol, proteins, and nucleic acids.20 As they can protect the encapsulated molecules from external factors, Exos are ideal nanovesicles for use when delivering miRNAs and other bioactive materials to target cells in an effort to modulate gene expression.21 Fibroblasts are able to readily internalize serum-derived Exos22 and are essential mediators of wound healing owing to their ability to promote collagen synthesis and localized remodeling of diverse tissue types.6 As such, we explored the ability of diabetes patient-derived Exos to alter fibroblast functionality in vitro. This experimental approach revealed that Dia-Exos suppressed fibroblast proliferation and induced their apoptotic death, thereby compromising wound healing. In prior studies, Exos were shown to be retained in wound sites for up to three days.23 We therefore injected Exos into wound sites in model mice every two days and monitored the impact of such treatment on overall wound healing, confirming the ability of Dia-Exos to compromise the wound repair process.
As miRNAs are present at high levels in circulation, fibroblasts likely interact with and are regulated by these non-coding RNAs. Herein, we determined that miR-20b-5p was overexpressed in Dia-Exos and was able to negatively regulate the functionality of fibroblasts. Following miR-20b-5p agonist transfection, fibroblast collagen synthesis and proliferation were markedly compromised, whereas they underwent apoptotic death at higher rates. Similar findings were also observed in vivo. MiR-20b-5p is a miR-17 family member that is thus related to miR-106a-363, miR-17-192, and the miR-106b-25 cluster.24 Dysfunction of the pancreatic islets is tightly linked to the onset of diabetes, and exosomal miRNAs have recently been shown to be differentially expressed under stress upon release from the islets.25 It is thus possible that exosomal miR-20b-5p in Dia-Exos is derived from islet cells.
VEGFA is a critical regulator of wound healing, tissue remodeling, and collagen production.26 Impaired VEGFA signaling activity is believed to be a key cause of disrupted wound healing in DFU, and several studies have shown VEGFA to be associated with fibroblasts.27 Herein, we identified VEGFA as a miR-20b-5p target gene such that when its expression was suppressed, fibroblast function and wound repair were adversely impacted.
Herein, we also explored the adverse impact of Dia-Exos on HSFs, and our results suggested that the transfer of miR-20b-5p into target cells may be the primary mechanism whereby these nanovesicles suppress normal wound healing. Therapeutic approaches that target these Dia-Exos and/or miR-20b-5p may therefore improve healing in diabetes patients.
Conclusion
In conclusion, we found that Dia-Exos suppress cutaneous wound repair by suppressing fibroblast functionality in vivo and in vitro. Overexpressing miR-20b-5p in Dia-Exos suppresses wound repair by inhibiting the expression of VEGFA. As such, Exos or similar nanoparticles loaded with miR-20b-5p inhibitors may offer therapeutic value as a means of enhancing wound repair in those with diabetes.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Takihata M, Terauchi Y. The efficacy and safety of luseogliflozin and sitagliptin depending on the sequence of administration in patients with type 2 diabetes mellitus: a randomized controlled pilot study. Expert Opin Pharmacother. 2019;20:2185–2194. doi:10.1080/14656566.2019.1656717
2. Zhu L, Zhong Q, Yang T, et al. Improved therapeutic effects on diabetic foot by human mesenchymal stem cells expressing MALAT1 as a sponge for microRNA-205-5p. Aging (Albany NY). 2019;11:12236–12245. doi:10.18632/aging.102562
3. Xiong Y, Chen L, Yan C, et al. Circulating exosomal miR-20b-5p inhibition restores wnt9b signaling and reverses diabetes-associated impaired wound healing. Small. 2020;16:e1904044. doi:10.1002/smll.201904044
4. Li B, Luan S, Chen J, et al. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814–826. doi:10.1016/j.omtn.2019.11.034
5. Liu XQ, Duan LS, Chen YQ, et al. lncRNA MALAT1 accelerates wound healing of diabetic mice transfused with modified autologous blood via the HIF-1alpha signaling pathway. Mol Ther Nucleic Acids. 2019;17:504–515. doi:10.1016/j.omtn.2019.05.020
6. Martella D, Paoli P, Pioner JM, et al. Liquid crystalline networks toward regenerative medicine and tissue repair. Small. 2017;13. doi:10.1002/smll.201702677
7. Demyanenko IA, Popova EN, Zakharova VV, et al. Mitochondria-targeted antioxidant SkQ1 improves impaired dermal wound healing in old mice. Aging (Albany NY). 2015;7:475–485. doi:10.18632/aging.100772
8. Ono M, Masaki A, Maeda A, et al. CCN4/WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via alpha5beta1 and TNFalpha. Matrix Biol. 2018;68:533–546. doi:10.1016/j.matbio.2018.01.004
9. Skotland T, Sagini K, Sandvig K, et al. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev. 2020;159:308–321. doi:10.1016/j.addr.2020.03.002
10. Zhao J, Liu C, Li Y, et al. Thermophoretic detection of exosomal microRNAs by nanoflares. J Am Chem Soc. 2020;142:4996–5001. doi:10.1021/jacs.9b13960
11. Fang SB, Zhang HY, Wang C, et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles. 2020;9:1723260. doi:10.1080/20013078.2020.1723260
12. Xiong Y, Yan C, Chen L, et al. IL-10 induces MC3T3-E1 cells differentiation towards osteoblastic fate in murine model. J Cell Mol Med. 2020;24:1076–1086. doi:10.1111/jcmm.14832
13. Shen T, Zheng Q, Luo H, et al. Exosomal miR-19a from adipose-derived stem cells suppresses differentiation of corneal keratocytes into myofibroblasts. Aging (Albany NY). 2020;12:4093–4110. doi:10.18632/aging.102802
14. Katayama M, Wiklander O, Fritz T, et al. Circulating exosomal miR-20b-5p Is elevated in Type 2 diabetes and could impair insulin action in human skeletal muscle. Diabetes. 2019;68:515–526. doi:10.2337/db18-0470
15. Demir L, Erten C, Somali I, et al. Metastases of renal cell carcinoma to the larynx and thyroid: two case reports on metastasis developing years after nephrectomy. Can Urol Assoc J. 2012;6:E209–12. doi:10.5489/cuaj.11255
16. Kumar S, Michael IJ, Park J, et al. Cloaked exosomes: biocompatible, durable, and degradable encapsulation. Small. 2018;14:e1802052. doi:10.1002/smll.201802052
17. Zong J, Jiang J, Shi P, et al. Fatty acid extracts facilitate cutaneous wound healing through activating AKT, ERK, and TGF-beta/Smad3 signaling and promoting angiogenesis. Am J Transl Res. 2020;12:478–492.
18. Xu G, Zhang B, Ye J, et al. Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression. Int J Biol Sci. 2019;15:2320–2329. doi:10.7150/ijbs.33750
19. Stefanius K, Servage K, de Souza SM, et al. Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. eLife. 2019;8. doi:10.7554/eLife.40226
20. Geng T, Song ZY, Xing JX, et al. Exosome derived from coronary serum of patients with myocardial infarction promotes angiogenesis through the miRNA-143/IGF-IR pathway. Int J Nanomed. 2020;15:2647–2658. doi:10.2147/IJN.S242908
21. Tao Y, Tang Y, Yang Z, et al. Exploration of serum exosomal LncRNA TBILA and AGAP2-AS1 as promising biomarkers for diagnosis of non-small cell lung cancer. Int J Biol Sci. 2020;16:471–482. doi:10.7150/ijbs.39123
22. Tan W, Zhang Y, Li M, et al. miR-27a-containing exosomes secreted by irradiated skin keratinocytes delayed the migration of unirradiated skin fibroblasts. Int J Biol Sci. 2019;15:2240–2255. doi:10.7150/ijbs.35356
23. Deng T, Zhang H, Yang H, et al. Exosome miR-155 derived from gastric carcinoma promotes angiogenesis by targeting the c-MYB/VEGF axis of endothelial cells. Mol Ther Nucleic Acids. 2020;19:1449–1459. doi:10.1016/j.omtn.2020.01.024
24. Tanzer A, Stadler PF. Molecular evolution of a microRNA cluster. J Mol Biol. 2004;339:327–335. doi:10.1016/j.jmb.2004.03.065
25. Saravanan PB, Vasu S, Yoshimatsu G, et al. Differential expression and release of exosomal miRNAs by human islets under inflammatory and hypoxic stress. Diabetologia. 2019;62:1901–1914. doi:10.1007/s00125-019-4950-x
26. Sjoqvist S, Ishikawa T, Shimura D, et al. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J Extracell Vesicles. 2019;8:1565264. doi:10.1080/20013078.2019.1565264
27. Shen H, Yu X, Yang F, et al. Reprogramming of normal fibroblasts into cancer-associated fibroblasts by miRNAs-mediated CCL2/VEGFA signaling. PLoS Genet. 2016;12:e1006244. doi:10.1371/journal.pgen.1006244
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.