Back to Journals » Cancer Management and Research » Volume 13
Inhibition of Bone Morphogenetic Protein 2 Suppresses the Stemness Maintenance of Cancer Stem Cells in Hepatocellular Carcinoma via the MAPK/ERK Pathway
Received 14 September 2020
Accepted for publication 24 December 2020
Published 27 January 2021 Volume 2021:13 Pages 773—785
DOI https://doi.org/10.2147/CMAR.S281969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Eileen O'Reilly
Juncheng Guo, Min Guo, Jinfang Zheng
Department of Hepatobiliary Surgery, Hainan General Hospital, Haikou, 570311 Hainan, People’s Republic of China
Correspondence: Jinfang Zheng
Hainan General Hospital, No. 19 Xinhua Road, Xiuying District, Haikou, 570311 Hainan Province, People’s Republic of China
Tel +86 13907571082
Email [email protected]
Background: Hepatocellular carcinoma (HCC) remains a life-threatening malignant tumor. Cancer stem cells (CSCs) harbor tumor-initiating capacity and can be used as a therapeutic target for human malignancies. Bone morphogenetic proteins (BMPs) play a regulatory role in CSCs. This study investigated the role and mechanism of BMP2 in CSCs in HCC.
Methods: BMP2 expression in HCC tissues and cells, and CSCs from HepG2 cells and SMMC7721 cells (HepG2-CSCs and SMMC7721-CSCs) was measured. The association between BMP2 expression and prognosis of HCC patients was analyzed. CSCs were interfered with BMP2 to evaluate the abilities of colony and tumor sphere formation, levels of stemness-related markers, epithelial–mesenchymal transition (EMT), and invasion and migration. Levels of MAPK/ERK pathway-related proteins in HepG2-CSCs were detected after BMP2 knockdown. The effect of the activated MAPK/ERK pathway on HepG2-CSCs was assessed. Finally, the effect of BMP2 inhibition on CSCs in HCC was verified in vivo.
Results: BMP2 showed obvious upregulation in HCC tissues and cells and was further upregulated in CSCs in HCC, with its higher expression indicative of worse prognosis. Silencing BMP2 inhibited colony and tumor sphere formation, levels of stemness-related markers, as well as EMT, invasion and migration of HepG2-CSCs and SMMC7721-CSCs. The MAPK/ERK pathway was suppressed after BMP2 knockdown, and its activation reversed the inhibitory effect of shBMP2 on hepatic CSCs. BMP2 accelerated tumor growth and EMT of CSCs in HCC in vivo.
Conclusion: We concluded that BMP2 knockdown inhibited the EMT, proliferation and invasion of CSCs in HCC, thereby hindering the stemness maintenance via suppressing the MAPK/ERK pathway.
Keywords: hepatocellular carcinoma, cancer stem cells, bone morphogenetic protein 2, stemness, epithelial-mesenchymal transition, MAPK/ERK pathway
Introduction
Hepatocellular carcinoma (HCC), defined as a predominant type of liver cancer, remains the leading cause of tumor-related deaths with the ever-increasing incidence and poor prognosis.1 The majority of patients with early-stage HCC are asymptomatic, and only 20–30% of HCC patients are diagnosed early when the initial treatment may be effective; most HCC patients diagnosed at an advanced stage are subjected to expensive surgical resection or transplantation, and what’s worse, more than 70% of them are unresectable HCC.2,3 It has shown that epithelial–mesenchymal transition (EMT) takes a principal part in driving HCC progression, as evidenced by endowing HCC cells with anti-apoptotic and migratory traits.4 Therefore, it is urgent to open new avenues for better HCC therapies.
Recently, there has been a growing consensus that targeting cancer stem cells (CSCs) is the most promising therapy for multiple incurable cancers.5 CSCs, also known as tumor-initiating cells, occupy a small group of tumorigenic cells in tumors, and harbor intrinsic stem cell-like characteristics, including the ability of self-renewal and differentiation.6 CSCs have been identified to make great contributions to tumor initiation, maintenance (malignant proliferation, invasion and metastasis) and recurrence even after apparently eradicating the primary tumor.7–9 Biomarkers for hepatic CSCs contribute to the diagnosis and prognosis prediction of HCC, indicating the application of hepatic CSCs in clinical management of HCC.10 EMT plasticity of CSCs is identified to be a therapeutic target in HCC.11–13 In a prior work, CSC self-renewal and proliferation are implicated in HCC tumorigenesis.14 However, the potential mechanism has not been fully elucidated.
Bone morphogenetic proteins (BMPs) have been proven to play a role in tissue morphogenesis, organogenesis and adult tissue homeostasis.15 BMPs regulate diverse cellular processes, such as proliferation, apoptosis and migration in many organs including liver, showing the potential to be a target for liver disease treatments.16 BMPs serve as a gene delivery system for gene therapy and confer a potential treatment target for HCC.17 Numerous studies have demonstrated that BMPs are tightly linked to CSC sustenance in diverse cancers, such as glioblastoma and breast carcinoma.18,19 The BMP signaling has been verified to promote hepatic CSC self-renewal and then drive HCC oncogenesis.20 As previously evidenced, BMP2 shows an abnormal upregulation in CSCs in prostate cancer.21 BMP2 positively regulates cancer cell malignant behavior and tumor growth in HCC.22 Nevertheless, the role of BMP2 in CSCs in HCC remains to be elucidated.
Hence, this study innovatively speculated that BMP2 may be intimately implicated in HCC via the underlying interactions of hepatic CSCs. Consequently, we performed a series of histological and molecular experiments to identify the regulatory mechanism of BMP2 in CSCs in HCC, with the purpose to provide new theoretical basis for HCC treatment.
Materials and Methods
Ethics Approval and Consent to Participate
This study got approval from the Ethics Committee of Hainan Provincial People’s Hospital (Hainan General Hospital) (Approve number: 2020 (No. 139)). Significant efforts were made to minimize both the number of animals and their suffering. All procedures were strictly conducted in conformity to the International Code of Ethics in Laboratory Animals and national regulations.
Cell Culturing
HCC cell lines (HCC-LM3, SMMC77721, Huh7, Hep3B and HepG2) and normal hepatic cell THLE3 supplied by Cell Bank of Chinese Academy of Sciences (Shanghai, China) were cultured in the Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Company, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco) at 37°C with 5% CO2.
Sorting, Identification and Treatment of CSCs in HCC
The HCC cells in exponential phase were detached with trypsin and washed 1–2 times with phosphate-buffered saline (PBS). Next, cell precipitation was collected, re-suspended with 2% FBS and mixed. The cell concentration was adjusted to 1 × 108 cells/mL, and then 100 μL cell resuspension was added into the centrifuge tube (15 mL), followed by the addition of 10 μL phycoerythrin-labeled cluster of differentiation (CD) 133 antibody (ab216323, Abcam Inc., Cambridge, MA, USA) or corresponding homologous control. The cells were fully mixed and underwent a 30-min incubation away from light at 4°C. After twice washes with PBS containing 2% FBS, the cells were re-suspended with 500 μL PBS containing 2% FBS. Finally, the CD133+ and CD133− cell clusters were detected and sorted using a flow cytometer.
The lentiviral interference vector of BMP2 [BMP2-short hairpin RNA (shRNA)] and its negative control (NC) (scramble) were constructed by Cyagen Biosciences Inc. (Guangzhou, China), and the titer determination was performed. The titer of lentiviral interference vector BMP2-shRNA and its NC was 4 × 108 CFU/mL. Next, the constructed vectors were transfected into hepatic CSCs. DIPQUO (HY-128591, MedChemExpress Co., Ltd., Monmouth Junction, NJ, USA) served as the MAPK/ERK pathway activator and KO-947 (HY-112181, MedChemExpress) served as the MAPK/ERK pathway inhibitor.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Takara Bio Inc., Kyoto, Japan). RNA concentration and purity were measured using an ultra-micro-spectrophotometer (Shanghai Puyuan Instrument Co., Ltd., Shanghai, China), and cDNA was synthetized from 1 μg RNA using the reverse transcription kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). The qPCR detection was performed using SYBR® Premix Ex TaqTM II (Takara) on ABI 7900HT fast PCR real-time system (Applied Biosystems, Foster City, CA, USA), with the reaction conditions being 10 min at 95°C (pre-denaturation), and 40 cycles of 10 s at 95°C (denaturation), 20 s at 60°C (annealing) and 34 s at 72°C (stretching). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal reference. The 2−ΔΔCt method was used for data analysis. The design and synthesis of all primers were finished by Sangon Biotech Co., Ltd. (Shanghai, China) (Table 1).
 |
Table 1 Primer Sequences for qRT-PCR |
Western Blot Analysis (WB)
The tissue homogenate and cells were lysed using enhanced RIPA lysis (Boster Biological Technology Co., Ltd., Wuhan, Hubei, China) containing protease inhibitors. Protein concentration was determined utilizing the bicinchoninic acid protein quantitative kit (Boster). The proteins were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for separation, transferred to polyvinylidene fluoride membranes using electro-transfer method, and then blocked with 5% bovine serum albumin for 2 h at room temperature for blocking the nonspecific binding. After incubated with the primary antibodies overnight at 4°C, the membranes were rinsed and underwent a 1-h incubation with horseradish peroxidase-labeled secondary antibody immunoglobulin G (IgG) at room temperature. Enhanced chemiluminescence working solution (EMD Millipore, Billerica, MA, USA) was used for membrane development. Image Pro Plus 6.0 (Media Cybernetics, Bethesda, Maryland, USA) was utilized to quantify the gray value of each band in the WB image. GAPDH served as the internal reference. Three independent experiments were done. The antibody information is shown in Table 2. All antibodies were obtained from Abcam Inc.
 |
Table 2 Antibodies Used in WB |
Sphere Formation Assay
The cells were detached with trypsin and then put in a serum-containing medium for trypsin neutralization, followed by a 5-min centrifugation at 800 rpm. Afterwards, cells were re-suspended in the stem cell culture medium and counted, and the cell concentration was adjusted. Subsequently, cells were seeded into 6-well ultra-low attachment plates (1 × 104 cells/well) and underwent a 2-week incubation at 37°C with 5% CO2. Cell sphere formation was observed and counted. The sphere-forming rate = cell sphere numbers per well/total cells × 100%.
Colony Formation Assay
CSCs from HepG2 cells (HepG2-CSCs) and SMMC7721 cells (SMMC7721-CSCs) were detached, re-suspended and counted. After being diluted to an appropriate concentration, the cell suspension (2 mL/well) was added into the 6-well plates. When cell colonies grew to an appropriate size, drugs of an appropriate volume were added and gently mixed. After 72-h further culture, the medium was removed. Following 3 washes (3 min/time) with 4°C pre-cooled PBS solution, cells were added with methanol (2 mL/well) and cross-linked for 15 min for fixing. After the methanol was removed, the cells were rinsed 3 times (3 min/time) in PBS and added with 10% Giemsa staining solution (2 mL/well) pre-diluted by PBS solution at room temperature. After 30 min, the staining solution was discarded. Cells were then subjected to 3 washes (3 min/time) in PBS, followed by natural drying. The colony numbers were counted under a microscope.
Cell Counting Kit-8 (CCK-8) Assay
HepG2-CSCs and SMMC7721-CSCs were seeded into the 96-well plates (1 × 106 cells/well), with 12 duplicated wells in each group. The cells were cultured for 1–7 days, with 3 duplicated wells at each time point. CCK-8 solution (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was added into the medium free of cells as the blank control. The plates were cultured at 37°C with 5% CO2, and 10 μL CCK-8 solution was added into the corresponding wells at each time point. After 4-h incubation, the optical density (OD) at 450 nm was measured using a microplate reader.
Transwell Assay
After 48-h infection, the Transwell assay was conducted utilizing Matrigel (YB356234; Yubo Biotechnology Co., Ltd., Shanghai, China) that were unfrozen at 4°C overnight before treatment for balance maintenance. Matrigel (200 μL) was diluted at 4°C using 200 μL serum-free medium. Next, the diluted Matrigel (50 μL) was added to the apical chamber of the plate for a 2–3 h incubation at 4°C. After cell detachment and counting, cell suspension (200 μL) was added into each apical chamber and the medium (800 μL) containing 20% FBS was added into each basolateral chamber. After that, the cells underwent a 20–24 h incubation at 37°C, followed by washes with PBS twice, 10-min immersion in formaldehyde, and washes with tap water 3 times. Next, the cells were stained with 0.1% violet for 30 min staining at room temperature and washed with PBS twice. Finally, the cells on the plate surface were wiped off using cotton cloth.
Tumor Xenograft Formation in Nude Mice
Ten nude mice [Shanghai Branch of Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China; SYXK (Shanghai) 2017–0014] were randomly allocated into the shBMP2 group (injected with HepG2-CSCs transfected with shBMP2) and the scramble group (injected with HepG2-CSCs transfected with scramble), with 5 mice in each group. Briefly, HepG2-CSCs in logarithmic growth phase stably transfected with shBMP2 or scramble were collected, detached with 0.25% trypsin and dispersed into single-cell suspension in PBS. Next, the suspension (2 × 106 cells) was injected subcutaneously into the right armpit of mice. The health status of nude mice was monitored every day after injection. The tumor size was measured every 4 days, and calculated by the following formula: V = W2 × L × 0.52.23 The nude mice were euthanized by intraperitoneal injection of pentobarbital sodium (≥100 mg/kg) on the 28th day after injection, and the tumors were removed under sterile conditions. After PBS washing, the tumor weights were recorded.
Statistical Analysis
SPSS 21.0 (IBM Corp. Armonk, NY, USA) was utilized for data analysis. Kolmogorov–Smirnov test verified that the data were in normal distribution. Data were represented as mean ± standard deviation. Independent sample t-test was utilized for comparison analysis between two groups, and one-way or two-way analysis of variance (ANOVA) for comparison analysis among multiple groups followed by Tukey’s multiple comparisons test. The p value was obtained from a two-tailed test. The p < 0.05 indicated the statistically significant difference and p < 0.01 represented the highly statistically significant difference.
Results
BMP2 Was Highly Expressed in HCC Tissues and Cells
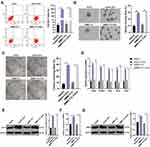
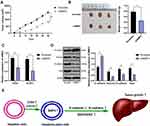
To explore the relationship between BMP2 and HCC, we first predicted that BMP2 expression in HCC tissues was upregulated relative to that in normal hepatic tissues (Figure 1A) through TCGA online visualization website (http://starbase.sysu.edu.cn/panCancer.php),24 and higher BMP2 expression in HCC tissues was indicative of worse prognosis of HCC patients (Figure 1B). Subsequently, BMP2 expression in HCC cells and normal hepatic cells was further verified using WB and qRT-PCR, as confirmed by a remarkably elevated BMP2 expression in HCC cells (HepG2, Hep3B, SMMC7721, HCC-LM3 and Huh7) compared to that in normal hepatic cells (Figure 1C and D) (all p < 0.05).
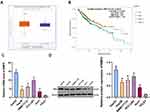 |
Figure 1 BMP2 is highly expressed in HCC tissues and cells. (A) The expression of BMP2 in HCC and normal liver tissues was analyzed using online visualization data from Starbase (http://starbase.sysu.edu.cn/panCancer.php) in TCGA; (B) The relationship between the expression of BMP2 in Starbase and the prognosis of HCC patients; (C) qRT-PCR was used to detect the mRNA expression of BMP2 in HCC cells and normal liver cells; (D) WB was used to detect the protein level of BMP2 in five HCC cells and normal liver cells. The cell experiment was repeated three times, and the data were expressed as mean ± standard deviation. Data in panels (C and D) were analyzed using one-way ANOVA and Tukey’s multiple comparisons test. *p < 0.05. |
BMP2 Expression Was Further Increased in CSCs in HCC
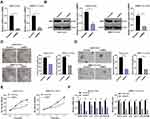
CSCs are critical in tumor survival, proliferation, metastasis and recurrence, which maintain tumor cell vitality through their self-renewal and unlimited proliferation.25 We proved that BMP2 was closely related to the prognosis of HCC patients. Therefore, we speculated there may be a certain correlation between BMP2 and CSCs in HCC. HepG2 and SMMC7721 cells are widely researched in hepatocellular carcinoma experiment.26,27 Hence, HepG2 and SMMC7721 cells with the highest BMP2 expression were selected, and then sorted for identifying CSCs in HCC (HepG2-CSCs and SMMC7721-CSCs) using a flow cytometer. We found that CD133 was positively expressed in HepG2-CSCs and SMMC7721-CSCs was dramatically increased compared with that in HepG2 cells and SMMC7721 cells (Figure 2A) (p < 0.01). The self-renewal ability of HepG2 cells, HepG2-CSCs, SMMC7721 cells and SMMC7721-CSCs was assessed using sphere formation assay and colony formation assay. Compared with HepG2 and SMMC7721 cells, HepG2-CSCs and SMMC7721-CSCs showed dramatically increased sphere formation rate and colony numbers (Figure 2B and C) (both p < 0.05). The mRNA expressions of CSC markers [CD44 and aldehyde dehydrogenase 1 (ALDH1)] and pluripotent transcription factors (Bmil, sex-determining region Y-box protein 2 (SOX2) and octamer binding transcription factor 4 (OCT4)] were elevated markedly in HepG2-CSCs and SMMC7721-CSCs relative to those in HepG2 and SMMC7721 cells (Figure 2D) (all p < 0.05). WB also confirmed that the protein level of CSC marker CD44 was noticeably increased in HepG2-CSCs and SMMC7721-CSCs (Figure 2E) (p < 0.05), demonstrating the stemness of HepG2-CSCs and SMMC7721-CSCs. BMP2 expression in HepG2-CSCs and SMMC7721-CSCs was found to be upregulated (Figure 2F and G) (both p < 0.05), indicating that BMP2 expression may be linked to CSCs in HCC.
BMP2 Knockdown Inhibited the Stemness of CSCs in HCC
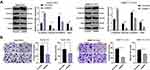
The lentiviral interference vector shBMP2 was constructed and transfected into the HepG2-CSCs and SMMC7721-CSCs to study the specific role of BMP2 in CSCs in HCC. The stemness changes of CSCs were detected using sphere formation assay and colony formation assay to explore the effect of shBMP2 on the stemness of CSCs in HCC. We first identified that BMP2 mRNA expression and protein level were obviously reduced after silencing BMP2 (Figure 3A and B) (all p < 0.05). Next, colony formation assay showed that inhibition of BMP2 markedly reduced colony numbers (Figure 3C) (p < 0.05). Meanwhile, silencing BMP2 also remarkably decreased the volume and number of tumor spheres, and then reduced the sphere-forming rate of HepG2-CSCs (Figure 3D) (all p < 0.01). CCK-8 assay revealed that silencing BMP2 inhibited the proliferation ability of HepG2-CsCs and SMMC7721-CSCs (Figure 3E) (all p < 0.01). As indicated by qRT-PCR, the mRNA expressions of stemness-related markers [Nanog, SOX2, OCT4, CD13, CD44 and epithelial Cell Adhesion Molecule (EpCAM)] in CSCs in HCC were obviously decreased (Figure 3F) (all p < 0.01). From all the above, we concluded that inhibition of BMP2 suppressed CSC stemness in HCC.
BMP2 Knockdown Inhibited the EMT and the Invasion and Migration of CSCs in HCC
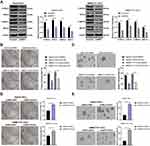
As evidenced previously, CSCs show a close relationship with EMT of tumor cells.28 Therefore, we speculated that BMP2 promoted CSC invasion and migration in HCC via regulating the EMT of CSCs in HCC. As shown by WB results, after inhibition of BMP2, the level of epithelial marker E-cadherin in CSCs in HCC was upregulated, while levels of mesenchymal markers (Vimentin and N-cadherin) and Snail were downregulated (Figure 4A) (all p < 0.05). Briefly, BMP2 played an important role in EMT of CSCs in HCC. In addition, EMT of tumor cells often leads to the enhancement of cell invasion and migration.29 Hence, CSC invasion and migration in HCC in each group were detected. As shown by Transwell assay, inhibition of BMP2 in vitro effectively suppressed CSC invasion and migration in HCC (Figure 4B) (both p < 0.05). In conclusion, BMP2 knockdown inhibited the EMT and the invasion and migration of CSCs in HCC.
BMP2 Knockdown Inhibited the Stemness Maintenance of CSCs in HCC via Inhibiting the MAPK/ERK Pathway
The MAPK/ERK pathway has been suggested to be involved in the proliferation and invasion of CSCs.30 Based on our previous experiments, BMP2 induced EMT changes and promoted CSC proliferation, migration and invasion in HCC. Therefore, we further speculated that BMP2 affected CSC proliferation, migration and invasion in HCC via regulating the MAPK/ERK pathway. The activation of the MAPK/ERK pathway was detected, and we observed that p-ERK1/2 and p-MEK1/2 levels were obviously decreased after BMP2 inhibition (Figure 5A) (all p < 0.05). MEK/ERK pathway inhibitor KO-947 notably decreased the sphere and colony formation of CSCs (Figure 5B and C) (all p < 0.05). Furthermore, DIPQUO was used to activate the MAPK/ERK pathway to observe the stemness of CSCs in HCC. We observed that the colony numbers were remarkably increased after DIPQUO treatment (Figure 5D) (p < 0.05). Similar results were also found in tumor sphere formation (Figure 5E) (p < 0.05). Overall, the MAPK/ERK pathway activation by DIPQUO reversed the inhibitory effects of BMP2 inhibition on colony and sphere formation of CSCs in HCC. Collectively, BMP2 knockdown inhibited the stemness maintenance of CSCs in HCC via suppressing the MAPK/ERK pathway.
BMP2 Knockdown Inhibited Tumor Growth and EMT of CSCs in HCC in Nude Mice
We have proven that BMP2 knockdown suppressed the MAPK/ERK pathway and inhibited CSC proliferation, migration and invasion in HCC in vitro. The effect of shBMP2 on CSCs in HCC in nude mice was further studied. According to tumor xenograft formation assay, mice treated with BMP2 knockdown showed remarkably reduced tumor volume and weight (Figure 6A and B) (both p < 0.05). Meanwhile, as shown by qRT-PCR results, the mRNA expressions of CSC markers (CD44 and ALDH1) were obviously decreased after BMP2 knockdown (Figure 6C) (both p < 0.05). Furthermore, WB verified the dramatically elevated E-cadherin level, and the reduced levels of Vimentin, N-cadherin and Snail in hepatic CSCs (Figure 6D) (all p < 0.05). From all the above, we concluded that suppression of BMP2 inhibited CSC EMT and stemness in HCC in vitro (Figure 6E).
Discussion
Currently, a few drugs have been approved for unresectable HCC; nevertheless, the response rate of these drugs is low and the guaranteed survival benefit (2–3 months) is limited.31 CSCs are essential driving force in diverse cancers,32 and BMP is identified to regulate CSCs.33 This study demonstrated that BMP2 depletion suppressed the EMT, proliferation, migration and invasion, thereby hindering stemness maintenance of CSCs in HCC via blocking the MAPK/ERK pathway.
BMP2 has been found to show a deregulated expression in multiple cancers, such as nasopharyngeal carcinoma, gastric cancer and pancreatic cancer, and its high expression is often closely linked to poor prognosis of patients.34–36 As shown by our results, BMP2 expression in HCC tissues and cells was remarkably upregulated, and its high expression indicated worse prognosis of HCC patients. In agreement with this, a previous study has also demonstrated the abnormal upregulation of BMP2 in HCC.22 CSCs, showing presence in primary cancers, are identified to drive cancer growth with the potent self-renewing ability, and consequently targeting CSCs has been recognized as a promising therapy for cancer treatment.25 Compared with HepG2 and SMMC7721 cells, HepG2-CSCs and SMMC7721-CSCs showed dramatically increased sphere formation rate and colony numbers, and markedly elevated levels of CSC markers (CD44 and ALDH1) and pluripotent transcription factors (Bmil, SOX2 and OCT4), demonstrating the stemness of HepG2-CSCs and SMMC7721-CSCs. In addition, BMP2 expression in HepG2-CSCs and SMMC7721-CSCs was remarkably upregulated, which suggested that BMP2 expression may be related to CSCs in HCC. Consistently, a previous study has demonstrated the aberrantly elevated expression of BMP2 in CSCs in prostate cancer.21
Growing studies have pointed out that hepatic CSCs make great contributions to the initiation, progression, and recurrence of HCC.37,38 Furthermore, the BMP signaling is implicated in CSC self-renewal and HCC progression.20 It is well accepted that Nanog, SOX2, OCT4, CD133, CD44, EpCAM and CD13, as well as high ALDH activity are functional markers for stemness of hepatic CSCs.39,40 We observed that inhibition of BMP2 dramatically reduced the colony and sphere of hepatic CSCs, accompanied by remarkably decreased levels of stemness-related markers (Nanog, SOX2, OCT4, CD13, CD44 and EpCAM) in CSCs in HCC. Consistently, numerous studies have provided evidence for the inhibitory effect of BMP2 depletion on CSCs in multiple cancers. For instance, BMP2 knockdown decreases CSC numbers and tumor initiation rate in ovarian cancer.20 Silencing BMP2 contributes to reducing sphere formation and downregulating stemness markers (CD133+ and EpCAM+) of colon CSCs.41 Nevertheless, the interplay of BMP2 with CSCs in HCC remained to be elucidated.
EMT is tightly bound up with the stemness maintenance of CSCs and can even confer CSC-like properties to normal tumor cells, including in HCC.42,43 EMT contributes to tumor cell migration and invasion.44 Additionally, EMT can be evaluated by the loss of E-cadherin and the presence of Vimentin, N-cadherin and Snail.45,46 As shown by our observations, BMP2 knockdown elevated E-cadherin level and reduced levels of Vimentin, N-cadherin and Snail; in addition, inhibition of BMP2 effectively suppressed the invasion and migration of CSCs in HCC. In agreement with these, BMP2 facilitates EMT and stemness of breast CSCs, thereby promoting tumor metastasis.47 Depletion of BMP2 suppressed EMT and stemness maintenance of colon CSCs, so as to inhibit colon cancer migration and invasion.41 The role of inhibited BMP2 was further verified in vivo in nude mice, as demonstrated by the reduced tumor volume and weight, and inhibited stemness and EMT of CSCs. Silencing BMP2 helps to prevent HCC cell migration and invasion.22 In conclusion, BMP2 knockdown inhibited the EMT and then the invasion and migration of CSCs in HCC.
As has been pointed out previously, the activated MAPK/ERK pathway is strongly linked to HCC progression.48 MAPK/ERK signal transduction is the most abundant process in tumors, and MAPK/ERK is closely related to the maintenance of CSCs.49 Reduction of MAPK/ERK signal transduction contributes to inhibiting CSC proliferation and migration.30 Blockade of the MAPK/ERK pathway helps to hinder the stemness, proliferation and in vivo tumor formation capacity of renal CSCs.50 Moreover, it is suggested that BMP2 shows a close relation with the MAPK/ERK pathway in osteoblastic differentiation.51 As indicated by our results, BMP2 knockdown markedly inhibited the MAPK/ERK pathway, while the activated MAPK/ERK pathway reversed the inhibitory effects of BMP2 knockdown on the stemness of CSCs in HCC. Consistently, the p38/MAPK signaling modulates hepatic CSCs stemness acquisition under tobacco smoke exposure.52 Inhibition of ERK prevents the migration and invasion, as well as CSC-like cell maintenance in HCC.53 Moreover, BMP2 mediates the MAPK signaling to promote HCC progression, tumor growth, and angiogenesis.22 Overall, we proved that inhibition of BMP2 suppressed the stemness maintenance of CSCs in HCC via blocking the MAPK/ERK pathway.
All in all, we proved that BMP2 knockdown inhibited CSC EMT, proliferation, migration and invasion, thus hindering the stemness maintenance of CSCs in HCC via blocking the MAPK/ERK pathway. Previous studies of BMP2 mostly focus on osteogenesis fields and function of its expression on tumor; based on this, this study innovatively explored the regulatory role of BMP2 in CSCs isolated from HCC tissues involving the MAPK/ERK pathway. These results discovered a novel CSC-based target for HCC patients. Although the present study provided therapeutic value for HCC treatment, the experiment results and clinical application need to be further verified.
Data Sharing Statement
All the data generated or analyzed during this study are included in this published article.
Acknowledgments
We would like to thank Dr. Nanyao Chen for comments that greatly improved the manuscript.
Funding
This work was supported by the Key Research and Development Plan of Hainan Province (2019RC373) and Supported by Hainan Natural Science Foundation (818MS161).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Kim DW, Talati C, Kim R. Hepatocellular carcinoma (HCC): beyond sorafenib-chemotherapy. J Gastrointest Oncol. 2017;8(2):256–265. doi:10.21037/jgo.2016.09.07
2. Mathew S, Ali A, Abdel-Hafiz H, et al. Biomarkers for virus-induced hepatocellular carcinoma (HCC). Infect Genet Evol. 2014;26:327–339. doi:10.1016/j.meegid.2014.06.014
3. Wang EA, Stein JP, Bellavia RJ, Broadwell SR. Treatment options for unresectable HCC with a focus on SIRT with Yttrium-90 resin microspheres. Int J Clin Pract. 2017;71(11):e12972. doi:10.1111/ijcp.12972
4. Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65(4):798–808. doi:10.1016/j.jhep.2016.05.007
5. Eun K, Ham SW, Kim H. Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep. 2017;50(3):117–125. doi:10.5483/bmbrep.2017.50.3.222
6. Chiba T, Iwama A, Yokosuka O. Cancer stem cells in hepatocellular carcinoma: therapeutic implications based on stem cell biology. Hepatol Res. 2016;46(1):50–57. doi:10.1111/hepr.12548
7. Albini A, Bruno A, Gallo C, Pajardi G, Noonan DM, Dallaglio K. Cancer stem cells and the tumor microenvironment: interplay in tumor heterogeneity. Connect Tissue Res. 2015;56(5):414–425. doi:10.3109/03008207.2015.1066780
8. Kharkar PS. Cancer stem cell (CSC) inhibitors: a review of recent patents (2012-2015). Expert Opin Ther Pat. 2017;27(7):753–761. doi:10.1080/13543776.2017.1325465
9. Li S, Li Q. Cancer stem cells and tumor metastasis (Review). Int J Oncol. 2014;44(6):1806–1812. doi:10.3892/ijo.2014.2362
10. Liu YC, Yeh CT, Lin KH. Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells. 2020;9:6. doi:10.3390/cells9061331
11. Jayachandran A, Dhungel B, Steel JC. Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. J Hematol Oncol. 2016;9(1):74. doi:10.1186/s13045-016-0307-9
12. Li Y, Chen G, Han Z, Cheng H, Qiao L, Li Y. IL-6/STAT3 signaling contributes to sorafenib resistance in hepatocellular carcinoma through targeting cancer stem cells. Onco Targets Ther. 2020;13:9721–9730. doi:10.2147/OTT.S262089
13. Modi SJ, Kulkarni VM. Discovery of VEGFR-2 inhibitors exerting significant anticancer activity against CD44+ and CD133+ cancer stem cells (CSCs): reversal of TGF-beta induced epithelial-mesenchymal transition (EMT) in hepatocellular carcinoma. Eur J Med Chem. 2020;207:112851. doi:10.1016/j.ejmech.2020.112851
14. Wu Y, Zhang J, Zhang X, Zhou H, Liu G, Li Q. Cancer stem cells: a potential Breakthrough in HCC-targeted therapy. Front Pharmacol. 2020;11:198. doi:10.3389/fphar.2020.00198
15. Sampath TK, Reddi AH. Discovery of bone morphogenetic proteins - a historical perspective. Bone. 2020;140:115548. doi:10.1016/j.bone.2020.115548
16. Herrera B, Sanchez A, Fabregat I. BMPS and liver: more questions than answers. Curr Pharm Des. 2012;18(27):4114–4125. doi:10.2174/138161212802430503
17. Wang X, Wang JG, Geng YY, et al. An enhanced anti-tumor effect of apoptin-cecropin B on human hepatoma cells by using bacterial magnetic particle gene delivery system. Biochem Biophys Res Commun. 2018;496(2):719–725. doi:10.1016/j.bbrc.2018.01.108
18. Garulli C, Kalogris C, Pietrella L, et al. Dorsomorphin reverses the mesenchymal phenotype of breast cancer initiating cells by inhibition of bone morphogenetic protein signaling. Cell Signal. 2014;26(2):352–362. doi:10.1016/j.cellsig.2013.11.022
19. Rampazzo E, Dettin M, Maule F, et al. A synthetic BMP-2 mimicking peptide induces glioblastoma stem cell differentiation. Biochim Biophys Acta Gen Subj. 2017;1861(9):2282–2292. doi:10.1016/j.bbagen.2017.07.001
20. Wang Y, Zhu P, Luo J, et al. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. EMBO J. 2019;38(17):e101110. doi:10.15252/embj.2018101110
21. Kanwal R, Shukla S, Walker E, Gupta S. Acquisition of tumorigenic potential and therapeutic resistance in CD133+ subpopulation of prostate cancer cells exhibiting stem-cell like characteristics. Cancer Lett. 2018;430:25–33. doi:10.1016/j.canlet.2018.05.014
22. Feng PC, Ke XF, Kuang HL, Pan LL, Ye Q, Wu JB. BMP2 secretion from hepatocellular carcinoma cell HepG2 enhances angiogenesis and tumor growth in endothelial cells via activation of the MAPK/p38 signaling pathway. Stem Cell Res Ther. 2019;10(1):237. doi:10.1186/s13287-019-1301-2
23. Kim CJ, Terado T, Tambe Y, et al. Anti-oncogenic activities of cyclin D1b siRNA on human bladder cancer cells via induction of apoptosis and suppression of cancer cell stemness and invasiveness. Int J Oncol. 2018;52(1):231–240. doi:10.3892/ijo.2017.4194
24. Li JH, Liu S, Zhou H, Qu LH, Yang JH. Starbase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–7. doi:10.1093/nar/gkt1248
25. Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234(6):8381–8395. doi:10.1002/jcp.27740
26. Sun L, Li PB, Yao YF, et al. Proteinase-activated receptor 2 promotes tumor cell proliferation and metastasis by inducing epithelial-mesenchymal transition and predicts poor prognosis in hepatocellular carcinoma. World J Gastroenterol. 2018;24(10):1120–1133. doi:10.3748/wjg.v24.i10.1120
27. Wu W, Zhang X, Liao Y, et al. miR-30c negatively regulates the migration and invasion by targeting the immediate early response protein 2 in SMMC-7721 and HepG2 cells. Am J Cancer Res. 2015;5(4):1435–1446.
28. Tanabe S, Quader S, Cabral H, Ono R. Interplay of EMT and CSC in cancer and the potential therapeutic strategies. Front Pharmacol. 2020;11:904. doi:10.3389/fphar.2020.00904
29. Zhang F, Duan C, Yin S, Tian Y. MicroRNA-379-5p/YBX1 axis regulates cellular EMT to suppress migration and invasion of nasopharyngeal carcinoma cells. Cancer Manag Res. 2020;12:4335–4346. doi:10.2147/CMAR.S253504
30. Erdogan S, Turkekul K. Neferine inhibits proliferation and migration of human prostate cancer stem cells through p38 MAPK/JNK activation. J Food Biochem. 2020;44(7):e13253. doi:10.1111/jfbc.13253
31. Caruso S, O’Brien DR, Cleary SP, Roberts LR, Zucman-Rossi J. Genetics of HCC: novel approaches to explore molecular diversity. Hepatology. 2020. doi:10.1002/hep.31394
32. Pan Y, Ma S, Cao K, et al. Therapeutic approaches targeting cancer stem cells. J Cancer Res Ther. 2018;14(7):1469–1475. doi:10.4103/jcrt.JCRT_976_17
33. Horne GA, Copland M. Approaches for targeting self-renewal pathways in cancer stem cells: implications for hematological treatments. Expert Opin Drug Discov. 2017;12(5):465–474. doi:10.1080/17460441.2017.1303477
34. Duan L, Ye L, Wu R, et al. Inactivation of the phosphatidylinositol 3-kinase/Akt pathway is involved in BMP9-mediated tumor-suppressive effects in gastric cancer cells. J Cell Biochem. 2015;116(6):1080–1089. doi:10.1002/jcb.25063
35. Li CS, Tian H, Zou M, et al. Secreted phosphoprotein 24 kD (Spp24) inhibits growth of human pancreatic cancer cells caused by BMP-2. Biochem Biophys Res Commun. 2015;466(2):167–172. doi:10.1016/j.bbrc.2015.08.124
36. Wang MH, Zhou XM, Zhang MY, et al. BMP2 promotes proliferation and invasion of nasopharyngeal carcinoma cells via mTORC1 pathway. Aging (Albany NY). 2017;9(4):1326–1340. doi:10.18632/aging.101230
37. Feng Y, Jiang W, Zhao W, Lu Z, Gu Y, Dong Y. miR-124 regulates liver cancer stem cells expansion and sorafenib resistance. Exp Cell Res. 2020;394(2):112162. doi:10.1016/j.yexcr.2020.112162
38. Yu H, Zhu X, Lin H, et al. A new risk model comprising genes highly correlated with CD133 identifies different tumor-immune microenvironment subtypes impacting prognosis in hepatocellular carcinoma. Aging (Albany NY). 2020;12(12):12234–12250. doi:10.18632/aging.103409
39. Castelli G, Pelosi E, Testa U. Liver cancer: molecular characterization, clonal evolution and cancer stem cells. Cancers (Basel). 2017;9(9). doi:10.3390/cancers9090127
40. Zhao Z, Bai S, Wang R, et al. Cancer-associated fibroblasts endow stem-like qualities to liver cancer cells by modulating autophagy. Cancer Manag Res. 2019;11:5737–5744. doi:10.2147/CMAR.S197634
41. Kim BR, Oh SC, Lee DH, et al. BMP-2 induces motility and invasiveness by promoting colon cancer stemness through STAT3 activation. Tumour Biol. 2015;36(12):9475–9486. doi:10.1007/s13277-015-3681-y
42. Jing L, Ruan Z, Sun H, et al. Epithelial-mesenchymal transition induced cancer-stem-cell-like characteristics in hepatocellular carcinoma. J Cell Physiol. 2019;234(10):18448–18458. doi:10.1002/jcp.28480
43. Liu X, Fan D. The epithelial-mesenchymal transition and cancer stem cells: functional and mechanistic links. Curr Pharm Des. 2015;21(10):1279–1291. doi:10.2174/1381612821666141211115611
44. Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11(1):28–39. doi:10.1002/1878-0261.12017
45. Liang G, Fang X, Yang Y, Song Y. Silencing of CEMIP suppresses Wnt/beta-catenin/Snail signaling transduction and inhibits EMT program of colorectal cancer cells. Acta Histochem. 2018;120(1):56–63. doi:10.1016/j.acthis.2017.11.002
46. Wei Y, Lv B, Xie J, et al. Plumbagin promotes human hepatoma SMMC-7721 cell apoptosis via caspase-3/vimentin signal-mediated EMT. Drug Des Devel Ther. 2019;13:2343–2355. doi:10.2147/DDDT.S204787
47. Huang P, Chen A, He W, et al. BMP-2 induces EMT and breast cancer stemness through Rb and CD44. Cell Death Discov. 2017;3:17039. doi:10.1038/cddiscovery.2017.39
48. Xiao S, Yang M, Yang H, Chang R, Fang F, Yang L. miR-330-5p targets SPRY2 to promote hepatocellular carcinoma progression via MAPK/ERK signaling. Oncogenesis. 2018;7(11):90. doi:10.1038/s41389-018-0097-8
49. da Cunha Jaeger M, Ghisleni EC, Cardoso PS, et al. HDAC and MAPK/ERK inhibitors cooperate To reduce viability and stemness in medulloblastoma. J Mol Neurosci. 2020;70(6):981–992. doi:10.1007/s12031-020-01505-y
50. Huang B, Fu SJ, Fan WZ, et al. PKCepsilon inhibits isolation and stemness of side population cells via the suppression of ABCB1 transporter and PI3K/Akt, MAPK/ERK signaling in renal cell carcinoma cell line 769P. Cancer Lett. 2016;376(1):148–154. doi:10.1016/j.canlet.2016.03.041
51. Sun X, Xie Z, Ma Y, et al. TGF-beta inhibits osteogenesis by upregulating the expression of ubiquitin ligase SMURF1 via MAPK-ERK signaling. J Cell Physiol. 2018;233(1):596–606. doi:10.1002/jcp.25920
52. Xie C, Zhu J, Wang X, et al. Tobacco smoke induced hepatic cancer stem cell-like properties through IL-33/p38 pathway. J Exp Clin Cancer Res. 2019;38(1):39. doi:10.1186/s13046-019-1052-z
53. Tsai CF, Hsieh TH, Lee JN, et al. Curcumin suppresses phthalate-induced metastasis and the proportion of Cancer Stem Cell (CSC)-like cells via the inhibition of AhR/ERK/SK1 signaling in hepatocellular carcinoma. J Agric Food Chem. 2015;63(48):10388–10398. doi:10.1021/acs.jafc.5b04415
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.