Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Inhaled corticosteroid use by exacerbations and eosinophils: a real-world COPD population
Authors Vestbo J , Vogelmeier CF, Small M, Siddall J, Fogel R , Kostikas K
Received 3 October 2018
Accepted for publication 15 March 2019
Published 16 April 2019 Volume 2019:14 Pages 853—861
DOI https://doi.org/10.2147/COPD.S189585
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Jørgen Vestbo,1 Claus F Vogelmeier,2 Mark Small,3 James Siddall,3 Robert Fogel,4 Konstantinos Kostikas4,5
1Division of Infection, Immunity & Respiratory Medicine, University of Manchester, Manchester, UK; 2Department for Pulmonary Medicine, Philipps-University of Marburg, Marburg, Germany; 3Respiratory Research, Adelphi Real World, Bollington, UK; 4Global Medical Affairs, Novartis Pharma AG, Basel, Switzerland; 5Respiratory Medicine Department, University of Ionnina Medical School, Ionnina, Greece
Background: Blood eosinophils may predict response to inhaled corticosteroids (ICS) in chronic obstructive pulmonary disease (COPD) where ICS is recommended in patients at high risk of exacerbations. The proportion of patients who may benefit the most from ICS-based therapy was quantified in a real-world population.
Materials and methods: European data from the Adelphi Real World Respiratory Disease Specific Programme™ 2017 survey were collected from consecutive COPD patients by participating physicians. Overall, 1,528 patients were assessable for Global Initiative for COPD (GOLD) 2017 status and were included in the analysis.
Results: More GOLD D patients had elevated eosinophil counts compared with GOLD B. The proportions of GOLD D patients with a history of ≥2 exacerbations and eosinophil counts of ≥150, ≥300, and ≥400 cells/μL were 81.2%, 39.4%, and 24.6%, respectively. In total, 10.6% of the patients had ≥300 eosinophils/μL and a history of ≥2 exacerbations. ICS-based therapy was received by 41.5% of GOLD B and 68.0% of GOLD D patients.
Conclusion: There was no apparent relation between ICS use and eosinophil blood count. There are differences in the distributions of patients with frequent exacerbations and/or high blood eosinophil counts and the use of ICS in COPD. These data may provide information for the implementation of future treatment recommendations.
Keywords: COPD, exacerbations, inhaled corticosteroids, eosinophils, observational study
Introduction
The clinical benefits versus risks of inhaled corticosteroids (ICS) in the treatment of chronic obstructive pulmonary disease (COPD) have been disputed. Initially widely prescribed, since 2011, they are now recommended for symptomatic patients at high risk of exacerbations by the Global Initiative for COPD (GOLD) Strategy.1,2 Putative concerns over ICS in the treatment of COPD include increased risks of pneumonia, oral candidiasis and hoarseness, and possibly hyperglycemia, osteoporotic fractures, and cataracts.3 It is therefore important to limit ICS exposure to those patients likely to benefit. Nevertheless, ICS remain widely prescribed in combination with a long-acting β2-agonist (LABA), or both a LABA and long-acting muscarinic agonist (LAMA) (“triple” therapy),4–7 and the over-prescription of ICS in COPD has been widely reported.4,6–10
The GOLD Strategy document revision of 2017 includes changes that reduce the recommended use of ICS.2 Recognizing the limitations of forced expiratory volume in 1 second (FEV1) in predicting the risk of exacerbations, spirometry has been removed from the ABCD classification process for deciding management. Consequently, patients with more symptoms who were also previously considered high risk (group D) solely due to impaired lung function have now been reclassified as low risk (group B), for whom ICS are not recommended.2
In GOLD 2017 group D, the preferred initial prescription for COPD patients without concomitant asthma is LABA plus LAMA therapy, with the addition of ICS in patients with continued exacerbations.2 However, it is probable that ICS responders form a subset of the COPD population. Evidence is emerging that blood eosinophil counts in COPD patients may predict response to ICS.11–18 For example, a secondary analysis of data from two parallel randomized controlled trials showed that reductions in exacerbations with fluticasone furoate/vilanterol, compared with vilanterol alone, were more prominent with increasing blood eosinophil counts.11 Similar findings were reported when comparing beclomethasone/formoterol with formoterol only.12 A post-hoc analysis of the large WISDOM study (n=2,296) also demonstrated that withdrawal of ICS in patients previously receiving triple therapy was associated with an increased risk of exacerbations only in those who had an eosinophil count ≥300 cells/µL and a history of exacerbations.18 Finally, when comparing triple therapy with tiotropium, the effect on risk of exacerbations was more pronounced in patients with an eosinophil count above 200 cells/µL or 2% than in patients with lower eosinophil counts.19 Data from the FLAME study showed that indacaterol/glycopyrronium provided superior or similar benefits to salmeterol/fluticasone in reducing the risk of exacerbations, regardless of blood eosinophil levels in patients with COPD;13 however, there was a significant interaction between treatment effect and blood eosinophil levels with the two treatments demonstrating similar efficacy in patients with blood eosinophils ≥300 cells/μL.17
It may therefore be possible to define which COPD patients may significantly benefit from ICS-based therapy based on exacerbation history and blood eosinophil counts. Such data may be extracted through analysis of a real-life data set. The Adelphi Real World Disease Specific Programme™ (DSP) is a large multinational, cross-sectional survey that generates observational real-world data from current clinical practice.
The aim of this analysis is to report the proportion of COPD patients in the DSP who had a history of 0, 1, or ≥2 exacerbations in the last 12 months, with additional stratification by blood eosinophil levels and GOLD 2017 grouping. The study will also describe the clinical characteristics and current treatment patterns of the COPD patients who may benefit from ICS-based therapy.
Methods
Cross-sectional data collected via physician and patient surveys undertaken by the Respiratory DSP 2017 data were collected in the first three months of the year and are representative of COPD patients presenting to their physician in a routine care setting. The methodology has been published previously.20,21 In brief, data were collected from primary care physicians (PCPs) and pulmonologists and their patients in five European countries (France, Germany, Italy, Spain, and the UK). To be eligible to participate, PCPs and pulmonologists had to manage >3 patients each week, be actively involved in COPD management and have been medically qualified between 1979 and 2012. Inclusion criteria for patients were a physician-confirmed airflow obstruction and a current diagnosis of COPD (including emphysema and/or chronic bronchitis).
Each participating physician completed a patient record form for the next five consecutive patients with COPD who consulted them for any reason (not necessarily COPD). The same patients then voluntarily completed details relating to their condition via a patient self-completion form (PSC). Although not truly random, the study physicians had no control over which of the eligible patients in their care presented in their clinic during the data collection period, and physician pre-selection of patients was thereby limited. Prescription of long-acting bronchodilators and ICS were not regulated in this study. Patients were excluded at the analysis stage if they were subsequently found to have a diagnosis or history of asthma.
Primary patient variables extracted for analysis were exacerbation frequency and severity in the previous 12 months, blood eosinophil count, and current treatment group. Exacerbations were physician-confirmed and were recorded regardless of severity. Exacerbations extracted for analysis were presented as moderate (treated with oral/systemic corticosteroids or antibiotics) or severe (requiring hospitalization). To qualify as high risk for exacerbations, as described by GOLD 2017, patients suffered ≥2 moderate or severe exacerbations, or ≥1 severe exacerbation (requiring hospitalization), in the previous 12 months.2 An eosinophil count was described as high according to two thresholds: ≥300 cells/µL and ≥400 cells/µL, as defined by previous studies.12,17,18,22 Descriptive variables, including GOLD 2017 group, COPD Assessment Test (CAT) score,23 modified Medical Research Council Dyspnea Scale (mMRC),24 and most recent post-bronchodilator FEV1% predicted, were also collected, as previously described.21
The full Respiratory DSP XV (2017) sample size conducted in the EU comprised of 600 physicians (300 PCPs and 300 pulmonologists). These physicians completed patient record form data on 3,003 COPD-only patients. Patients with a concomitant diagnosis of asthma were excluded from the analysis (n=127), producing a maximum study population of 2,876 with full exacerbation and treatment history in the last 12 months.
Data are presented as patient proportions and summary statistics.
The DSP was conducted as a survey adhering to market research guidelines and codes of conduct according to the International Chamber of Commerce/European Society for Opinion and Marketing Research international code on observational research. Before completing the voluntary PSC, patients were asked to provide written consent. Physicians and patients provided anonymized data. The survey was submitted to the Freiburger Ethic-Kommission International (FEKI) where approval was granted on 25th January 2017 (FEKI Code 017/1014).
Results
Patient baseline characteristics
Of the 2,876 patients included, 1,563 supplied the accompanying self-completion form and represent the maximum study population where patient-reported data is given. Of these, 1,528 were assessable for GOLD 2017 group status, calculated by using physician-reported recent history of exacerbations and patient-reported CAT scores (Figure 1). Baseline characteristics are presented in Table 1.
 | Table 1 Baseline characteristics of all EU patients with derived GOLD classification (n=1,528) |
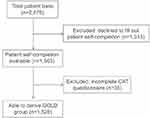 | Figure 1 Patient disposition.Abbreviation: CAT, COPD Assessment Test. |
The mean age of the population was 66 years; approximately 70% of patients were male. Almost all were current or ex-smokers, with high past or present tobacco consumption. As expected, the patient population had many comorbidities, approximately two-thirds presenting with hypertension alone. Patients in groups A, B, C, and D constituted 12.0%, 61.1%, 0.9%, and 26.0% of the population, respectively, and did not differ significantly between primary care physicians and pulmonologists. As patients were predominantly GOLD B or D, we focused on comparisons between these two groups.
Regarding post-bronchodilator FEV1% predicted, 33.8% of GOLD D patients had values <50%. In GOLD B patients, the corresponding figure was 11.0%.
Exacerbation rates by GOLD 2017 group
The proportions of COPD patients who had a history of 0, 1, 2, or ≥3 exacerbations in the last 12 months in GOLD groups B and D are shown in Figure 2. As per definition, all GOLD B patients experienced <2 exacerbations (mean, 0.2) during this period. In contrast, the mean number of exacerbations in GOLD D was 2.3, and 83.8% of GOLD D patients experienced ≥2 exacerbations. This figure additionally shows that almost 80% of GOLD B patients experienced no exacerbations over the study period, and, by definition, no patients in this group suffered a severe exacerbation. In the GOLD D group, one-quarter of patients experienced ≥3 exacerbations, and 58% suffered ≥1 severe exacerbation. In total, 41.6% of GOLD D patients experienced moderate exacerbations only.
Eosinophil blood count distribution by GOLD group and exacerbation frequencies
Where both data variables were available, mean eosinophil counts in GOLD B and D groups were 257.3 (n=205) and 294.0 (n=147) cells/µL, respectively. Numerically, greater proportions of patients in GOLD D group had elevated cell counts compared with the GOLD B group (≥150 cells/µL, 79.7% vs 73.8%; ≥300 cells/µL, 37.8% vs 30.5%; ≥400 cells/µL, 23.6% vs 19.2%).
Figure 3 shows the proportions of COPD patients with histories of 0, 1, or ≥2 of total and severe exacerbations by blood eosinophil levels. There was a numerical trend for higher proportions of patients with eosinophil counts of ≥300 cells/µL with a history of 1 (41.0%) or ≥2 (39.4%) exacerbations compared with no exacerbations (25.0%). In contrast, there was no apparent relation for eosinophil blood count distribution by the number of previous severe exacerbations. The proportions of patients with a history of 1 or ≥2 severe exacerbations and an eosinophil count of ≥300 cells/µL were 35.8% and 29.3%, respectively. The proportion of patients with an eosinophil blood count of ≥300 cells/µL and with a history of ≥2 moderate/severe exacerbations in the overall study population was 10.6%.
Current treatment patterns by GOLD group and eosinophil blood count distribution
Current treatment patterns by GOLD group are shown in Figure 4. ICS-based therapy was being received by 41.5% of GOLD B patients and 68.0% of GOLD D patients. There was no clear difference between patients seen by primary care physicians or pulmonologists, although primary care physicians tended to treat patients classified as GOLD B with more ICS, 45.7% vs 38.5%. Almost half of GOLD D patients were receiving triple therapy. In total, 56.2% of GOLD B patients were receiving mono or dual bronchodilator therapy. A minority of GOLD D patients (8.6%) were taking mono or short-acting only bronchodilator therapy.
The percentages of patients receiving ICS therapy by exacerbation rates (any) and by eosinophil blood count distribution are shown in Figure 5. The data show that ICS use increased with higher rates of exacerbations, but that there was no apparent relation between ICS use and eosinophil blood count.
Discussion
This study, performed in a large real-world data set of patients with COPD, provided detailed information regarding clinical characteristics, exacerbation history, and eosinophil blood count distribution across GOLD 2017 groups B and D. It also showed that a minority of patients overall presented both as high risk (GOLD group D) and with high blood eosinophil levels (≥300 cells/µL) and that many “low-risk” patients (GOLD group B) and blood eosinophil levels <300 cells/µL received ICS.
The current study population predominantly fell into GOLD 2017 highly symptomatic groups B and D, as reflected by a mean CAT symptom score of >20. In the present study, as previously noted for this DSP and other populations, these data may indicate that COPD is frequently undiagnosed unless the patient is highly symptomatic.21,25,26
The GOLD 2017 group distribution in the current study differs from the GOLD 2011 distribution in a previous study of this DSP21 in that the proportion of GOLD B patients was considerably larger and that for GOLD D patients was correspondingly smaller. This resulted from patients previously considered group D due to impaired lung function alone under the GOLD 2011 classification being reclassified as group B under the new GOLD classification.2 This has been observed in other cohorts.27,28
According to the GOLD 2017 Strategy, ICS-based treatment is preferred only for patients classified as group D.2 In the present study, these patients were not only more likely to experience ≥2 exacerbations and/or be hospitalized in the previous 12 months, by definition, but were also more burdened by COPD symptoms, poor lung function, and comorbidities. More than half of group D patients suffered ≥1 severe exacerbation. Each exacerbation requiring hospitalization has been shown to cause a decline in health and raise the risk of further severe exacerbations.29 A parallel worsening in symptoms, airflow limitation, and exacerbation incidence in more severe disease has previously been noted for this DSP.21 A link between COPD risk under the 2011 GOLD Strategy and incidence of comorbidities has been reported in other studies.25,30
There was a clear contrast between group D patients and group B patients, many of whom experienced no exacerbations, confirming their low-risk status. Although the mean eosinophil blood count and the proportion of patients with blood counts ≥300 cells/µL were higher in group D patients versus group B patients; the differences in blood count distribution between these groups were not marked. Further examination showed that there was a similar modest difference in blood count distribution between patients experiencing <2 and ≥2 exacerbations, as may be expected from the GOLD group data. The greatest differences in blood eosinophil count distribution were seen between that for patients with no exacerbations and those for patients with 1 or ≥2 in the previous 12 months, which is not reflected by GOLD grouping.2 There was no apparent relation between eosinophil blood count distribution and number of severe exacerbations.
In a Danish study, compared with lower eosinophil levels, a blood count of ≥340 cells/µL was associated with a 76% adjusted increase in risk for severe exacerbations and a 15% increase for moderate exacerbations.31 More recently, a retrospective survey found that, after adjustment, significantly greater numbers of exacerbations were found in patients with blood eosinophil counts of ≥300, ≥400, and ≥500 cells/µL compared with those with counts lower than each of these respective thresholds.32 However, in the FLAME study, there was no relation found between blood eosinophil count and the rate of moderate/severe exacerbations in the overall study population.13 It should be noted, however, that in this latter study, for patients with blood eosinophil counts of <300 cells/µL, indacaterol/glycopyrronium significantly reduced the rate of moderate/severe exacerbations compared with salmeterol/fluticasone in patients with 1 and ≥2 exacerbations (p<0.001 and p=0.038, respectively), whereas the effects of both treatments were similar in patients with blood eosinophil counts of ≥300 cells/µL, independent of exacerbation history.17 The study data therefore support the contention that blood eosinophil levels are a marker of response to ICS.
It is possible that a clear relation between eosinophil blood count and exacerbation history was not observed in the present study due to the widespread prescription of ICS, which may have suppressed exacerbations – or eosinophils – in some patients. Indeed, some patients classified as group B may have been “masked” group D patients, whose exacerbations were controlled by the long-term (ie, >12 months) administration of ICS. Data from the FLAME study again showed long-term ICS therapy to have had a minimal effect on blood eosinophil levels,13 but given the large number of studies examining baseline eosinophil counts and response to ICS, there is a scarcity of studies examining the effects of different ICSs at different doses on blood eosinophils.
Data from the present study show that treatment with ICS bears no relation to eosinophil blood count distribution. This may be expected as currently there is no strong recommendation for considering eosinophil levels when deciding treatment.2 However, the present data show that ICS-based treatment was being received by over half of all patients with blood counts of <300 cells/µL, and even <150 cells/μL, who may be less likely to benefit from these therapies.18 Indeed, the proportion of patients with an eosinophil blood count of ≥300 cells/µL and a history of ≥2 moderate/severe exacerbations was under 11% of the total COPD population in our study.
Recent evidence has shown that it is possible in appropriate patients receiving long-acting bronchodilators, to withdraw ICS without increasing the overall risk of exacerbations. The WISDOM study showed that most patients with eosinophil counts <300 cells/µL receiving triple therapy were able to discontinue ICS without experiencing increased exacerbations.18 An analysis of data from the DACCORD observational study showed that in appropriate patients in real-life clinical practice, it was possible to change treatment from ICS/LABA or triple therapy to LABA/LAMA therapy without increased exacerbations.33
In 2017, two new fixed-dose combination (FDC) triple therapy formulations were licensed.34,35 The active comparators in the pivotal trials for these formulations were ICS/LABA or LAMA alone. The first comparison between FDC triple therapy and a LABA/LAMA combination included symptomatic patients with ≥1 exacerbation in the previous year and found that FDC triple therapy was superior to LABA/LAMA in reducing the rate of moderate-to-severe exacerbations by 15%, the effect being more pronounced in those with a baseline eosinophil count of ≥200 cells/µL (19%) versus those with lower counts (13%).17 The second comparison found that FDC was superior to LABA/LAMA in reducing the rate of moderate-to-severe exacerbations (25% reduction). The effect was also more pronounced in this study in patients with a baseline eosinophil count of ≥150 cells/µL (32%) compared to those with lower counts (12%).36
Regarding study limitations, given the difficulties in differentiating diagnoses of specific respiratory diseases, it is conceivable an unknown proportion of patients may have been incorrectly diagnosed with COPD. However, this is common to most real-world surveys and unlikely a significant problem. In addition, the GOLD A-D classification is meant mainly to decide initial treatment and we cannot preclude that a number of patients in Group B are actually not Group D patients originally who have subsequently responded to treatment. Furthermore, the sample collected is not a truly random sample of COPD patients. Therefore, it may inaccurately represent the overall COPD patient population. Lastly, for tests not routinely conducted or available, a sizable amount of missing data is to be expected. Therefore, generalizability is unknown as the assumption that the missing data reflects the data collected cannot be confirmed. Despite these limitations, real-world data collected without pre-selection of patients and mandatory measurement of disease characteristics make a valuable contribution to understand COPD disease.
Conclusion
In conclusion, this real-world study data indicate that there are differences in the distributions of patients with frequent exacerbations and/or high blood eosinophil counts and the use of ICS in COPD. Only a minority of the overall patient population presented both as high risk (≥2 exacerbations) and with high (≥300 cells/µl) blood eosinophil levels. These data may provide information for the implementation of future treatment recommendations.
Acknowledgments
The authors wish to thank Neil Fisher, PhD, of Helicon Medical Writing Ltd (Welling, UK) for providing medical writing support which was in accordance with Good Publication Practice (GPP3) guidelines and funded by Novartis. JV is supported by the NIHR Manchester Biomedical Research Centre. Data collection was undertaken by Adelphi Real World as part of a syndicated survey, entitled the Respiratory Disease Specific Programme, subscribed to by multiple pharmaceutical companies of including Novartis. The original survey was designed and the data were collected independently of Novartis. The analysis described here using data from the survey was funded by Novartis and all authors contributed to the analysis and interpretation of the data, the writing of the report and the decision to submit the paper for publication.
Disclosure
JV reports personal fees from AstraZeneca, grants, Boehringer-Ingelheim, Chiesi, and Novartis, outside the submitted work; CV reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, received personal fees from CSL Behring, Chiesi, GlaxoSmithKline, Grifols, Menarini, Mundipharma, Novartis, Teva, Bayer Schering Pharma AG, MSD, and Pfizer, outside the submitted work; MS reports non-personal fees (work by Adelphi from Novartis), during the conduct of the study; and is a full time employee of Adelphi Real World. JS reports nothing from Novartis, during the conduct of the study; and is a full time employee of Adelphi Real World. RF is an employee of and own stock in Novartis Pharmaceuticals Corporation. KK was an employee of Novartis at the time of conduct of this study. The authors report no other conflicts of interest in this work.
References
1. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
2.
3. Matera MG, Cardaci V, Cazzola M, Rogliani P. Safety of inhaled corticosteroids for treating chronic obstructive pulmonary disease. Expert Opin Drug Saf. 2015;14(4):533–541. doi:10.1517/14740338.2015.1001363
4. Koblizek V, Pecen L, Zatloukal J, et al. Real-life GOLD 2011 implementation: the management of COPD lacks correct classification and adequate treatment. PLoS One. 2014;9(11):e111078. doi:10.1371/journal.pone.0111078
5. Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217. doi:10.2147/COPD.S91694
6. Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47–53. doi:10.1016/j.rmed.2015.12.004
7. Safka KA, Wald J, Wang H, McIvor L, McIvor A. GOLD stage and treatment in COPD: a 500 patient point prevalence study. Chronic Obstr Pulm Dis. 2016;4(1):45–55. doi:10.15326/jcopdf.4.1.2016.0126
8. Chalmers JD, Tebboth A, Gayle A, Ternouth A, Ramscar N. Determinants of initial inhaled corticosteroid use in patients with GOLD A/B COPD: a retrospective study of UK general practice. NPJ Prim Care Respir Med. 2017;27(1):43. doi:10.1038/s41533-017-0040-z
9. Franssen FM, Spruit MA, Wouters EF. Determinants of polypharmacy and compliance with GOLD guidelines in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:493–501. doi:10.2147/COPD.S24443
10. Fitch K, Iwasaki K, Pyenson B, Plauschinat C, Zhang J. Variation in adherence with Global Initiative for Chronic Obstructive Lung Disease (GOLD) drug therapy guidelines: a retrospective actuarial claims data analysis. Curr Med Res Opin. 2011;27(7):1425–1429. doi:10.1185/03007995.2011.583230
11. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X
12. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi:10.1164/rccm.201502-0235LE
13. Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med. 2017;195(9):1189–1197. doi:10.1164/rccm.201701-0193OC
14. Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016;47(5):1374–1382. doi:10.1183/13993003.01370-2015
15. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi:10.1164/rccm.201108-1553OC
16. Hinds DR, DiSantostefano RL, Le HV, Pascoe S. Identification of responders to inhaled corticosteroids in a chronic obstructive pulmonary disease population using cluster analysis. BMJ Open. 2016;6(6):e010099. doi:10.1136/bmjopen-2015-010099
17. Papi A, Kostikas K, Wedzicha JA, et al. Dual bronchodilation response by exacerbation history and eosinophilia in the FLAME study. Am J Respir Crit Care Med. 2018;197(9):1223–1226.
18. Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi:10.1016/S2213-2600(16)00100-4
19. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi:10.1016/S0140-6736(17)30188-5
20. Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes – a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi:10.1185/03007990802457040
21. Vestbo J, Vogelmeier C, Small M, Higgins V. Understanding the GOLD 2011 strategy as applied to a real-world COPD population. Respir Med. 2014;108(5):729–736. doi:10.1016/j.rmed.2014.03.002
22. Brightling CE, Bleecker ER, Panettieri RA
23. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
24. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586.
25. Raluy-Callado M, Lambrelli D, MacLachlan S, Khalid JM. Epidemiology, severity, and treatment of chronic obstructive pulmonary disease in the United Kingdom by GOLD 2013. Int J Chron Obstruct Pulmon Dis. 2015;10:925–937. doi:10.2147/COPD.S82064
26. Johns DP, Walters JA, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thorac Dis. 2014;6(11):1557–1569. doi:10.3978/j.issn.2072-1439.2014.08.18
27. Marcoa R, Rodrigues DM, Dias M, et al. Classification of chronic obstructive pulmonary disease (COPD) according to the new Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: comparison with GOLD 2011. COPD. 2018; 15(1):21–26.
28. Tudoric N, Koblizek V, Miravitlles M, et al. GOLD 2017 on the way to a phenotypic approach? Analysis from the Phenotypes of COPD in Central and Eastern Europe (POPE) Cohort. Eur Respir J. 2017;49(4):1602518. doi:10.1183/13993003.02518-2016
29. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi:10.1136/thoraxjnl-2011-201518
30. Jones PW, Nadeau G, Small M, Adamek L. Characteristics of a COPD population categorised using the GOLD framework by health status and exacerbations. Respir Med. 2014;108(1):129–135. doi:10.1016/j.rmed.2013.08.015
31. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965–974. doi:10.1164/rccm.201509-1869OC
32. Zeiger RS, Tran TN, Butler RK, et al. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2018;6(3):944–954.
33. Worth H, Buhl R, Criee CP, Kardos P, Lossi NS, Vogelmeier CF. GOLD treatment pathways in ‘real life’: an analysis of the DACCORD observational study. Respir Med. 2017;2017(131):77–84. doi:10.1016/j.rmed.2017.08.008
34. TRELEGY ELLIPTA (fluticasone furoate, umeclidinium, and vilanterol inhalation powder), for oral inhalation. Highlights of Prescribing Information. GlaxoSmithKline & Innoviva; 2017. [cited]. Available from:
35. Trimbow 87 micrograms/5 micrograms/9 micrograms pressurised inhalation, solution. Summary of Product Characteristics. Chiesi Limited. 2017.
36.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




