Back to Journals » Medical Devices: Evidence and Research » Volume 11
Inhibition of bacterial attachment and biofilm formation by a novel intravenous catheter material using an in vitro percutaneous catheter insertion model
Authors Pathak R , Bierman SF, d'Arnaud P
Received 29 August 2018
Accepted for publication 9 October 2018
Published 19 December 2018 Volume 2018:11 Pages 427—432
DOI https://doi.org/10.2147/MDER.S183409
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Dr Rahul Pathak
Views: 1044
Rahul Pathak,1 Steve F Bierman,2 Pieter d’Arnaud3
1University of Central Florida College of Medicine, Lakeland, FL 33813, USA; 2Access Scientific, LLC, San Diego, CA 92121, USA; 3Instat Consulting, Inc., Chatham, NJ 07928, USA
Introduction: Despite sterile barrier precautions and vigorous skin antisepsis, percutaneous insertion of intravenous catheters has been shown to result in attachment to the catheter surface of bacteria residing in the deep structures of the skin. Such attachment poses the risk of biofilm formation and eventual catheter-related bloodstream infection (CRBSI). This study was undertaken to assess whether the non-coated surface treatment of a unique catheter material (ChronoFlex C® with BioGUARD™) could inhibit bacterial attachment and biofilm formation.
Methods: A novel in vitro model and fluorescence microscopy were used to compare two intravascular catheter materials with respect to bacterial attachment and biofilm formation. The control material was a commonly used polyurethane. The study material was a unique copolymer, treated so as to remove surface additives, alter hydrophobicity and create surface micro-patterning. Outcomes were assessed using both a membrane potential indicator and a cell death reporter with appropriate fluorescent channels. Thus, bacterial cells attached to the catheter surface (living and dead) were imaged without mechanical disruption.
Results: Both bacterial attachment and biofilm formation are significantly inhibited by the study catheter material. In fact, over 5 times more bacteria were able to attach and grow on the control polyurethane material than on the study material (P=0.0020). Moreover, those few bacteria that were able to attach to the study material had a 1.5 times greater likelihood of dying.
Conclusion: Using a novel in vitro percutaneous catheter insertion model, ChronoFlex C with BioGUARD is proven to significantly inhibit bacterial attachment and biofilm formation as compared with a commonly used polyurethane catheter material.
Keywords: biofilm, CRBSI, CLABSI, catheter-related infections, catheter-associated infections, bacterial resistance
Introduction
In 1998, Livesley et al demonstrated that percutaneous insertion of catheters – despite rigorous aseptic technique, maximum barrier protection and chlorhexidine skin antisepsis – resulted in staphylococcal “impaction” (ie, attachment) on the external surface of catheters in 16.7% of cases.1 Once attached, of course, numerous factors including material characteristics, type of bacteria and hematologic conditions mediate the progression from attachment to cell-to-cell adhesion, biofilm formation, maturation and eventually planktonic dispersion into the bloodstream.2–4 While disquieting, bacterial contamination upon insertion may well explain, at least in part, the observation by Crnich and Maki5 and Trimsit et al6 as to the similarity between central venous catheter (CVC) and arterial line bloodstream infection rates: namely, despite their obvious differences, both are placed percutaneously and have similar average dwell times. It may further explain rising bloodstream infections rates (predominantly staphylococcal) when peripheral IV catheters are allowed to dwell an average of 6 days as opposed to shorter time periods.5,7,8
Livesley further demonstrated that catheter insertion through an introducer sheath can prevent skin contamination of the catheter’s external surface during insertion.1 However, introducer sheaths entail their own risks. If they do not peel away, the external surfaces of the sheaths may be expected to acquire bacteria during percutaneous insertion. On the other hand, if they do peel away, the to-and-fro movement of the sheath and catheter during sheath removal may well damage the vessel intima, provoking thrombophlebitis;9 the larger hole left by the sheath, once removed, may result in leakage and back bleeding, especially during computerized tomography power injection of the contrast media; also, when used for CVC placement, an open, unvalved introducer sheath certainly enhances the risks of bleeding and air embolism.10
Coating the catheters with antimicrobials, in “pre-CVC bundle” studies, has been shown to reduce associated bloodstream infections roughly to half.11 However, it is not without risk. Anaphylaxis to chlorhexidine-containing coatings has been reported, and with chlorhexidine usage on the rise, so goes chlorhexidine sensitivity and allergy.12
What would be ideal is a percutaneous catheter somehow resistant to bacterial attachment and biofilm formation, but devoid of special coatings or embedded substances. Unfortunately, polyurethane – the polymer out of which most intravenous catheters are now made – is the exact opposite of this ideal.13 Nouman et al have recently demonstrated that during natural aging, additives in commercially used polyurethanes bloom to the surface, and that these additives then “facilitate bacterial attachment”.14
The present study was undertaken to determine whether a novel catheter material (ChronoFlex C®; AdvanSource Biomaterials, Wilmington, MA, USA), which undergoes proprietary processing intended to discourage bacterial attachment and biofilm formation, could outperform a commonly used polyurethane catheter with respect to bacterial attachment and biofilm formation, using an in vitro percutaneous catheter insertion model. The study catheter is neither coated nor impregnated; its base material is a unique copolymer consisting of both polyurethane and polycarbonate. According to the catheter manufacturer (Access Scientific, LLC., San Diego, CA, USA), it is treated in such a way as to remove surface additives, alter hydrophobicity and create surface micro-patterning – measures which have all been shown to discourage microbial attachment.14–17
Materials and methods
Bacillus subtilis NCIB 3610 trpC2 pheA1 espA::Pspac-hy-epsA-0 (Bs) was selected, based on its evolutionarily shared attachment and biofilm formation mechanisms with numerous biofilm-forming bacteria.18–20 In other words, with respect to bacterial attachment, adhesion and biofilm formation, Bs is a nonpathogenic model organism for Gram-positive bacteria, including Staphylococcus aureus.21B. subtilis is well characterized, allowing one to determine using fluorescent microscopy techniques whether failed biofilm formation is the result of impaired attachment, growth or matrix production.22
Control catheters were made of a polyurethane material: 18 guage (PowerGlide™; C.R. Bard, Inc., Salt Lake City, UT, USA). Study catheters were made of the copolymer ChronoFlex C (polycarbonate, polyurethane): 17 guage (note: no 18 guage is manufactured; POWERWAND™ Access Scientific, LLC.).
The percutaneous catheter insertion model consisted of a central Bs biofilm culture, an underlying pH-adjusted (pH=7.0) agar/nutrient substrate (~3–4 mm thick) and an orifice through the bottom of the Petri dish covered with a Parafilm® (Bemis NA; Neenah, WI, USA) (Figure 1). This model was designed so as to simulate skin, such that a catheter passing through it – from the bottom to top – must first penetrate the epidermal layer (the Parafilm), then the dermis (the agar) and, finally, enter the hypodermal layer (Bs colony) wherein pockets of bacteria are known to reside within the various deeper structures (eg, sweat glands or follicles) despite chlorhexidine antisepsis.23 All catheters were placed in this manner in accordance with the manufacturers’ directions for use.
  | Figure 1 Percutaneous model. |
Upon withdrawal of the needle (and dilator, in the case of study catheter), the internal lumen of the catheter was evacuated using pressurized, filtered air. (This was done in order to study the biofilm formed on the exterior of the catheter only, since bloodstream infections associated with short-term percutaneously placed catheters – as opposed to the catheters placed through introducer sheaths – are associated more commonly with external surface biofilm.)24 Catheters were then cut into segments and incubated at 30°C for 48 hours. Thereafter, the catheter segments were exposed to Sytox™ (Thermo Fisher Scientific; Waltham, MA, USA); a commercially available dye that reports directly on cell death and to Thioflavin-T (ThT; Abcam, Cambridge, MA, USA); a Nernstian membrane potential indicator dye that reports on cell viability, allowed to incubate for 1 hour and imaged using a phase contrast microscope (Olympus IX81 or IX83): the yellow fluorescent protein (YFP) channel was used for Sytox and the cyan fluorescent protein (CFP) fluorescent channel for ThT.25 Thus, bacterial cells attached to the catheter surface (living and dead) were imaged without mechanical disruption, as often happens with “semi-quantitative” methods (eg, the roll plate technique).
Total fluorescence was then meticulously recorded over the entire external catheter surface of the distal tip of each catheter, for each indicator, over nine separate trial runs.
P-values were calculated using paired t-test for the means, signed-rank test for the medians and t-test for ratios for the geometric means (ie, folds). The test for normality was based on the Shapiro–Wilk test.
Results
Bacterial attachment and biofilm formation
In Table 1, Comparison 1 summarizes total CFP channel fluorescence – reflecting live bacterial attachment and biofilm formation – over the distal tip (2 cm) of both control and study catheters for all nine runs. The study catheter exhibited on average significant inhibition of bacterial attachment and biofilm formation as compared with the control catheter P=0.0133 based on a paired t-test of the differences, 95%CL 4.578E9-2.915E10, mean difference=1.687E10.
On average, the amount of attached, biofilm-producing cells on the polyurethane control catheter was 5.407 times greater than on the study catheter (P=0.0020). Figure 2 (ThT uptake using CFP channel) illustrates typical catheter segments as seen with the CFP channel using phase-contrast fluorescent microscopy. Exuberant biofilm growth can be seen extending broadly over a large area of the control catheter’s external surface. In contrast, the study catheter can be seen to host a relative paucity of bacteria.
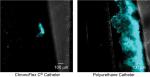  | Figure 2 CFP fluorescence. Abbreviation: CFP, cyan fluorescent protein. |
Bacterial attachment and cell death
In Table 1, Comparison 2 compares total YFP channel fluorescence – reflecting attachment and subsequent cell death – of both control and study catheters. Cell death was on average greater on the study catheter than on the control polyurethane catheter; however, this trend did not achieve statistical significance (P=0.1533). Nevertheless, on average, ~1.5-fold increase in bacterial cell death was observed on the study catheter as compared with the control catheter.
In sum, all catheters encountered actively growing Bs colonies upon “percutaneous” insertion. Yet, despite incubation in conditions favorable for attachment and growth, the study catheter exhibited significantly less bacterial attachment and biofilm formation than the control catheter. Moreover, when bacteria were able to attach to the study catheter, they were 1.5 times more likely to die as compared with attached bacteria on the polyurethane control catheter.
Discussion
This study utilizes a novel in vitro model and fluorescence microscopy to compare two intravascular catheter materials with respect to bacterial attachment and biofilm formation. The control material is a commonly used polyurethane. The study material is a unique copolymer consisting of polycarbonate and polyurethane, treated so as to remove surface additives, alter hydrophobicity and create surface micro-patterning – measures intended to discourage bacterial colonization and bloodstream infection. In clinical use, catheters made of the study material have consistently yielded zero bloodstream infections despite extensive use in a variety of challenging clinical settings.26–30 However, none of these reports feature catheter-related bloodstream infection as a primary endpoint; also, all but one of these studies lack the rigor of a randomized controlled trial (RCT). Therefore, it seemed prudent to begin with a controlled in vitro trial comparing these two materials prior to embarking on an RCT.
The results show that both bacterial attachment and biofilm formation are significantly inhibited by the study catheter material. In fact, over 5 times more bacteria were able to attach and grow on the control polyurethane material as compared with the study material (P=0.0020). Moreover, those bacteria that were able to attach to the study catheter material had 1.5 times greater likelihood of dying – possibly due to their inability to congregate with other cells and form a viable community.
If confirmed clinically in an RCT, these results may have far-reaching implications for vascular access. Presently, despite enormous strides in infection prevention, catheter-related/catheter-associated bloodstream infection remains a major scourge in hospitals worldwide.31,32 For example, bloodstream infection rates for CVCs range from 1% to 3%; similar rates exist for arterial catheters, and percutaneously placed polyurethane midlines have recently reported bloodstream infection rates as high as 3.2%.33–37 Even peripheral IV catheters, which were once removed in 72–96 hours (thereby limiting the time for biofilm to reach maturity), have recently been reported to have an associated bloodstream infection rate of 1.64 per 1,000 hospital admissions.7 The vast majority of these catheters are made of polyurethane, the very material recently shown to bloom additives with normal aging – additives proven to “facilitate bacterial attachment”.13,14
To the best of our knowledge, this study demonstrates for the first time how removal of the aforementioned additives, coupled with both micro-patterning and altered surface hydrophobicity (ie, the BioGUARD™ [Access Scientific, San Diego, CA, USA] secondary processes, performed by the catheter manufacturer), can inhibit bacterial viability on the surface of an extruded tube – specifically, an intravascular catheter. It has been known for some time that flat surfaces, when subjected to micro-patterning and altered hydrophobicity, can “create energetic conditions unfavorable for bacterial attachment and induce repulsive surface–bacterial interaction forces that impair attachment and subsequent biofilm formation”.15,16 It has also been shown, again on flat surfaces, that increased hydrophobicity and decreased availability of contact area can contribute to a reduction in bacterial adhesion.15 The question has been how to translate these findings into real-world applications. Figure 3 shows a scanning electron micrograph of the study catheter’s surface micro-patterning. This surface appears to offer little purchase for one or two microbes, much less a microbial community. If our results are confirmed clinically, one can easily imagine how this technology might be put to use across the entire range of infection-prone intravascular devices, including all central venous access devices, midlines, extended-dwell catheters and so on.
  | Figure 3 SEM of the study catheter material micro-patterning. Abbreviation: SEM, scanning electron micrograph. |
Limitations
While the present study models percutaneous catheter contamination, it should be noted that other routes of contamination exist. For example, intravascular catheters can also become contaminated from hematogenous spread of microorganisms and from accidents of mishandling. Certainly, no direct inference should be made from the present study as to the other potential mechanisms of catheter infection.
Additionally, care should be exercised when extrapolating the in vitro results to in vivo clinical circumstances. Proteins and formed elements present in blood, for example, are not present in the experimental model. Further, the bacteria used in this experiment, B. subtilis, are not the actual pathogens encountered in usual device-related bloodstream infections. Nevertheless, a compelling literature attests to the shared characteristics of the study bacteria and more pathogenic species, such as Staphylococcus aureus, especially with respect to attachment and biofilm formation.18–21
A similar study for in vivo catheters following careful removal could confirm our findings. Also, a study looking at bacterial attachment and biofilm formation on the intraluminal surface of both control and study catheters would provide additional insights and information. Finally, a study comparing the study material to available central lines and arterial lines with regard to biofilm formation both in vivo and in vitro would also be very informative.
Conclusion
Using a novel in vitro percutaneous catheter insertion model, ChronoFlex C with BioGUARD is proven to significantly inhibit bacterial attachment and biofilm formation as compared with a commonly used polyurethane catheter material. These findings may offer insight into the low bloodstream infection rates previously reported with catheters made of this material. A large-scale, randomized controlled clinical trial comparing bloodstream infections associated with extended-dwell catheters made of the study catheter material vs a polyurethane catheter material is now indicated.
Acknowledgments
The authors would like to gratefully acknowledge Samantha Hammack, Research Assistant (University of California, San Diego, CA, USA) for her superb contribution and meticulous execution of the study protocol.
Funding for this research was provided in part by Access Scientific, LLC, including statistical analysis by Instat Consulting, Inc.
Disclosure
Steve Bierman MD is the Chief Medical Officer of Access Scientific, LLC. Pieter d’Arnaud is an employee of Instat Consulting, Inc. The authors report no other conflicts of interest in this work.
References
Livesley MA, Tebbs SE, Moss HA, Faroqui MH, Lambert PA, Elliott TS. Use of pulsed field gel electrophoresis to determine the source of microbial contamination of central venous catheters. Eur J Clin Microbiol Infect Dis. 1998;17(2):108–112. | ||
Heilmann C. Adhesion mechanisms of staphylococci. Adv Exp Med Biol. 2011;715:105–123. | ||
Høiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1–S25. | ||
Xu LC, Siedlecki CA. Bacterial adhesion and interaction with biomaterial surfaces. Chapter 13. In: Hutmacher D, Chrzanowski W, editors, Biointerfaces: Where Material Meets Biology. The Royal Society of Chemistry; 2015:363–398. | ||
Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. II. Long-term devices. Clin Infect Dis. 2002;34(10):1362–1368. | ||
Timsit JF, Rupp M, Bouza E, et al. A state of the art review on optimal practices to prevent, recognize, and manage complications associated with intravascular devices in the critically ill. Intensive Care Med. 2018:742–759. | ||
Guembe M, Pérez-Granda MJ, Capdevila JA, et al. Nationwide study on peripheral-venous-catheter-associated-bloodstream infections in internal medicine departments. J Hosp Infect. 2017;97(3):260–266. | ||
Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. I. Pathogenesis and short-term devices. Clin Infect Dis. 2002;34(9):1232–1242. | ||
Steele D, Norris CM. Cutting peripherally inserted central catheters may lead to increased rates of catheter-related deep vein thrombosis. J Infus Nurs. 2014;37(6):466–472. | ||
Brull SJ, Prielipp RC. Vascular air embolism: a silent hazard to patient safety. J Crit Care. 2017;42:255–263. | ||
Casey AL, Mermel LA, Nightingale P, Elliott TS. Antimicrobial central venous catheters in adults: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(12):763–776. | ||
Weng M, Zhu M, Chen W, Miao C. Life-threatening anaphylactic shock due to chlorhexidine on the central venous catheter: a case series. Int J Clin Exp Med. 2014;7(12):5930–5936. | ||
Nouman M, Jubeli E, Saunier J, Yagoubi N. Exudation of additives to the surface of medical devices: impact on biocompatibility in the case of polyurethane used in implantable catheters. J Biomed Mater Res A. 2016;104(12):2954–2967. | ||
Nouman M, Saunier J, Jubeli E, Yagoubi N. Additive blooming in polymer materials: consequences in the pharmaceutical and medical field. Polym Degrad Stab. 2017;143:239–252. | ||
Feng G, Cheng Y, Wang SY, Borca-Tasciuc DA, Worobo RW, Moraru CI. Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: how small is small enough? NPJ Biofilms Microbiomes. 2015;1:15022. | ||
Xu LC, Siedlecki CA. 9 – Antibacterial polyurethanes. In: Cooper SL, Guan J, editors. Advances in Polyurethane Biomaterials. Woodhead Publishing; 2016:247–284. | ||
Wallace A, Albadawi H, Patel N, et al. Anti-fouling strategies for central venous catheters. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S246–S257. | ||
Balasubramanian D, Harper L, Shopsin B, Torres VJ. Staphylococcus aureus pathogenesis in diverse host environments. Pathog Dis. 2017;75(1):ftx005. | ||
Kuroda M, Ohta T, Uchiyama I, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357(9264):1225–1240. | ||
Roux CM, Demuth JP, Dunman PM. Characterization of components of the Staphylococcus aureus mRNA degradosome holoenzyme-like complex. J Bacteriol. 2011;193(19):5520–5526. | ||
Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol. 2013;11(3):157–168. | ||
van Gestel J, Vlamakis H, Kolter R. New tools for comparing microscopy images: quantitative analysis of cell types in Bacillus subtilis. J Bacteriol. 2015;197(4):699–709. | ||
Karpanen TJ, Worthington T, Conway BR, Hilton AC, Elliott TS, Lambert PA. Penetration of chlorhexidine into human skin. Antimicrob Agents Chemother. 2008;52(10):3633–3636. | ||
Safdar N, Maki DG. The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med. 2004;30(1):62–67. | ||
Johnson MB, Criss AK. Fluorescence microscopy methods for determining the viability of bacteria in association with mammalian cells. J Vis Exp. 2013;79. | ||
Warrington WG, Aragon Penoyer D, Kamps TA, van Hoeck EH. Outcomes of using a modified Seldinger technique for long term intravenous therapy in hospitalized patients with difficult venous access. J Vasc Access. 2012;17(1):24–30. | ||
Caparas JV, Hu JP. Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access. 2014;15(4):251–256. | ||
Caparas JV, Hu JP. Safe administration of long-term vancomycin through a novel midline catheter: a response to letter to the editor. J Vasc Access. 2016;17(4):e92. | ||
Caparas JV, Hung H-S. Vancomycin administration through a novel midline catheter: summary of a 5-year, 1086-patient experience in an urban community hospital. J Vasc Access. 2017;22(1):38–41. | ||
Pathak R, Patel A, Enuh H, Adekunle O, Shrisgantharajah V, Diaz K. The incidence of central line-associated bacteremia after the introduction of midline catheters in a ventilator unit population. Infect Dis Clin Pract (Baltim Md). 2015;23(3):131–134. | ||
Moureau N. Reducing risks of bloodstream infections. Mater Manag Health Care. 2009;18(11):26–28. | ||
Latif A, Halim MS, Pronovost PJ. Eliminating infections in the ICU: CLABSI. Curr Infect Dis Rep. 2015;17(7):491. | ||
Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. | ||
Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741. | ||
Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918. | ||
Dumont C, Getz O, Miller S. Evaluation of midline vascular access: a descriptive study. Nursing. 2014;44(10):60–66. | ||
Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

